Cataract Prevalence in Patients with Cutaneous Melanoma in Lithuanian Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ophthalmological Examination
2.3. Dermatological Examination
2.4. Questionnaire Data
2.5. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivanov, I.V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. Ultraviolet Radiation Oxidative Stress Affects Eye Health. J. Biophotonics 2018, 11, e201700377. [Google Scholar] [CrossRef] [PubMed]
- Zinflou, C.; Rochette, P.J. Ultraviolet A-Induced Oxidation in Cornea: Characterization of the Early Oxidation-Related Events. Free. Radic. Biol. Med. 2017, 108, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, E.; Nechalioti, P.M.; Georgopoulou, K.E.; Goniotakis, G.; Roussaki Schulze, A.V.; Zafiriou, E.; Kouretas, D. Systemic Oxidative Stress Parameters in Skin Cancer Patients and Patients with Benign Lesions. Stresses 2023, 3, 785–812. [Google Scholar] [CrossRef]
- Raimondi, S.; Suppa, M.; Gandini, S. Melanoma Epidemiology and Sun Exposure. Acta Derm. Venereol. 2020, 100, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Oliveria, S.A.; Saraiya, M.; Geller, A.C.; Heneghan, M.K.; Jorgensen, C. Sun Exposure and Risk of Melanoma. Arch. Dis. Child. 2006, 91, 131–138. [Google Scholar] [CrossRef]
- Šemeklis, L.; Kapitanovaitė, L.; Butrimas, G.; Briedė, K.; Dubinskaitė, A.; Žemaitienė, R.; Valiukevičienė, S. Iris Pigmented Lesions and Risk of Cutaneous Melanoma: Case–Control Study in Lithuania. J. Pers. Med. 2024, 14, 530. [Google Scholar] [CrossRef]
- Pesudovs, K.; Lansingh, V.C.; Kempen, J.H.; Tapply, I.; Fernandes, A.G.; Cicinelli, M.V.; Arrigo, A.; Leveziel, N.; Briant, P.S.; Vos, T.; et al. Global Estimates on the Number of People Blind or Visually Impaired by Cataract: A Meta-Analysis from 2000 to 2020. Eye 2024, 38, 2229–2231. [Google Scholar] [CrossRef]
- Varssano, D.; Friedman, M.; Goldstein, M.; Bar-Sela, S.; Sella, T.; Shalev, V.; Chodick, G. Association between Cataract and Keratinocytic Skin Cancers or Melanoma: Speculating on the Common Role of Sun and Ultraviolet Radiation Exposures. Ophthalmic Epidemiol. 2017, 24, 336–340. [Google Scholar] [CrossRef]
