Relationship Between the Risk of Obstructive Sleep Apnea and Cardiovascular Health in Middle-Aged Korean Men and Women: A Nationwide Study
Abstract
1. Introduction
2. Materials and Methods
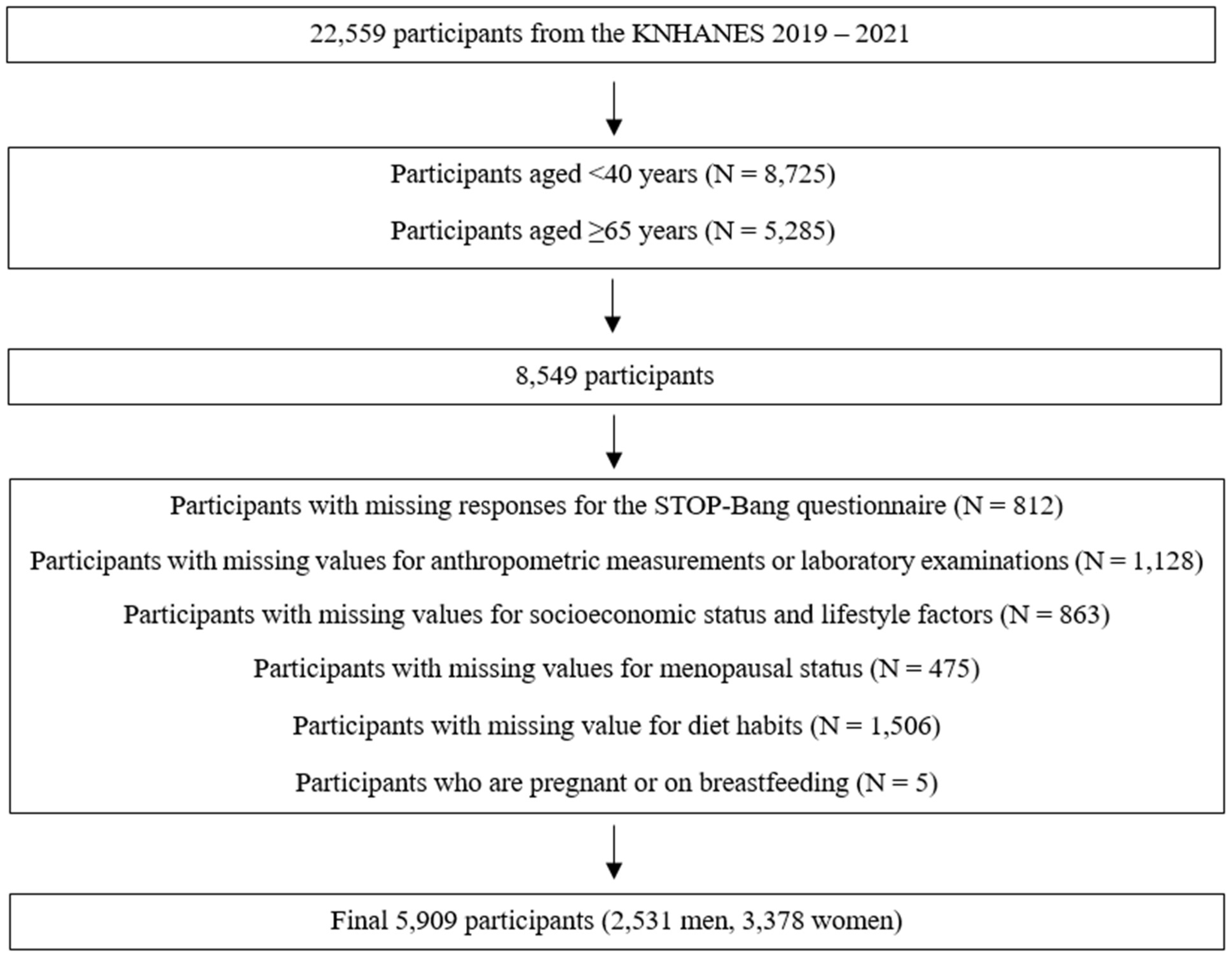
2.1. Study Population
2.2. Sociodemographic and Lifestyle Characteristics
2.3. Anthropometric and Laboratory Variables
2.4. Classification of OSA Risk
2.5. Definitions of CVH
2.6. Statistical Analysis
3. Results
3.1. Basic Participant Characteristics by CVH Status
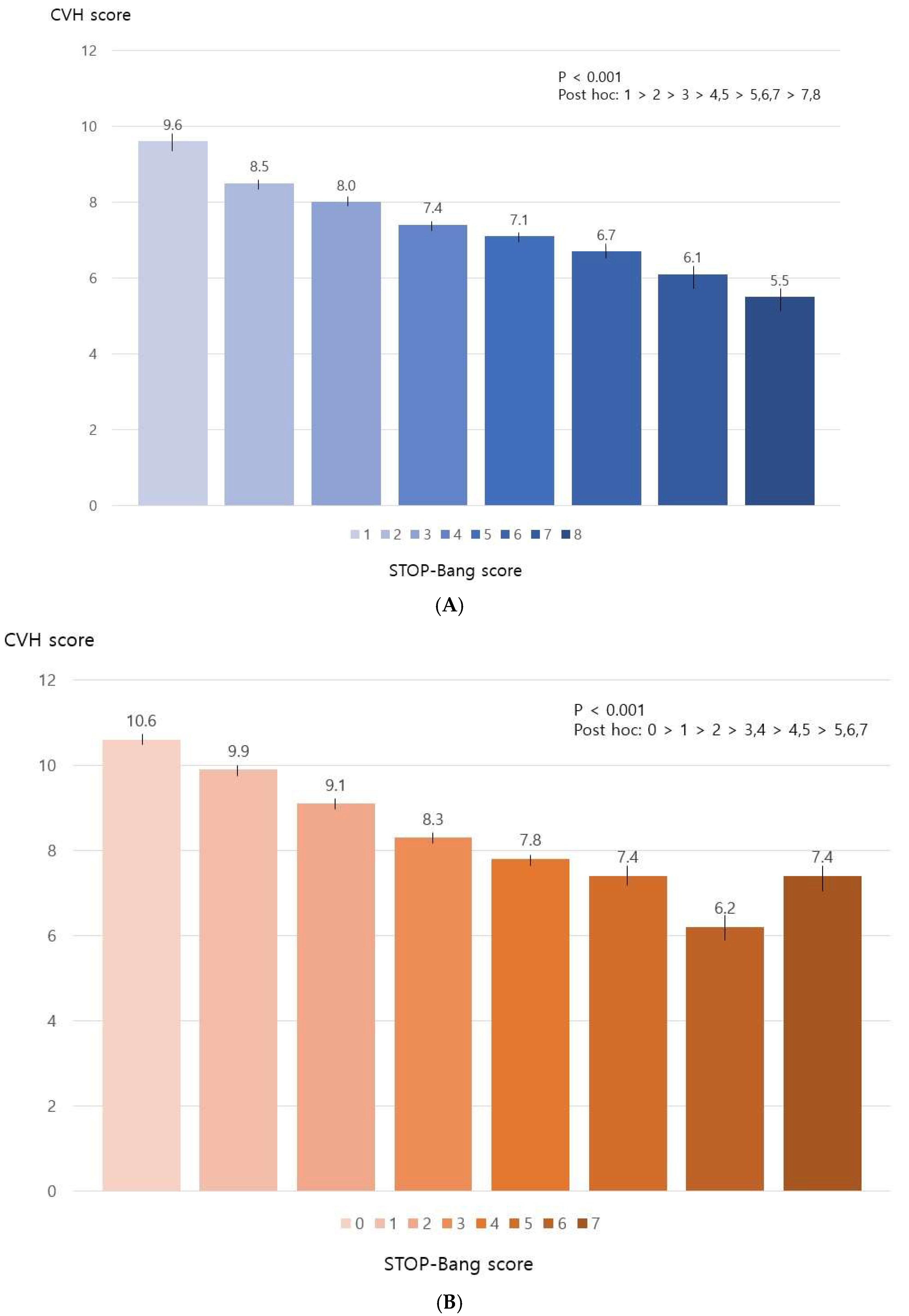
3.2. STOP-Bang Scores and CVH
3.3. Factors Associated with Poor CVH
3.4. Association Between Moderate-to-High Risk of OSA and CVH Metrics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Francula-Zaninovic, S.; Nola, I.A. Management of Measurable Variable Cardiovascular Disease’ Risk Factors. Curr. Cardiol. Rev. 2018, 14, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Jiang, M.; Fan, Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: A meta-analysis. Int. J. Cardiol. 2016, 214, 279–283. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, S. Association between ideal cardiovascular health metrics and risk of cardiovascular events or mortality: A meta-analysis of prospective studies. Clin. Cardiol. 2017, 40, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, M.; Jankovic, J.; Mandic-Rajcevic, S.; Dumic, I.; Hanna, R.D.; Nordstrom, C.W. Ideal Cardiovascular Health and Risk of Cardiovascular Events or Mortality: A Systematic Review and Meta-Analysis of Prospective Studies. J. Clin. Med. 2023, 12, 4417. [Google Scholar] [CrossRef]
- Cho, S.M.J.; Lee, H.; Kim, H.C. Sex- and Age-Specific Trends in Cardiovascular Health in Korea, 2007–2018. Korean Circ. J. 2021, 51, 922–935. [Google Scholar] [CrossRef]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef]
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef]
- Arnaud, C.; Bochaton, T.; Pépin, J.L.; Belaidi, E. Obstructive sleep apnoea and cardiovascular consequences: Pathophysiological mechanisms. Arch. Cardiovasc. Dis. 2020, 113, 350–358. [Google Scholar] [CrossRef]
- Dong, J.Y.; Zhang, Y.H.; Qin, L.Q. Obstructive sleep apnea and cardiovascular risk: Meta-analysis of prospective cohort studies. Atherosclerosis 2013, 229, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Khazaie, H.; Abolfathi, M.; Ghasemi, H.; Shabani, S.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. The effect of obstructive sleep apnea on the increased risk of cardiovascular disease: A systematic review and meta-analysis. Neurol. Sci. 2022, 43, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Strausz, S.; Havulinna, A.S.; Tuomi, T.; Bachour, A.; Groop, L.; Mäkitie, A.; Koskinen, S.; Salomaa, V.; Palotie, A.; Ripatti, S.; et al. Obstructive sleep apnoea and the risk for coronary heart disease and type 2 diabetes: A longitudinal population-based study in Finland. BMJ Open 2018, 8, e022752. