Atrial Fibrillation in Patients with Very High Risk for Stroke and Adverse Events—Insights from the Observational ARENA Study
Abstract
1. Introduction
2. Materials and Methods
Statistics
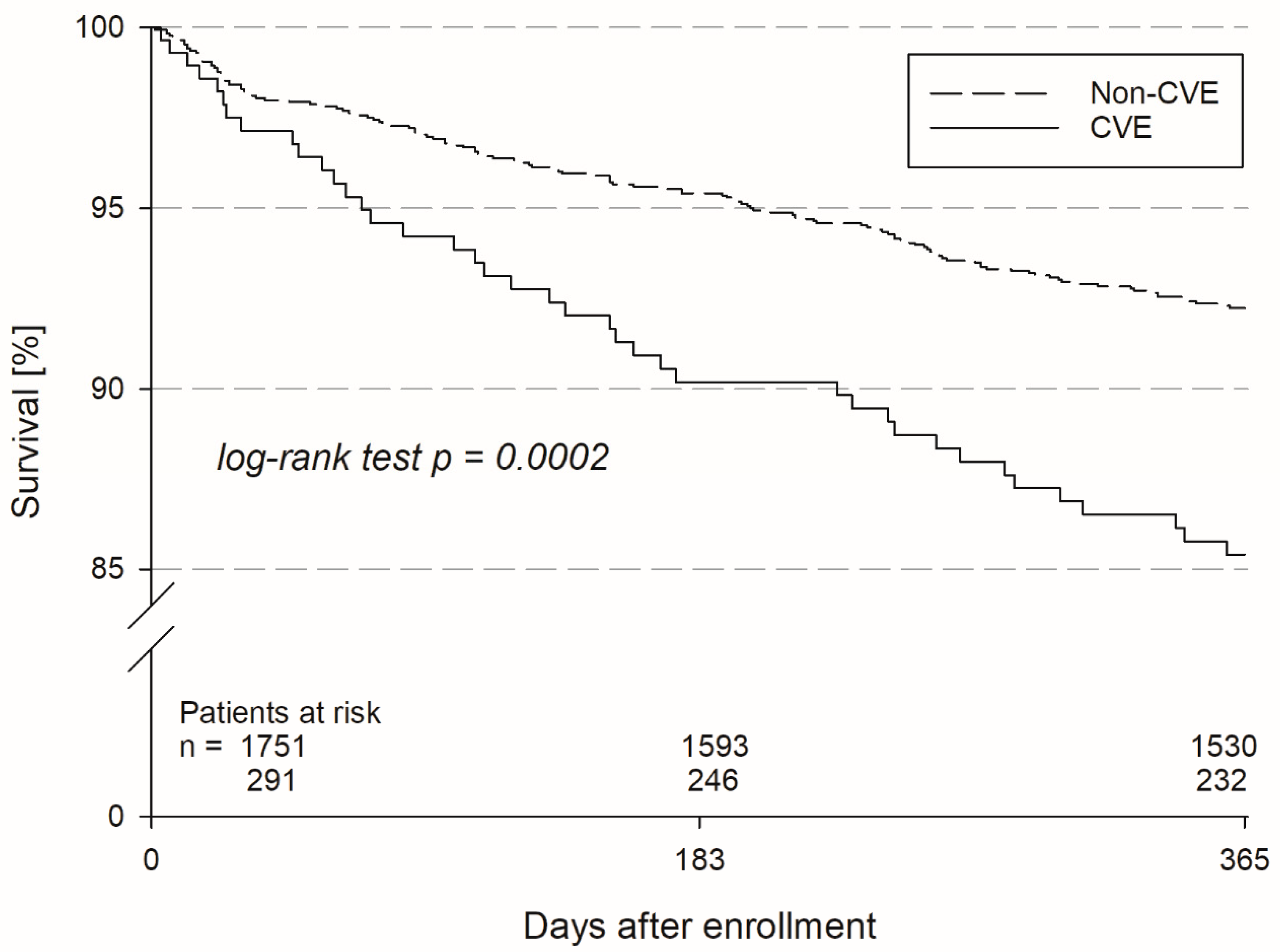
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.; Odier, C.; Rutgers, M.; Reichhart, M.; Maeder, P.; Meuli, R.; Wintermark, M.; Maghraoui, A.; Faouzi, M.; Croquelois, A.; et al. The Acute STroke Registry and Analysis of Lausanne (ASTRAL): Design and baseline analysis of an ischemic stroke registry including acute multimodal imaging. Stroke 2010, 41, 2491–2498. [Google Scholar] [CrossRef]
- Brachmann, J.; Morillo, C.A.; Sanna, T.; Di Lazzaro, V.; Diener, H.C.; Bernstein, R.A.; Rymer, M.; Ziegler, P.D.; Liu, S.; Passman, R.S. Uncovering Atrial Fibrillation Beyond Short-Term Monitoring in Cryptogenic Stroke Patients: Three-Year Results From the Cryptogenic Stroke and Underlying Atrial Fibrillation Trial. Circ. Arrhythm. Electrophysiol. 2016, 9, e003333. [Google Scholar] [CrossRef]
- Zhou, Z.; You, S.; Sakamoto, Y.; Xu, Y.; Ding, S.; Xu, W.; Li, W.; Yu, J.; Wang, Y.; Harris, K.; et al. Covert Cerebrovascular Changes in People with Heart Disease: A Systematic Review and Meta-Analysis. Neurology 2024, 102, e209204. [Google Scholar] [CrossRef]
- EAFT Study Group. Silent brain infarction in nonrheumatic atrial fibrillation. European Atrial Fibrillation Trial. Neurology 1996, 46, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Halperin, J.L. Improving stroke risk stratification in atrial fibrillation. Am. J. Med. 2010, 123, 484–488. [Google Scholar] [CrossRef]
- Nielsen, P.B.; Skjoth, F.; Overvad, T.F.; Larsen, T.B.; Lip, G.Y.H. Female Sex Is a Risk Modifier Rather Than a Risk Factor for Stroke in Atrial Fibrillation: Should We Use a CHA(2)DS(2)-VA Score Rather Than CHA(2)DS(2)-VASc? Circulation 2018, 137, 832–840. [Google Scholar] [CrossRef]
- Tomasdottir, M.; Friberg, L.; Hijazi, Z.; Lindback, J.; Oldgren, J. Risk of ischemic stroke and utility of CHA(2) DS(2) -VASc score in women and men with atrial fibrillation. Clin. Cardiol. 2019, 42, 1003–1009. [Google Scholar] [CrossRef]
- Szymanski, F.M.; Lip, G.Y.; Filipiak, K.J.; Platek, A.E.; Hrynkiewicz-Szymanska, A.; Opolski, G. Stroke Risk Factors Beyond the CHA(2)DS(2)-VASc Score: Can We Improve Our Identification of “High Stroke Risk” Patients With Atrial Fibrillation? Am. J. Cardiol. 2015, 116, 1781–1788. [Google Scholar] [CrossRef]