- Vashist, P.; Tandon, R.; Murthy, G.V.S.; Barua, C.K.; Deka, D.; Singh, S.; Gupta, V.; Gupta, N.; Wadhwani, M.; Singh, R.; et al. Association of Cataract and Sun Exposure in Geographically Diverse Populations of India: The CASE Study. First Report of the ICMR-EYE SEE Study Group. PLoS ONE 2020, 15, e0227868. [Google Scholar] [CrossRef]
- Modenese, A.; Gobba, F. Cataract Frequency and Subtypes Involved in Workers Assessed for Their Solar Radiation Exposure: A Systematic Review. Acta Ophthalmol. 2018, 96, 779–788. [Google Scholar] [CrossRef]
- Haag, R.; Sieber, N.; Heßling, M. Cataract Development by Exposure to Ultraviolet and Blue Visible Light in Porcine Lenses. Medicina 2021, 57, 535. [Google Scholar] [CrossRef] [PubMed]
- Galichanin, K.; Löfgren, S.; Söderberg, P. Cataract after Repeated Daily in Vivo Exposure to Ultraviolet Radiation. Health Phys. 2014, 107, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Chang, Y.J.; Shieh, C.F.; Yu, J.H.; Yang, M.C. Relationship between Practices of Eye Protection against Solar Ultraviolet Radiation and Cataract in a Rural Area. PLoS ONE 2021, 16, e0255136. [Google Scholar] [CrossRef]
- Sharma, S.; Lang, C.; Khadka, J.; Inacio, M.C. Association of Age-Related Cataract with Skin Cancer in an Australian Population. Investig. Ophthalmol. Vis. Sci. 2020, 61, 48. [Google Scholar] [CrossRef]
- Keung, E.Z.; Gershenwald, J.E. The Eighth Edition American Joint Committee on Cancer (AJCC) Melanoma Staging System: Implications for Melanoma Treatment and Care. Expert. Rev. Anticancer. Ther. 2018, 18, 775. [Google Scholar] [CrossRef] [PubMed]
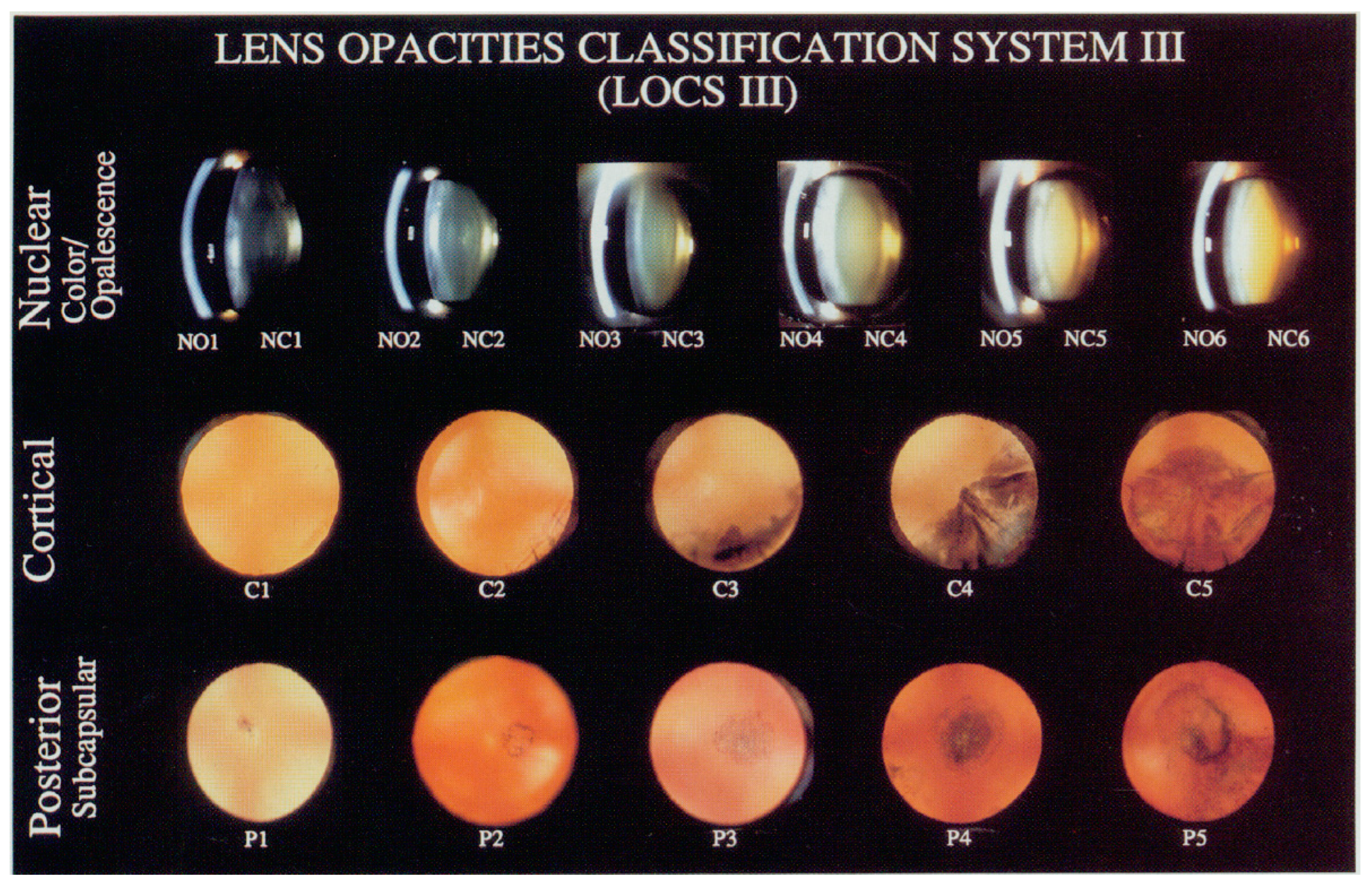
- Chylack Jr, L.T.; Leske, M.C.; McCarthy, D.; Khu, P.; Kashiwagi, T.; Sperduto, R. Lens Opacities Classification System II (LOCS II). Arch Ophthalmol 1989, 107, 991–997. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Valiukeviciene, S.; Gollnick, H.; Stang, A. Body-Site Distribution of Common Acquired Melanocytic Nevi Associated with Severe Sunburns among Children in Lithuania. Int. J. Dermatol. 2007, 46, 1242–1249. [Google Scholar] [CrossRef]
- Institute of Hygiene. Lithuanian Health Statistics. Available online: https://stat.hi.lt (accessed on 25 October 2024).