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.K.; Brown, J.W.; Kwok, C.S.; Niruban, A.; Myint, P.K. Association of obstructive sleep apnea with risk of serious cardiovascular events: A systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 720–728. [Google Scholar] [CrossRef]
- Xie, C.; Zhu, R.; Tian, Y.; Wang, K. Association of obstructive sleep apnoea with the risk of vascular outcomes and all-cause mortality: A meta-analysis. BMJ Open 2017, 7, e013983. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Gaines, J.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Bixler, E.O. Obstructive sleep apnea and the metabolic syndrome: The road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med. Rev. 2018, 42, 211–219. [Google Scholar] [CrossRef]
- Korean Standard Statistical Classification Portal. The Korean Standard Classification of Occupations (KSCO). Available online: https://kssc.kostat.go.kr (accessed on 15 January 2024).
- World Health Organization. International Guide for Monitoring Alcohol Consumption and Related Harm; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Hoang, M.T.; Lee, H.; Kim, H.C. Spousal concordance of ideal cardiovascular health metrics: Findings from the 2014–2019 Korea National Health and Nutrition Examination Survey. Clin. Hypertens. 2022, 28, 41. [Google Scholar] [CrossRef]
- Byun, J.I.; Kim, D.H.; Kim, J.S.; Shin, W.C. Usefulness of Using Alternative Body-Mass Index and Neck Circumference Criteria for STOP-Bang Questionnaire in Screening South Korean Obstructive Sleep Apnea Patients. Sleep Med. Res. 2020, 11, 38–43. [Google Scholar] [CrossRef]
- Makarem, N.; Castro-Diehl, C.; St-Onge, M.P.; Redline, S.; Shea, S.; Lloyd-Jones, D.; Ning, H.; Aggarwal, B. Redefining Cardiovascular Health to Include Sleep: Prospective Associations with Cardiovascular Disease in the MESA Sleep Study. J. Am. Heart Assoc. 2022, 11, e025252. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.E.; Herbert, B.M.; Stokes, N.; Brooks, M.M.; Needham, B.L.; Magnani, J.W. Educational Attainment, Race, and Ethnicity as Predictors for Ideal Cardiovascular Health: From the National Health and Nutrition Examination Survey. J. Am. Heart Assoc. 2022, 11, e023438. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S. Mechanisms of cardiovascular disease in obstructive sleep apnoea. J. Thorac. Dis. 2018, 10, S4201–S4211. [Google Scholar] [CrossRef] [PubMed]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W.M.; Kelli, H.M.; Lisko, J.C.; Varghese, T.; Shen, J.; Sandesara, P.; Quyyumi, A.A.; Taylor, H.A.; Gulati, M.; Harold, J.G.; et al. Socioeconomic Status and Cardiovascular Outcomes: Challenges and Interventions. Circulation 2018, 137, 2166–2178. [Google Scholar] [CrossRef]