- Baumann, S.; Grau, A.; Senges, J.; Schneider, S.; Alonso, A.; Katus, H.A.; Thomas, D.; Waldecker, B.; Haass, M.; Zahn, R.; et al. ARENA-Project atrial fibrillation in the Rhein-Neckar region. Herz 2020, 45, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, S.; Kamel, H. Stratifying Stroke Risk in Atrial Fibrillation: Beyond Clinical Risk Scores. Stroke 2017, 48, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G. Atrial fibrillation and stroke prevention. N. Engl. J. Med. 2003, 349, 1015–1016. [Google Scholar] [CrossRef] [PubMed]
- Brambatti, M.; Connolly, S.J.; Gold, M.R.; Morillo, C.A.; Capucci, A.; Muto, C.; Lau, C.P.; Van Gelder, I.C.; Hohnloser, S.H.; Carlson, M.; et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014, 129, 2094–2099. [Google Scholar] [CrossRef]
- Hirsh, B.J.; Copeland-Halperin, R.S.; Halperin, J.L. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: Mechanistic links and clinical inferences. J. Am. Coll. Cardiol. 2015, 65, 2239–2251. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef]
- Overvad, T.F.; Nielsen, P.B.; Larsen, T.B.; Sogaard, P. Left atrial size and risk of stroke in patients in sinus rhythm. A systematic review. Thromb. Haemost. 2016, 116, 206–219. [Google Scholar] [CrossRef]
- Chiang, C.E.; Naditch-Brule, L.; Murin, J.; Goethals, M.; Inoue, H.; O’Neill, J.; Silva-Cardoso, J.; Zharinov, O.; Gamra, H.; Alam, S.; et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: Insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ. Arrhythm. Electrophysiol. 2012, 5, 632–639. [Google Scholar] [CrossRef]
- Dretzke, J.; Chuchu, N.; Agarwal, R.; Herd, C.; Chua, W.; Fabritz, L.; Bayliss, S.; Kotecha, D.; Deeks, J.J.; Kirchhof, P.; et al. Predicting recurrent atrial fibrillation after catheter ablation: A systematic review of prognostic models. Europace 2020, 22, 748–760. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Kheirkhahan, M.; Brachmann, J. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 379, 492. [Google Scholar] [CrossRef]
- Packer, D.L.; Mark, D.B.; Lee, K.L. Catheter Ablation Compared With Drug Therapy for Atrial Fibrillation-Reply. JAMA 2019, 322, 1106. [Google Scholar] [CrossRef] [PubMed]
- Sposato, L.A.; Hilz, M.J.; Aspberg, S.; Murthy, S.B.; Bahit, M.C.; Hsieh, C.Y.; Sheppard, M.N.; Scheitz, J.F.; World Stroke Organisation, B.; Heart Task, F. Post-Stroke Cardiovascular Complications and Neurogenic Cardiac Injury: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 2768–2785. [Google Scholar] [CrossRef]