- Deng, Y.; Yang, D.; Yu, J.M.; Xu, J.X.; Hua, H.; Chen, R.T.; Wang, N.; Ou, F.R.; Liu, R.X.; Wu, B.; et al. The Association of Socioeconomic Status with the Burden of Cataract-Related Blindness and the Effect of Ultraviolet Radiation Exposure: An Ecological Study. Biomed. Environ. Sci. 2021, 34, 101–109. [Google Scholar] [CrossRef]
- Tang, Y.; Ji, Y.; Ye, X.; Wang, X.; Cai, L.; Xu, J.; Lu, Y. The Association of Outdoor Activity and Age-Related Cataract in a Rural Population of Taizhou Eye Study: Phase 1 Report. PLoS ONE 2015, 10, e0135870. [Google Scholar] [CrossRef]
- Verma, C.; Lehane, J.; Neale, R.E.; Janda, M. Review of Sun Exposure Guidance Documents in Australia and New Zealand. Public Health Res. Pract. 2022, 32, e3212202. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, R.; Zanin Poletto, G.; Guarneri, F.; Conforti, C.; Guarneri, C.; Hofmann-Wellenhof, R.; Zalaudek, I. Facial Naevus Count in the Identification of Patients at Higher Risk of Melanoma. Br. J. Dermatol. 2023, 189, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, D.; Nufer, K.L.; Betz-Stablein, B.; Soyer, H.P.; Janda, M. Body Site Distribution of Acquired Melanocytic Naevi and Associated Characteristics in the General Population of Caucasian Adults: A Scoping Review. Dermatol. Ther. 2022, 12, 2453–2488. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; Buettner, P.G.; Maclennan, R. Body-Site Distribution of Melanocytic Nevi in Young Australian Children. Arch. Dermatol. 1999, 135, 47–52. [Google Scholar] [CrossRef]
- Ivert, L.U.; Dal, H.; Rodvall, Y.; Lindelöf, B. Analysis of the Stockholm Public Health Cohort: Exploring How Ultraviolet Radiation and Other Factors Associate with Skin Cancer. J. Ski. Cancer 2024, 2024, 7142055. [Google Scholar] [CrossRef]
- Liu, M.; Lan, Y.; Zhang, H.; Wu, M.; Zhang, X.; Leng, L.; Zheng, H.; Li, J. Analysing the Causal Relationship between Potentially Protective and Risk Factors and Cutaneous Melanoma: A Mendelian Randomization Study. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 102–111. [Google Scholar] [CrossRef]
- Solar Ultraviolet (UV) Radiation Exposure. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/uv-radiation (accessed on 25 October 2024).