- Dupre, M.E.; George, L.K.; Liu, G.; Peterson, E.D. The cumulative effect of unemployment on risks for acute myocardial infarction. Arch. Intern. Med. 2012, 172, 1731–1737. [Google Scholar] [CrossRef]
- Méjean, C.; Droomers, M.; Van Der Schouw, Y.T.; Sluijs, I.; Czernichow, S.; Grobbee, D.E.; Bueno-de-Mesquita, H.B.; Beulens, J.W. The contribution of diet and lifestyle to socioeconomic inequalities in cardiovascular morbidity and mortality. Int. J. Cardiol. 2013, 168, 5190–5195. [Google Scholar] [CrossRef]
- Wong, C.W.; Kwok, C.S.; Narain, A.; Gulati, M.; Mihalidou, A.S.; Wu, P.; Alasnag, M.; Myint, P.K.; Mamas, M.A. Marital status and risk of cardiovascular diseases: A systematic review and meta-analysis. Heart 2018, 104, 1937–1948. [Google Scholar] [CrossRef]
- Piano, M.R. Alcohol’s Effects on the Cardiovascular System. Alcohol Res. 2017, 38, 219–241. [Google Scholar]
- Kim, Y.; Nho, S.J.; Woo, G.; Kim, H.; Park, S.; Kim, Y.; Park, O.; Oh, K. Trends in the prevalence and management of major metabolic risk factors for chronic disease over 20 years: Findings from the 1998–2018 Korea National Health and Nutrition Examination Survey. Epidemiol. Health 2021, 43, e2021028. [Google Scholar] [CrossRef]
- Nam, G.E.; Kim, Y.H.; Han, K.; Jung, J.H.; Rhee, E.J.; Lee, W.Y. Obesity Fact Sheet in Korea, 2020: Prevalence of Obesity by Obesity Class from 2009 to 2018. J. Obes. Metab. Syndr. 2021, 30, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Geer, J.H.; Hilbert, J. Gender Issues in Obstructive Sleep Apnea. Yale J. Biol. Med. 2021, 94, 487–496. [Google Scholar] [PubMed]
- Woodward, M. Cardiovascular Disease and the Female Disadvantage. Int. J. Environ. Res. Public Health 2019, 16, 1165. [Google Scholar] [CrossRef]
- Huxley, R.R.; Woodward, M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: A systematic review and meta-analysis of prospective cohort studies. Lancet 2011, 378, 1297–1305. [Google Scholar] [CrossRef]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Diabetes as risk factor for incident coronary heart disease in women compared with men: A systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014, 57, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Wong, C.X.; Hsiao, A.J.; Altman, D.G.; Peters, S.A.; Woodward, M.; Odutayo, A.A. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: Systematic review and meta-analysis of cohort studies. BMJ 2016, 532, h7013. [Google Scholar] [CrossRef]