- Becker, L.; Alonso, A.; Kruska, M.; Baumann, S.; Grassl, N.; Lesch, H.; Eisele, P.; Sieburg, T.; Behnes, M.; Schupp, T.; et al. Acute ischemic stroke and troponin elevation: Update of the Mannheim clinical algorithm. Inn. Med. 2024, 65, 830–839. [Google Scholar] [CrossRef]
- Chen, L.Y.; Leening, M.J.; Norby, F.L.; Roetker, N.S.; Hofman, A.; Franco, O.H.; Pan, W.; Polak, J.F.; Witteman, J.C.; Kronmal, R.A.; et al. Carotid Intima-Media Thickness and Arterial Stiffness and the Risk of Atrial Fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study, Multi-Ethnic Study of Atherosclerosis (MESA), and the Rotterdam Study. J. Am. Heart Assoc. 2016, 5, e002907. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, R.A.; Kamel, H.; Granger, C.B.; Piccini, J.P.; Sethi, P.P.; Katz, J.M.; Vives, C.A.; Ziegler, P.D.; Franco, N.C.; Schwamm, L.H.; et al. Effect of Long-term Continuous Cardiac Monitoring vs Usual Care on Detection of Atrial Fibrillation in Patients With Stroke Attributed to Large- or Small-Vessel Disease: The STROKE-AF Randomized Clinical Trial. JAMA 2021, 325, 2169–2177. [Google Scholar] [CrossRef]
- Paciaroni, M.; Agnelli, G.; Caso, V.; Silvestrelli, G.; Seiffge, D.J.; Engelter, S.; De Marchis, G.M.; Polymeris, A.; Zedde, M.L.; Yaghi, S.; et al. Causes and Risk Factors of Cerebral Ischemic Events in Patients With Atrial Fibrillation Treated With Non-Vitamin K Antagonist Oral Anticoagulants for Stroke Prevention. Stroke 2019, 50, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Rubiera, M.; Aires, A.; Antonenko, K.; Lemeret, S.; Nolte, C.H.; Putaala, J.; Schnabel, R.B.; Tuladhar, A.M.; Werring, D.J.; Zeraatkar, D.; et al. European Stroke Organisation (ESO) guideline on screening for subclinical atrial fibrillation after stroke or transient ischaemic attack of undetermined origin. Eur. Stroke J. 2022, 7, VI. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Kraus, J.; Ebert, A.; Nikolayenko, V.; Kruska, M.; Sandikci, V.; Lesch, H.; Duerschmied, D.; Platten, M.; Baumann, S.; et al. Left atrial area index provides the best prediction of atrial fibrillation in ischemic stroke patients: Results from the LAETITIA observational study. Front. Neurol. 2023, 14, 1237550. [Google Scholar] [CrossRef]
- Sanna, T.; Diener, H.C.; Passman, R.S.; Crystal, A.F.S.C. Cryptogenic stroke and atrial fibrillation. N. Engl. J. Med. 2014, 371, 1261. [Google Scholar] [CrossRef]
| CVE + AF Patients | AF Patients | p-Value | |
|---|---|---|---|
