Low PAPPA and Its Association with Adverse Pregnancy Outcomes in Twin Pregnancies: A Systematic Review of the Literature and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection
2.4. Data Collection
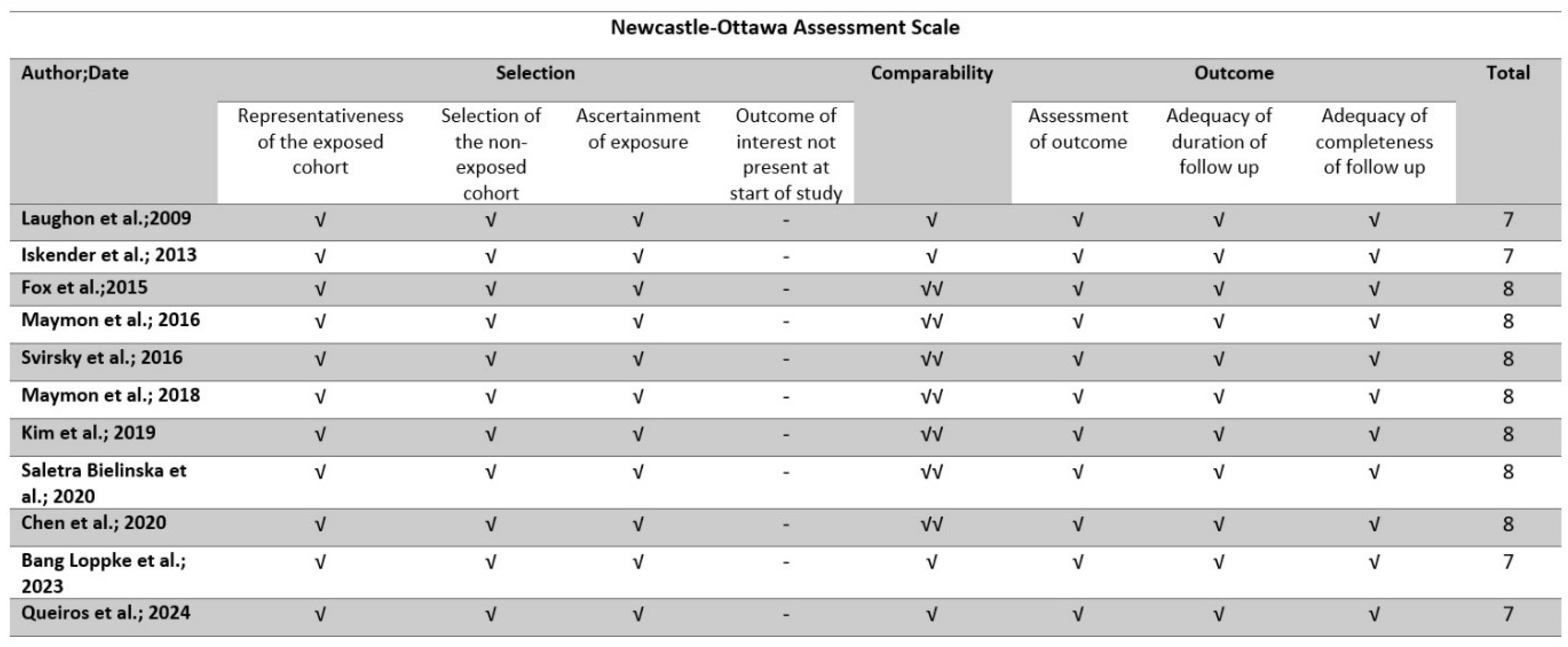
2.5. Quality Assessment
2.6. Data Synthesis
2.7. Prediction Intervals
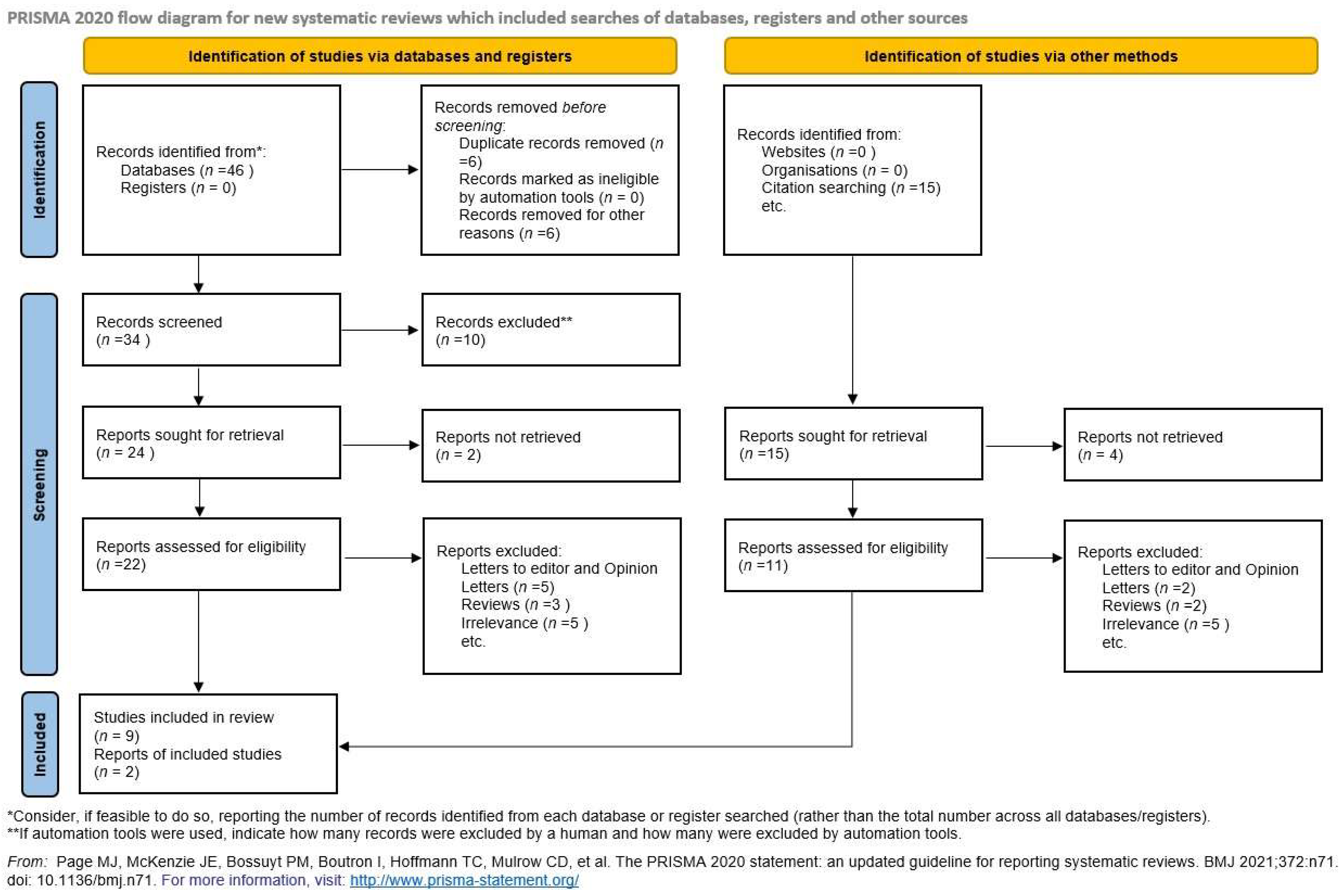
3. Results
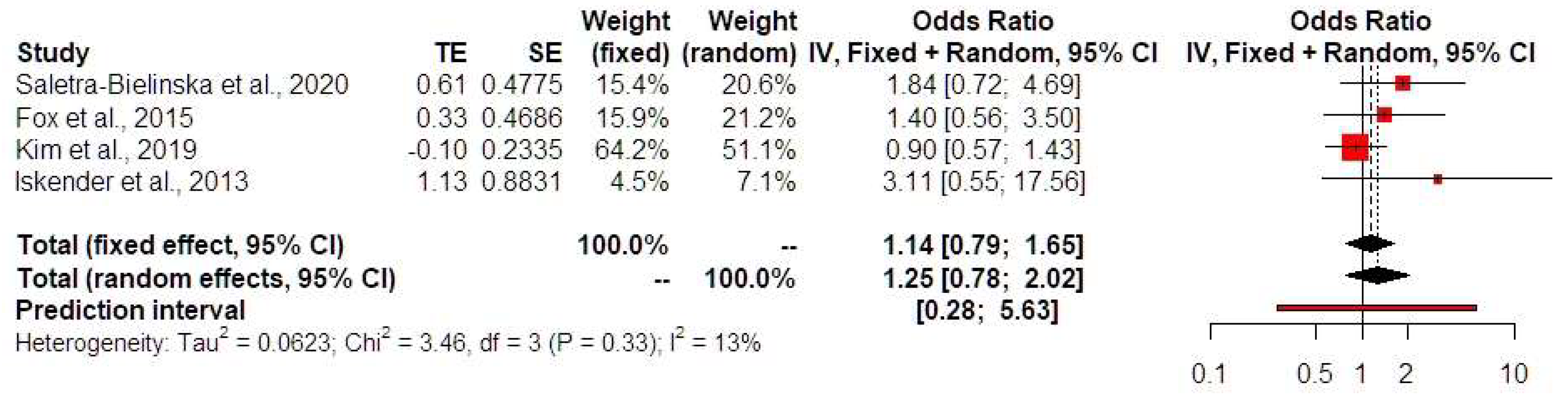
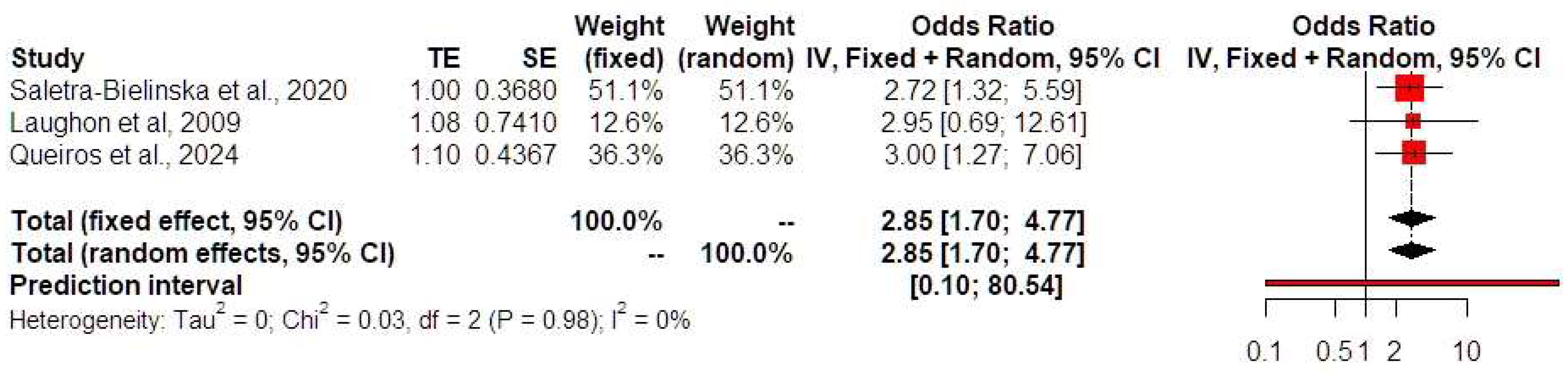
Synthesis of Results
4. Discussion
4.1. Low PAPP-A and PTB
4.2. Low PAPP-A and SGA/FGR
4.3. Low PAPP-A and Hypertensive Disorders of Pregnancy
4.4. Low PAPP-A and GDM
4.5. Low PAPP-A and Birth Weight Discordance
4.6. Low PAPP-A and Intrauterine Fetal Demise
4.7. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Kupka, M.S.; Ferraretti, A.P.; de Mouzon, J.; Erb, K.; D’Hooghe, T.; Castilla, J.A.; Calhaz-Jorge, C.; De Geyter, C.; Goossens, V.; European IVF-Monitoring Consortium, for the European Society of Human Reproduction and Embryology. Assisted reproductive technology in Europe, 2010: Results generated from European registers by ESHRE. Hum. Reprod. 2014, 29, 2099–2113. [Google Scholar] [CrossRef] [PubMed]
- Chien, P. Multiple pregnancy and assisted conception treatment. BJOG 2020, 127, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Santolaya, J. Twins-twice more trouble? Clin. Obstet. Gynecol. 2012, 55, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Rouvali, A.; Sapantzoglou, I.; Stavros, S.; Antsaklis, P.; Theodora, M.; Daskalakis, G. Adverse pregnancy outcomes in twins and their association with the conception method. HJOG 2022, 21, 161–166. [Google Scholar] [CrossRef]