- Backholer, K.; Peters, S.A.; Bots, S.H.; Peeters, A.; Huxley, R.R.; Woodward, M. Sex differences in the relationship between socioeconomic status and cardiovascular disease: A systematic review and meta-analysis. J. Epidemiol. Community Health 2017, 71, 550–557. [Google Scholar] [CrossRef]
- Gleeson, M.; McNicholas, W.T. Bidirectional relationships of comorbidity with obstructive sleep apnoea. Eur. Respir. Rev. 2022, 31, 210256. [Google Scholar] [CrossRef]
- Nagappa, M.; Liao, P.; Wong, J.; Auckley, D.; Ramachandran, S.K.; Memtsoudis, S.; Mokhlesi, B.; Chung, F. Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0143697. [Google Scholar] [CrossRef]
| Men (n = 2531) | Women (n = 3378) | |||||||
|---|---|---|---|---|---|---|---|---|
| Poor CVH (n = 1149) | Intermediate CVH (n = 1312) | Ideal CVH (n = 70) | p-Value | Poor CVH (n = 580) | Intermediate CVH (n = 2206) | Ideal CVH (n = 592) | p-Value | |
| Age group (years) | ||||||||
| 40–44 | 213 (45.0) | 248 (52.4) | 12 (2.5) | 0.205 | 56 (9.0) | 386 (62.0) | 181 (29.1) | <0.001 |
| 45–49 | 212 (43.0) | 270 (54.8) | 11 (2.2) | 81 (11.9) | 437 (64.0) | 165 (24.2) | ||
| 50–54 | 226 (50.3) | 211 (47.0) | 12 (2.7) | 125 (19.1) | 439 (67.2) | 89 (13.6) | ||
| 55–59 | 238 (44.1) | 281 (52.0) | 21 (3.9) | 141 (20.0) | 471 (66.8) | 93 (13.2) | ||
| 60–64 | 260 (45.1) | 302 (52.4) | 14 (2.4) | 177 (24.8) | 473 (66.2) | 64 (9.0) | ||
| Are of residence | ||||||||
| Urban | 887 (44.0) | 1073 (53.3) | 54 (2.7) | 0.004 | 439 (15.9) | 1809 (65.4) | 516 (18.7) | 0.003 |
| Rural | 262 (50.7) | 239 (46.2) | 16 (3.1) | 141 (23.0) | 397 (64.7) | 76 (12.4) | ||
| Household income | ||||||||
| First quartile (low) | 127 (52.5) | 108 (44.6) | 7 (2.9) | 0.082 | 85 (27.3) | 195 (62.7) | 31 (10.0) | <0.001 |
| Second quartile | 246 (46.2) | 273 (51.2) | 14 (2.6) | 154 (18.7) | 538 (65.5) | 130 (15.8) | ||
| Third quartile | 360 (46.1) | 404 (51.7) | 17 (2.2) | 165 (16.4) | 671 (66.6) | 172 (17.1) | ||
| Fourth quartile (high) | 416 (42.7) | 527 (54.1) | 32 (3.3) | 176 (14.2) | 802 (64.8) | 259 (20.9) | ||
| Educational level | ||||||||
| <Middle school graduate | 195 (54.0) | 161 (44.6) | 5 (1.4) | <0.001 | 188 (29.5) | 402 (63.1) | 47 (7.4) | <0.001 |
| High school graduate | 444 (48.3) | 457 (49.7) | 19 (2.1) | 253 (18.3) | 913 (65.9) | 219 (15.8) | ||
| ≥College graduate | 510 (40.8) | 694 (55.5) | 46 (3.7) | 139 (10.3) | 891 (65.7) | 326 (24.0) | ||
| Occupation | ||||||||
| None | 183 (46.3) | 199 (50.4) | 13 (3.3) | 0.069 | 222 (16.3) | 898 (66.0) | 241 (17.7) | <0.001 |
| Manual work | 594 (47.1) | 637 (50.6) | 29 (2.3) | 254 (21.8) | 747 (64.2) | 163 (14.0) | ||
| Non-manual work | 372 (42.5) | 476 (54.3) | 28 (3.2) | 104 (12.2) | 561 (65.8) | 188 (22.0) | ||
| Marital status | ||||||||