| Characteristics | Control Group n = 182 | Case Group n = 180 | p-Value |
|---|---|---|---|
| Sex, % (n 1) | 0.892 | ||
| Male | 37.9 (69) | 37.2 (67) | |
| Female | 62.1 (113) | 62.8 (113) | |
| Total | 100.0 (182) | 100.0 (180) | |
| Age (years), mean (SD 2) | |||
| Male | 57.97 (8.2) | 60.70 (13.5) | 0.155 |
| Female | 57.34 (7.83) | 58.88 (11.89) | 0.249 |
| Total | 57.58 (7.95) | 59.56 (12.51) | 0.072 |
| Age groups (years), % (n) | 0.974 | ||
| ≤50 | 23.6 (43) | 22.8 (41) | |
| 51–60 | 25.8 (47) | 26.7 (48) | |
| ≥61 | 50.5 (92) | 50.6 (91) | |
| Skin color, % (n) | <0.001 | ||
| Fair | 29.5 (52) | 48.6 (87) | |
| Medium | 65.9 (116) | 40.8 (73) | |
| Olive | 4.5 (8) | 10.6 (19) | |
| Skin type (Fitzpatrick scale), % (n) | 0.061 | ||
| Type I | 15.3 (27) | 21.7 (39) | |
| Type II | 39.5 (70) | 36.1 (65) | |
| Type III | 30.5 (53) | 21.1 (38) | |
| Type IV | 14.7 (26) | 21.1 (38) | |
| Hair color, % (n) | 0.134 | ||
| Light brown | 51.7 (91) | 62.0 (111) | |
| Dark brown | 30.5 (54) | 25.1 (45) | |
| Black | 14.7 (26) | 12.8 (23) | |
| Familiar anamnesis of CM, % (n) | 0.541 | ||
| Yes | 2.2 (4) | 3.3 (6) | |
| No | 97.8 (178) | 96.7 (174) | |
| Wears sunglasses, % (n) | <0.001 | ||
| Yes | 63.5 (115) | 43.6 (78) | |
| No | 36.5 (66) | 56.4 (101) | |
| Severe sunburn, % (n) | |||
| Did not have severe sunburn | 69.8 (127) | 10.0 (18) | <0.001 |
| Had sunburn redness | 14.8 (27) | 23.3 (42) | 0.004 |
| Had peeled skin after sunburn | 13.2 (24) | 38.9 (70) | <0.001 |
| Had skin blisters after sunburn | 2.2 (4) | 27.8 (50) | <0.001 |
| Area | Control Group n = 182 | Case Group n = 180 | p-Value |
|---|---|---|---|
| Face, mean (SD) | |||
| Diameter 2–5 mm | 0.23 (0.607) | 1.12 (1.814) | <0.001 |
| Diameter ≥ 5 mm | 0.17 (0.733) | 0.36 (0.837) | 0.024 |
| Total | 0.4 (0.951) | 1.48 (2.213) | <0.001 |
| Outer surface of upper arm, mean (SD) | |||
| Diameter 2–5 mm | 1.39 (3.659) | 3.52 (4.718) | <0.001 |
| Diameter ≥ 5 mm | 0.06 (0.354) | 0.37 (1.246) | 0.002 |
| Total | 1.45 (3.821) | 3.89 (4.967) | <0.001 |
| Outer surface of lower arm, mean (SD) | |||
| Diameter 2–5 mm | 0.34 (1.045) | 1.57 (2.701) | <0.001 |
| Diameter ≥ 5 mm | 0.06 (0.42) | 0.03 (0.18) | 0.509 |
| Total | 0.4 (1.278) | 1.61 (2.757) | <0.001 |
| Outer surface of hands, mean (SD) | |||
| Diameter 2–5 mm | 0.07 (0.411) | 0.13 (0.485) | 0.246 |
| Diameter ≥ 5 mm | 0.04 (0.402) | 0.01 (0.075) | 0.273 |
| Total | 0.11 (0.626) | 0.13 (0.489) | 0.358 |
| LOCS III Grade | Control Group n = 182 | Case Group n = 180 | p-Value |
|---|---|---|---|
| Nuclear opacity (NO), % (n 1) | |||
| 1 | 11.2 (20) | 6.3 (11) | 0.104 |
| 2 | 46.1 (82) | 35.1 (61) | 0.035 |
| 3 | 34.3 (61) | 46.0 (80) | 0.025 |
| ≥4 | 8.4 (15) | 12.6 (22) | 0.197 |
| Nuclear color (NC), % (n) | |||
| 1 | 11.2 (20) | 6.9 (12) | 0.157 |
| 2 | 45.5 (81) | 31.6 (55) | 0.007 |
| 3 | 32.6 (58) | 44.8 (78) | 0.018 |
| ≥4 | 10.7 (19) | 16.7 (29) | 0.101 |
| Cortical (C), % (n) | |||
| 1 | 47.8 (85) | 14.9 (26) | <0.001 |
| 2 | 34.3 (61) | 65.5 (114) | <0.001 |
| 3 | 14.6 (26) | 16.1 (28) | 0.699 |
| ≥4 | 3.4 (6) | 3.4 (6) | 0.968 |
| Posterior subcapsular (P), % (n) | |||
| 1 | 64.0 (114) | 21.8 (38) | <0.001 |