| Number of patients, n (%) | 292 (14.2) | 1769 (85.8) | |
| Demographics | |||
| Age, years, mean (SD) | 74.6 (9.0) | 71.7 (11.5) | <0.001 |
| Sex, male, n (%) | 166/287 (57.8) | 1072/1755 (61.1) | 0.30 |
| Cerebrovascular event | n/a | ||
| Acute stroke at baseline, n (%) | 47/291 (16.2) | ||
| Acute TIA at baseline n (%) | 11/291 (3.8) | ||
| Previous stroke, n (%) | 183/291 (62.9) | ||
| Previous TIA, n (%) | 50/291 (17.2) | ||
| Vascular risk factors | |||
| Arterial hypertension, n (%) | 231/291 (79.4) | 1332/1720 (77.4) | 0.47 |
| Diabetes mellitus, n (%) | 86/291 (29.6) | 442/1720 (25.7) | 0.17 |
| Current smoker, n (%) | 14/291 (4.8) | 159/1720 (9.2) | 0.013 |
| Cardiovascular comorbidities | |||
| Congestive heart failure, n (%) | 60/289 (20.8) | 349/1682 (20.7) | 1.00 |
| CAD, n (%) | 127/289 (43.9) | 691/1682 (41.1) | 0.36 |
| Previous MI, n (%) | 55/289 (19.0) | 276/1682 (16.4) | 0.27 |
| Hypertensive cardiomyopathy, n (%) | 42/289 (14.5) | 313/1682 (18.6) | 0.096 |
| PAD | 33/291 (11.3) | 87/1720 (5.1) | <0.001 |
| Echocardiography | |||
| TTE performed, n (%) | 238/292 (81.5) | 1443/1769 (81.6) | 0.98 |
| LVEF, % (IQR) | 55 (44; 60) | 55 (45; 60) | 0.055 |
| LA diameter, mm (IQR) | 46 (43; 50) | 44 (40; 49) | 0.001 |
| LA diameter > 45 mm, n (%) | 75/131 (57.3) | 344/797 (43.2) | 0.003 |
| CVE + AF Patients | AF Patients | p-Value | |
|---|---|---|---|
| Number of patients, n (%) | 292 (14.2) | 1769 (85.8) | |
| AF characteristics | |||
| Paroxysmal AF, n (%) | 172/290 (59.3) | 1001/1722 (58.1) | 0.71 |
| Persistent AF, n (%) | 52/290 (17.9) | 424/1722 (24.6) | 0.013 |
| Long-lasting persistent AF, n (%) | 66/290 (22.8) | 297/1722 (17.2) | 0.024 |
| Newly diagnosed AF, n (%) | 33/292 (11.3) | 284/1745 (16.3) | 0.03 |
| Duration of AF, years, median, IQR § | 5 (3; 9) | 4 (2; 9) | 0.16 |
| AF at baseline, n (%) | 125/288 (43.4) | 968/1710 (56.6) | <0.001 |
| Heart rate, median (IQR) | 75 (63; 87) | 78 (65; 97) | 0.002 |
| CHA2DS2-VASc score, mean (SD) | 5.3 (1.5) | 3.3 (1.6) | <0.001 |
| HAS-BLED score, mean (SD) | 3.0 (1.0) | 2.0 (1.0) | <0.001 |
| EHRA class | <0.001 | ||
| I | 153/285 (53.7) | 683/1661 (41.1) | |
| II | 104/285 (36.5) | 710/1661 (42.7) | |
| III | 24/285 (8.4) | 251/1661 (15.1) | |
| IV | 4/285 (1.4) | 17/1661 (1.0) | |
| AF treatment | |||
| Oral anticoagulation, n (%) | 231/286 (80.8) | 1299/1726 (75.3) | 0.043 |
| VKA, n (%) | 63/286 (22.0) | 355/1726 (20.6) | 0.57 |
| DOAC, n (%) | 168/286 (58.7) | 944/1726 (54.7) | 0.20 |