| Married/cohabitating | 916 (44.2) | 1097 (52.9) | 60 (2.9) | 0.088 | 457 (16.4) | 1817 (65.2) | 511 (18.3) | 0.002 |
| Unmarried/divorced/separated/widowed | 233 (50.9) | 215 (46.9) | 10 (2.2) | 123 (20.7) | 389 (65.6) | 81 (13.7) | ||
| High risk drinking | ||||||||
| Non-drinker | 329 (38.8) | 477 (56.2) | 43 (5.1) | <0.001 | 379 (16.3) | 1539 (66.3) | 405 (17.4) | 0.147 |
| <1/month | 176 (43.8) | 218 (54.2) | 8 (2.0) | 77 (15.9) | 311 (64.3) | 96 (19.8) | ||
| ≥1/month | 644 (50.3) | 617 (48.2) | 19 (1.5) | 124 (21.7) | 356 (62.3) | 91 (15.9) | ||
| Menopausal status | ||||||||
| Premenopausal | N/A | N/A | N/A | N/A | 176 (11.6) | 974 (64.0) | 373 (24.5) | <0.001 |
| Postmenopausal | N/A | N/A | N/A | 404 (21.8) | 1232 (66.4) | 219 (11.8) | ||
| BMI (kg/m2) | 26.5 (0.1) | 23.7 (0.1) | 21.6 (0.2) | <0.001 | 27.2 (0.2) | 23.4 (0.1) | 21.2 (0.1) | <0.001 |
| SBP (mmHg) | 126.0 (0.5) | 117.7 (0.5) | 107.5 (1.3) | <0.001 | 126.5 (0.8) | 115.9 (0.4) | 106.1 (0.5) | <0.001 |
| DBP (mmHg) | 83.1 (0.3) | 77.8 (0.2) | 72.5 (0.8) | <0.001 | 79.8 (0.4) | 75.3 (0.2) | 69.6 (0.3) | <0.001 |
| Fasting glucose (mg/dL) | 115.2 (1.1) | 100.7 (0.5) | 93.7 (0.9) | <0.001 | 115.4 (1.7) | 97.9 (0.5) | 90.6 (0.3) | <0.001 |
| Total cholesterol (mg/dL) | 202.8 (1.4) | 190.0 (1.0) | 181.2 (3.7) | <0.001 | 211.7 (2.3) | 202.2 (0.9) | 185.1 (1.2) | <0.001 |
| Men (n = 2531) | Women (n = 3378) | |||||||
|---|---|---|---|---|---|---|---|---|
| Poor CVH (n = 1149) | Intermediate CVH (n = 1312) | Ideal CVH (n = 70) | p-Value | Poor CVH (n = 580) | Intermediate CVH (n = 2206) | Ideal CVH (n = 592) | p-Value | |
| Snoring | ||||||||
| No | 747 (42.0) | 970 (54.6) | 61 (3.4) | <0.001 | 448 (15.3) | 1934 (65.9) | 554 (18.9) | <0.001 |
| Yes | 402 (53.4) | 342 (45.4) | 9 (1.2) | 132 (29.9) | 272 (61.5) | 38 (8.6) | ||
| Daytime tiredness, fatigue, or sleepiness | ||||||||
| No | 778 (43.6) | 953 (53.4) | 55 (3.1) | 0.009 | 375 (16.5) | 1490 (65.7) | 403 (17.8) | 0.364 |
| Yes | 371 (49.8) | 359 (48.2) | 15 (2.0) | 205 (18.5) | 716 (64.5) | 189 (17.0) | ||
| Observed cessation of breathing during sleep | ||||||||
| No | 892 (43.4) | 1102 (53.7) | 59 (2.9) | <0.001 | 545 (16.8) | 2131 (65.6) | 574 (17.7) | 0.007 |
| Yes | 257 (53.8) | 210 (43.9) | 11 (2.3) | 35 (27.3) | 75 (58.6) | 18 (14.1) | ||
| High blood pressure | ||||||||
| No | 567 (34.8) | 998 (61.3) | 64 (3.9) | <0.001 | 286 (11.1) | 1718 (66.7) | 570 (22.1) | <0.001 |
| Yes | 582 (64.5) | 314 (34.8) | 6 (0.7) | 294 (36.6) | 488 (60.7) | 22 (2.7) | ||
| BMI (kg/m2) | ||||||||
| ≤30 | 1012 (42.9) | 1279 (54.2) | 70 (3.0) | <0.001 | 489 (15.3) | 2110 (66.1) | 592 (18.6) | <0.001 |
| >30 | 137 (80.6) | 33 (19.4) | 0 (0.0) | 91 (48.7) | 96 (51.3) | 0 (0.0) | ||