- Cavoretto, P.I.; Farina, A.; Salmeri, N.; Syngelaki, A.; Tan, M.Y.; Nicolaides, K.H. First trimester risk of preeclampsia and rate of spontaneous birth in patients without preeclampsia. Am. J. Obstet. Gynecol. 2024, 231, 452.e1–452.e7. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.K.; Bilagi, A.; Devani, P.; Kilby, M.D. Association of serum PAPP-A levels in first trimester with small for gestational age and adverse pregnancy outcomes: Systematic review and meta-analysis. Prenat. Diagn. 2017, 37, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Svirsky, R.; Levinsohn-Tavor, O.; Feldman, N.; Klog, E.; Cuckle, H.; Maymon, R. First- and second-trimester maternal serum markers of pre-eclampsia in twin pregnancy. Ultrasound Obstet. Gynecol. 2016, 47, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Saletra-Bielińska, A.; Kosińska-Kaczyńska, K.; Szymusik, I.; Kaczyński, B.; Brawura-Biskupski-Samaha, R.; Kozłowski, S.; Jarmużek, P.; Walasik, I.; Wielgoś, M. Both Low and High PAPP-A Concentrations in the First Trimester of Pregnancy Are Associated with Increased Risk of Delivery before 32 Weeks in Twin Gestation. J. Clin. Med. 2020, 9, 2099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laughon, S.K.; Rebarber, A.; Rolnitzky, L.; Fink, L.; Saltzman, D.H. Decreased first-trimester maternal serum free-beta subunit human chorionic gonadotropin and preterm birth in twin gestations. Am. J. Perinatol. 2009, 26, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Iskender, C.; Tarım, E.; Çok, T.; Yalcınkaya, C.; Kalaycı, H.; Yanık, F.B. Obstetrical complications associated with first-trimester screening markers in twin pregnancies. J. Obstet. Gynaecol. Res. 2013, 39, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Rosner, J.Y.; Fox, N.S.; Saltzman, D.; Klauser, C.K.; Rebarber, A.; Gupta, S. Abnormal Biochemical Analytes Used for Aneuploidy Screening and Adverse Pregnancy Outcomes in Twin Gestations. Am. J. Perinatol. 