| Beta-blocker, n (%) | 218/289 (75.4) | 1309/1709 (76.6) | 0.67 |
| Calcium channel blocker (Verapamil-type), n (%) | 36/289 (12.5) | 128/1709 (7.5) | 0.004 |
| Cardiac glycoside, n (%) | 32/289 (11.1) | 209/1709 (12.2) | 0.58 |
| Class I antiarrhythmic drug, n (%) | 5/286 (1.7) | 73/1662 (4.4) | 0.035 |
| Class II antiarrhythmic drug, n (%) | 13/286 (4.5) | 111/1662 (7.0) | 0.12 |
| Previous cardioversion, n (%) | 45/286 (15.7) | 414/1705 (24.3) | 0.001 |
| Previous ablation, n (%) | 25/287 (8.7) | 215/1703 (12.6) | 0.060 |
| Previous PM/ICD implantation, n (%) | 86/291 (29.6) | 430/1741 (24.7) | 0.078 |
| aCVE + AF Patients | pCVE + AF Patients | AF Patients | p-Value | |
|---|---|---|---|---|
| Number of patients, n (%) | 58 (2.8) | 234 (11.4) | 1769 (85.8) | |
| Cardiovascular comorbidities | ||||
| Any structural heart disease, n (%) | 26/58 (44.8) | 196/231 (84.8) | 1315/1682 (78.2) | <0.001 |
| CAD, n (%) | 14/58 (24.1) | 113/231 (48.9) | 691/1682 (41.1) | 0.002 |
| NYHA class I | 53/57 (93.9) | 68/228 (29.8) | 609/1663 (36.6) | <0.001 |
| Echocardiography | ||||
| LVEF, % (IQR) | 55 (54; 55) | 56 (49; 60) | 55 (45;60) | 0.051 |
| LA diameter > 45 mm, n (%) | 10/17 (58.8) | 65/114 (57.0) | 344/797 (43.2) | 0.011 |
| AF characteristics | ||||
| Paroxysmal AF, n (%) | 47/58 (81.0) | 125/232 (53.9) | 1001/1722 (58.1) | <0.001 |
| Persistent AF, n (%) | 11/58 (19.0) | 107/232 (46.1) | 721 (41.2) | |
| EHRA class I, n (%) | 53/57 (93.0) | 100/228 (43.9) | 683/1661 (41.1) | <0.001 |
| One-year event rates † | ||||
| 1-year mortality, % (KM) | 14.5 (7.5–26.9) | 14.6 (10.6–20.0) | 7.8 (6.6–9.2) | <0.001 |
| MACE, % (KM) | 14.6 (7.5–26.9) | 15.1 (11.0–25.5) | 8.1 (6.9–9.5) | <0.001 |
| MACCE, % (KM) | 18.1 (10.2–31.0) | 16.0 (11.7–21.5) | 8.1 (6.9–9.5) | <0.001 |
| MACCE + major bleeding, % (KM) | 18.1 (10.2–31.0) | 16.4 (12.1–22.0) | 8.5 (7.2–9.9) | <0.001 |
| CVE + AF Patients | AF Patients | p-Value | |
|---|---|---|---|
| Number of patients, n (%) | 292 (14.2) | 1769 (85.8) | |
| One-year event rates † | |||
| 1-year mortality, % (KM) | 14.6 (10.9–19.4) | 7.8 (6.6–9.2) | <0.001 |
| MACE, % (KM) | 15.0 (11.2–19.8) | 8.1 (6.9–9.5) | <0.001 |
| MACCE, % (KM) | 16.4 (12.5–21.4) | 8.1 (6.9–9.5) | <0.001 |
| MACCE + major bleeding, % (KM) | 16.8 (12.8–21.8) | 8.5 (7.2–9.9) | <0.001 |
| Patients alive at 1-year follow-up, n | 209 | 1397 | |
| Adverse events | |||
| Stroke, n (%) | 4/139 (2.9) | 0/976 (0.0) | <0.001 |