| Age (years) | ||||||||
| ≤50 | 475 (45.2) | 550 (52.4) | 25 (2.4) | 0.585 | 155 (10.8) | 902 (63.0) | 374 (26.1) | <0.001 |
| >50 | 674 (45.5) | 762 (51.5) | 45 (3.0) | 425 (21.8) | 1304 (67.0) | 218 (11.2) | ||
| Neck circumference (cm) | ||||||||
| ≤36.3 | 92 (18.2) | 371 (73.5) | 42 (8.3) | <0.001 | 489 (15.2) | 2129 (66.3) | 592 (18.4) | <0.001 |
| >36.3 | 1057 (52.2) | 941 (46.4) | 28 (1.4) | 91 (54.2) | 77 (45.8) | 0 (0.0) | ||
| Risk of obstructive sleep apnea | ||||||||
| Low (0–2) | 135 (24.9) | 372 (68.6) | 35 (6.5) | <0.001 | 372 (13.1) | 1885 (66.6) | 572 (20.2) | <0.001 |
| Moderate (3–4) | 616 (44.8) | 729 (53.0) | 31 (2.3) | 176 (35.2) | 306 (61.2) | 18 (3.6) | ||
| High (5–8) | 398 (64.9) | 211 (34.4) | 4 (0.7) | 32 (65.3) | 15 (30.6) | 2 (4.1) | ||
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) | p | * Adjusted OR (95% CI) | Crude OR (95% CI) | † Adjusted OR (95% CI) | ||||
| Age group (years) | ||||||||
| 40–44 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 45–49 | 0.96 (0.73–1.26) | 0.747 | 0.92 (0.69–1.23) | 0.584 | 1.17 (0.74–1.84) | 0.510 | 1.04 (0.66–1.64) | 0.875 |
| 50–54 | 1.29 (0.96–1.73) | 0.093 | 0.84 (0.61–1.17) | 0.307 | 2.07 (1.35–3.18) | 0.001 | 1.38 (0.83–2.30) | 0.218 |
| 55–59 | 1.02 (0.77–1.36) | 0.875 | 0.62 (0.46–0.84) | 0.002 | 2.19 (1.48–3.22) | <0.001 | 1.11 (0.63–1.95) | 0.718 |
| 60–64 | 1.03 (0.79–1.35) | 0.839 | 0.58 (0.42–0.80) | 0.001 | 2.83 (1.93–4.15) | <0.001 | 1.16 (0.64–2.13) | 0.624 |
| Area of residence | ||||||||
| Urban | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Rural | 1.40 (1.11–1.75) | 0.004 | 1.26 (1.00–1.59) | 0.054 | 1.56 (1.17–2.07) | 0.003 | 1.25 (0.92–1.70) | 0.158 |
| Household income | ||||||||
| Fourth quartile | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Third quartile | 1.13 (0.91–1.41) | 0.264 | 1.11 (0.87–1.42) | 0.388 | 1.17 (0.90–1.53) | 0.239 | 1.01 (0.77–1.32) | 0.965 |
| Second quartile | 1.13 (0.88–1.45) | 0.337 | 1.03 (0.78–1.36) | 0.852 | 1.62 (1.24–2.12) | 0.001 | 1.25 (0.92–1.70) | 0.152 |
| First quartile | 1.60 (1.15–2.23) | 0.006 | 1.37 (0.92–2.05) | 0.118 | 2.38 (1.69–3.37) | <0.001 | 1.57 (1.05–2.36) | 0.029 |
| Educational level | ||||||||
| ≥College graduate | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| High school graduate | 1.33 (1.10–1.61) | 0.004 | 1.29 (1.02–1.62) | 0.033 | 2.00 (1.55–2.57) | <0.001 | 1.57 (1.18–2.11) | 0.002 |
| <Middle school graduate | 1.76 (1.34–2.31) | <0.001 | 1.93 (1.38–2.71) | <0.001 | 3.93 (2.94–5.25) | <0.001 | 2.20 (1.48–3.26) | <0.001 |
| Occupation | ||||||||
| None | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Manual work | 0.99 (0.75–1.30) | 0.934 | 1.05 (0.77–1.43) | 0.768 | 1.50 (1.19–1.90) | 0.001 | 1.36 (1.05–1.74) | 0.018 |