| 2 | 27.0 (48) | 69.5 (121) | <0.001 |
| 3 | 7.9 (14) | 5.2 (9) | 0.307 |
| ≥4 | 1.1 (2) | 3.4 (6) | 0.143 |
| Odds Ratio | 95% Confidence Interval | p-Value | ||
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Sex, female (reference) | 0.97 | 0.64 | 1.49 | 0.892 |
| Age | 1.02 | 0.99 | 1.04 | 0.073 |
| Age groups, ≤50 (reference) | ||||
| 51–60 | 1.07 | 0.60 | 1.93 | 0.819 |
| ≥61 | 1.04 | 0.62 | 1.74 | 0.889 |
| CMN numbers on face, mean * | ||||
| Diameter 2–5 mm | 2.20 | 1.65 | 2.94 | <0.001 |
| Diameter ≥ 5 mm | 1.37 | 0.98 | 1.90 | 0.062 |
| Total | 1.25 | 1.13 | 1.39 | <0.001 |
| CMN numbers on the outer surface of upper arm, mean * | ||||
| Diameter 2–5 mm | 1.16 | 1.08 | 1.24 | <0.001 |
| Diameter ≥ 5 mm | 2.32 | 1.29 | 4.19 | 0.005 |
| Total | 1.05 | 1.02 | 1.07 | <0.001 |
| CMN numbers on the outer surface of lower arm, mean * | ||||
| Diameter 2–5 mm | 1.58 | 1.31 | 1.89 | <0.001 |
| Diameter ≥ 5 mm | 0.81 | 0.40 | 1.64 | 0.562 |
| Total | 1.04 | 1.00 | 1.09 | 0.047 |
| CMN numbers on the outer surface of hands, mean * | ||||
| Diameter 2–5 mm | 1.33 | 0.81 | 2.17 | 0.264 |
| Diameter ≥ 5 mm | 0.49 | 0.11 | 2.19 | 0.352 |
| Total | 1.29 | 1.02 | 1.61 | 0.030 |
| Lens nuclear opacity (NO) evaluated by LOCS III scale, grade ≤ 2 (reference) * | ||||
| ≥3 | 1.82 | 1.15 | 2.88 | 0.010 |
| Lens nuclear color (NC) evaluated by LOCS III scale, grade ≤ 2 (reference) * | ||||
| ≥3 | 2.02 | 1.29 | 3.16 | 0.002 |
| Lens cortical (C) layers evaluated by LOCS III scale, grade 1 (reference) * | ||||
| ≥2 | 5.24 | 3.11 | 8.84 | <0.001 |
| Lens posterior subcapsular (P) layers evaluated by LOCS III scale, grade 1 (reference) * | ||||
| ≥2 | 6.34 | 3.93 | 10.25 | <0.001 |
| Wears sunglasses * | 2.18 | 1.42 | 3.35 | <0.001 |
| History of severe sunburn; did not have severe sunburn (reference) * | ||||
| Had sunburn redness | 10.91 | 5.44 | 21.85 | <0.001 |
| Had peeled skin after sunburn | 22.32 | 11.18 | 44.56 | <0.001 |
| Had skin blisters after sunburn | 86.21 | 27.67 | 268.59 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šemeklis, L.; Kapitanovaitė, L.; Butrimas, G.; Briedė, K.; Račkauskaitė, L.; Žemaitienė, R.; Valiukevičienė, S. Cataract Prevalence in Patients with Cutaneous Melanoma in Lithuanian Population. J. Clin. Med. 2024, 13, 6717. https://doi.org/10.3390/jcm13226717
Šemeklis L, Kapitanovaitė L, Butrimas G, Briedė K, Račkauskaitė L, Žemaitienė R, Valiukevičienė S. Cataract Prevalence in Patients with Cutaneous Melanoma in Lithuanian Population. Journal of Clinical Medicine. 2024; 13(22):6717. https://doi.org/10.3390/jcm13226717
Chicago/Turabian StyleŠemeklis, Lukas, Laura Kapitanovaitė, Grinvydas Butrimas, Kamilija Briedė, Laura Račkauskaitė, Reda Žemaitienė, and Skaidra Valiukevičienė. 2024. "Cataract Prevalence in Patients with Cutaneous Melanoma in Lithuanian Population" Journal of Clinical Medicine 13, no. 22: 6717. https://doi.org/10.3390/jcm13226717
APA StyleŠemeklis, L., Kapitanovaitė, L., Butrimas, G., Briedė, K., Račkauskaitė, L., Žemaitienė, R., & Valiukevičienė, S. (2024). Cataract Prevalence in Patients with Cutaneous Melanoma in Lithuanian Population. Journal of Clinical Medicine, 13(22), 6717. https://doi.org/10.3390/jcm13226717