| TIA, n (%) | 1/136 (0.7) | 6/976 (0.6) | 0.87 |
| MI, n (%) | 1/135 (0.7) | 5/976 (0.5) | 0.74 |
| Major bleeding, n (%) | 0/139 (0.0) | 4/988 (0.4) | 0.45 |
| AF treatment | |||
| Oral anticoagulation, n (%) | 128/138 (92.8) | 817/991 (82.4) | 0.002 |
| VKA, n (%) | 22/137 (16.1) | 146/985 (14.8) | 0.7 |
| DOAC, n (%) | 105/137 (76.6) | 665/985 (67.5) | 0.031 |
| Beta-blocker, n (%) | 103/138 (74.6) | 723/984 (73.5) | 0.77 |
| Calcium channel blocker (Verapamil-type), n (%) | 27/138 (19.6) | 200/984 (20.3) | 0.84 |
| Cardiac glycoside, n (%) | 12/138 (8.7) | 118/984 (12.0) | 0.26 |
| Class I antiarrhythmic drug, n (%) | 3/136 (2.2) | 35/981 (3.6) | 0.41 |
| Class II antiarrhythmic drug, n (%) | 9/136 (6.6) | 71/981 (7.2) | 0.79 |
| LAA occluder, n (%) | 1/142 (0.7) | 2/1008 (0.2) | 0.27 |
| Cardioversion, n (%) | 7/142 (4.9) | 85/1006 (8.4) | 0.15 |
| Ablation, n (%) | 5/142 (3.5) | 84/1007 (8.3) | 0.044 |
| PM implantation, n (%) | 3/142 (2.1) | 13/1007 (1.3) | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, A.; Akin, I.; Hochadel, M.; Borggrefe, M.; Lesch, H.; Grau, A.; Zahn, R.; Lugenbiel, P.; Schwarzbach, C.J.; Sueselbeck, T.; et al. Atrial Fibrillation in Patients with Very High Risk for Stroke and Adverse Events—Insights from the Observational ARENA Study. J. Clin. Med. 2024, 13, 6645. https://doi.org/10.3390/jcm13226645
Alonso A, Akin I, Hochadel M, Borggrefe M, Lesch H, Grau A, Zahn R, Lugenbiel P, Schwarzbach CJ, Sueselbeck T, et al. Atrial Fibrillation in Patients with Very High Risk for Stroke and Adverse Events—Insights from the Observational ARENA Study. Journal of Clinical Medicine. 2024; 13(22):6645. https://doi.org/10.3390/jcm13226645
Chicago/Turabian StyleAlonso, Angelika, Ibrahim Akin, Matthias Hochadel, Martin Borggrefe, Hendrik Lesch, Armin Grau, Ralf Zahn, Patrick Lugenbiel, Christopher Jan Schwarzbach, Tim Sueselbeck, and et al. 2024. "Atrial Fibrillation in Patients with Very High Risk for Stroke and Adverse Events—Insights from the Observational ARENA Study" Journal of Clinical Medicine 13, no. 22: 6645. https://doi.org/10.3390/jcm13226645
APA StyleAlonso, A., Akin, I., Hochadel, M., Borggrefe, M., Lesch, H., Grau, A., Zahn, R., Lugenbiel, P., Schwarzbach, C. J., Sueselbeck, T., Senges, J., & Fastner, C. (2024). Atrial Fibrillation in Patients with Very High Risk for Stroke and Adverse Events—Insights from the Observational ARENA Study. Journal of Clinical Medicine, 13(22), 6645. https://doi.org/10.3390/jcm13226645