| Non-manual work | 0.87 (0.66–1.15) | 0.315 | 1.18 (0.83–1.66) | 0.355 | 0.70 (0.53–0.93) | 0.013 | 1.14 (0.82–1.60) | 0.429 |
| Marital status | ||||||||
| Married/cohabitating | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Unmarried/divorced/separated/widowed | 1.31 (1.05–1.63) | 0.017 | 1.31 (1.02–1.67) | 0.033 | 1.27 (0.98–1.63) | 0.067 | 0.96 (0.71–1.29) | 0.772 |
| High risk drinking | ||||||||
| Non-drinker | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| <1/month | 1.25 (0.94–1.65) | 0.125 | 1.40 (1.04–1.89) | 0.026 | 1.06 (0.77–1.46) | 0.707 | 1.14 (0.81–1.60) | 0.460 |
| ≥1/month | 1.58 (1.28–1.95) | <0.001 | 1.56 (1.25–1.95) | <0.001 | 1.42 (1.09–1.86) | 0.011 | 1.43 (1.07–1.92) | 0.016 |
| Menopausal status | ||||||||
| Premenopausal | N/A | N/A | 1.00 | 1.00 | ||||
| Postmenopausal | 1.94 (1.57–2.41) | <0.001 | 1.13 (0.74–1.73) | 0.574 | ||||
| Risk of obstructive sleep apnea | ||||||||
| Low (0–2) | 1.00 | 1.00 | ||||||
| Moderate (3–4) | 2.69 (2.08–3.49) | <0.001 | 3.21 (2.47–4.19) | <0.001 | ||||
| High (5–8) | 6.54 (4.81–8.90) | <0.001 | 12.88 (6.29–26.38) | <0.001 | ||||
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) | * Adjusted OR (95% CI) | Crude OR (95% CI) | * Adjusted OR (95% CI) | |||||
| Age group (years) | ||||||||
| 40–44 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 45–49 | 0.94 (0.72–1.23) | 0.657 | 0.91 (0.66–1.26) | 0.567 | 1.42 (0.84–2.390 | 0.189 | 1.10 (0.63–1.93) | 0.727 |
| 50–54 | 4.62 (3.18–6.72) | <0.001 | 5.88 (3.80–9.08) | <0.001 | 4.50 (2.87–7.07) | <0.001 | 3.40 (2.16–5.37) | <0.001 |
| 55–59 | 5.20 (3.62–7.47) | <0.001 | 7.95 (5.29–11.95) | <0.001 | 5.75 (3.68–9.00) | <0.001 | 4.16 (2.61–6.63) | <0.001 |
| 60–64 | 5.33 (3.59–7.92) | <0.001 | 7.32 (4.76–11.26) | <0.001 | 8.25 (5.48–12.44) | <0.001 | 5.07 (3.32–7.75) | <0.001 |
| Smoking status | ||||||||
| Never smoker | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Former smoker | 1.78 (1.34–2.35) | <0.001 | 1.56 (1.11–2.20) | 0.011 | 1.07 (0.68–1.68) | 0.778 | 1.40 (0.82–2.41) | 0.217 |
| Current smoker | 1.16 (0.87–1.54) | 0.315 | 1.09 (0.77–1.53) | 0.622 | 1.70 (1.08–2.66) | 0.021 | 2.50 (1.43–4.36) | 0.001 |
| Diet | ||||||||
| Ideal | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Intermediate | 0.71 (0.50–1.02) | 0.060 | 0.72 (0.49–1.06) | 0.095 | 1.19 (0.89–1.59) | 0.232 | 0.91 (0.65–1.28) | 0.592 |
| Poor | 0.77 (0.46–1.27) | 0.301 | 0.82 (0.44–1.54) | 0.536 | 1.18 (0.73–1.92) | 0.506 | 1.17 (0.66–2.10) | 0.591 |
| Physical activity | ||||||||
| Ideal | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Intermediate | 0.84 (0.67–1.05) | 0.123 | 0.72 (0.49–1.06) | 0.298 | 1.07 (0.83–1.39) | 0.592 | 1.11 (0.83–1.48) | 0.472 |