2015, 32, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Maymon, R.; Trahtenherts, A.; Svirsky, R.; Melcer, Y.; Madar-Shapiro, L.; Klog, E.; Meiri, H.; Cuckle, H. Developing a new algorithm for first and second trimester preeclampsia screening in twin pregnancies. Hypertens. Pregnancy 2017, 36, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Maymon, R.; Meiri, H.; Svirski, R.; Weiner, E.; Cuckle, H. Maternal serum screening marker levels in twin pregnancies affected by gestational diabetes. Arch. Gynecol. Obstet. 2019, 299, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Jung, I.; Heo, S.J.; Chang, S.W.; Cho, H.Y. A preeclampsia risk prediction model based on maternal characteristics and serum markers in twin pregnancy. J. Matern. Fetal Neonatal Med. 2021, 34, 3623–3628. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, D.; Liu, Y.; Zhou, J.; Zou, G.; Zhang, Y.; Guo, M.; Duan, T.; Van Mieghem, T.; Sun, L. Screening for preeclampsia in low-risk twin pregnancies at early gestation. Acta Obstet. Gynecol. Scand. 2020, 99, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Løppke, F.B.; Schou, K.V.; Ekelund, C.K.; Rode, L.; Tabor, A.; Sundberg, K. First-trimester biomarkers and ultrasound biometries in relation to growth discordance in monochorionic diamniotic twins. J. Matern. Fetal Neonatal Med. 2023, 36, 2184223. [Google Scholar] [CrossRef] [PubMed]
- Queirós, A.; Gomes, L.; Pereira, I.; Charepe, N.; Plancha, M.; Rodrigues, S.; Cohen, Á.; Alves, M.; Papoila, A.L.; Simões, T. First-trimester serum biomarkers in twin pregnancies and adverse obstetric outcomes-a single center cohort study. Arch. Gynecol. Obstet. 2024, 310, 315–325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Veroniki, A.A.; Jackson, D.; Viechtbauer, W.; Bender, R.; Bowden, J.; Knapp, G.; Kuss, O.; Higgins, J.P.; Langan, D.; Salanti, G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods 2016, 7, 55–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Publications Committee, Society for Maternal-Fetal Medicine; Sibai, B.M. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am. J. Obstet. Gynecol. 2011, 205, 191–198. [Google Scholar] [CrossRef] [PubMed]
- ACOG. Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, E1–E25. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli, A.L.; Dekker, G.; Magee, L.; Roberts, J.; Sibai, B.M.; Steyn, W.; Zeeman, G.G.; Brown, M.A. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014, 4, 97–104. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet. Gynecol. 2013, 122 Pt 1, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Wender-Ożegowska, E.; Bomba-Opoń, D.; Brązert, J.; Celewicz, Z.; Czajkowski, K.; Gutaj, P.; Malinowska-Polubiec, A.; Zawiejska, A.; Wielgoś, M. Standards of Polish Society of Gynecologists and Obstetricians in management of women with diabetes. Ginekol. Pol. 2018, 89, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, A.; Wilson, R.D.; Society of Obstetricians and Gynaecologists of Canada Genetics Committee. Obstetrical complications associated with abnormal maternal serum markers analytes. J. Obstet. Gynaecol. Can. 2008, 30, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Pummara, P.; Tongsong, T.; Wanapirak, C.; Sirichotiyakul, S.; Luewan, S. Association of first-trimester pregnancy-associated plasma protein A levels and idiopathic preterm delivery: A population-based screening study. Taiwan. J. Obstet. Gynecol. 2016, 55, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R. Prediction of preterm birth in twin gestations using biophysical and biochemical tests. Am. J. Obstet. Gynecol. 2014, 211, 583–595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khalil, A.; Cowans, N.J.; Spencer, K.; Goichman, S.; Meiri, H.; Harrington, K. First-trimester markers for the prediction of pre-eclampsia in women with a-priori high risk. Ultrasound Obstet. Gynecol. 