| Poor | 0.97 (0.76–1.23) | 0.784 | 0.82 (0.44–1.54) | 0.274 | 1.18 (0.92–1.50) | 0.187 | 1.01 (0.76–1.33) | 0.970 |
| BMI (kg/m2) | ||||||||
| <23 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 23–24.9 | 2.47 (1.87–3.24) | <0.001 | 3.22 (2.29–4.53) | <0.001 | 1.64 (1.19–2.25) | 0.003 | 1.22 (0.86–1.73) | 0.259 |
| ≥25 | 5.05 (3.91–6.53) | <0.001 | 6.38 (4.63–8.78) | <0.001 | 5.85 (4.53–7.56) | <0.001 | 3.69 (2.78–4.89) | <0.001 |
| Blood pressure | ||||||||
| Ideal | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Intermediate | 3.89 (3.11–4.88) | <0.001 | 2.63 (2.03–3.39) | <0.001 | 8.34 (6.15–11.33) | <0.001 | 5.07 (3.66–7.03) | <0.001 |
| Poor | 16.98 (5.68–50.76) | <0.001 | 15.57 (5.23–46.40) | <0.001 | 12.06 (7.21–20.18) | <0.001 | 8.96 (5.34–15.03) | <0.001 |
| Total cholesterol | ||||||||
| <200 mg/dL (untreated) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 200–239 mg/dL or treated to goal | 1.47 (1.18–1.83) | 0.001 | 1.34 (1.03–1.74) | 0.030 | 1.92 (1.51–2.45) | <0.001 | 1.10 (0.83–1.46) | 0.496 |
| ≥240 mg/dL | 1.21 (0.86–1.69) | 0.273 | 1.13 (0.74–1.73) | 0.562 | 1.40 (0.99–1.99) | 0.058 | 0.80 (0.54–1.18) | 0.265 |
| Fasting glucose | ||||||||
| <100 mg/dL (untreated) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 100–125 mg/dL or treated to goal | 2.03 (1.62–2.55) | <0.001 | 1.30 (1.00–1.70) | 0.053 | 3.31 (2.65–4.13) | <0.001 | 1.84 (1.41–2.38) | <0.001 |
| ≥126 mg/dL | 4.84 (2.55–9.20) | <0.001 | 2.11 (1.03–4.30) | 0.040 | 4.45 (2.48–7.99) | <0.001 | 1.58 (0.80–3.12) | 0.187 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.Y.; Kim, J.H.; Kim, Y. Relationship Between the Risk of Obstructive Sleep Apnea and Cardiovascular Health in Middle-Aged Korean Men and Women: A Nationwide Study. J. Clin. Med. 2024, 13, 6702. https://doi.org/10.3390/jcm13226702
Kang SY, Kim JH, Kim Y. Relationship Between the Risk of Obstructive Sleep Apnea and Cardiovascular Health in Middle-Aged Korean Men and Women: A Nationwide Study. Journal of Clinical Medicine. 2024; 13(22):6702. https://doi.org/10.3390/jcm13226702
Chicago/Turabian StyleKang, Seo Young, Jung Hwan Kim, and Yunmi Kim. 2024. "Relationship Between the Risk of Obstructive Sleep Apnea and Cardiovascular Health in Middle-Aged Korean Men and Women: A Nationwide Study" Journal of Clinical Medicine 13, no. 22: 6702. https://doi.org/10.3390/jcm13226702
APA StyleKang, S. Y., Kim, J. H., & Kim, Y. (2024). Relationship Between the Risk of Obstructive Sleep Apnea and Cardiovascular Health in Middle-Aged Korean Men and Women: A Nationwide Study. Journal of Clinical Medicine, 13(22), 6702. https://doi.org/10.3390/jcm13226702