2010, 35, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B.; Meiri, H.; Gizurarson, S.; Osol, G.; Sammar, M. Placental protein 13 (PP13): A new biological target shifting individualized risk assessment to personalized drug design combating pre-eclampsia. Hum. Reprod. Update 2013, 19, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Sapantzoglou, I.; Rouvali, A.; Koutras, A.; Chatziioannou, M.I.; Prokopakis, I.; Fasoulakis, Z.; Zachariou, E.; Douligeris, A.; Mortaki, A.; Perros, P.; et al. sFLT1, PlGF, the sFLT1/PlGF Ratio and Their Association with Pre-Eclampsia in Twin Pregnancies-A Review of the Literature. Medicina 2023, 59, 1232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Plows, J.F.; Stanley, J.L.; Philip, N.; Baker Reynolds, C.; Vicker, M. The pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Dugoff, L.; Hobbins, J.C.; Malone, F.D.; Porter, T.F.; Luthy, D.; Comstock, C.H.; Hankins, G.; Berkowitz, R.L.; Merkatz, I.; Craigo, S.D.; et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: A population-based screening study (the FASTER Trial). Am. J. Obstet. Gynecol. 2004, 191, 1446–1451. [Google Scholar] [CrossRef]
- Văduva, C.C.; Constantinescu, C.; Ţenovici, M.; Văduva, A.R.; Niculescu, M.; DiŢescu, D.; Albu, C.C.; Albu, D.F. Delayed interval delivery in twin pregnancy—Case reports. Rom. J. Morphol. Embryol. 2016, 57, 1089–1098. [Google Scholar] [PubMed]
- Fathian, A.; Miller, R.; Wolf, E. Analysis of first trimester markers, PAPP-A and free-hCG, and adverse outcomes in twin pregnancies. Am. J. Obstet. Gynecol. 2014, 210, S135. [Google Scholar] [CrossRef]
- Wang, H.S.; Perry, L.A.; Kanisius, J.; Iles, R.K.; Holly, J.M.; Chard, T. Purification and assay of insulin-like growth factor-binding protein-1: Measurement of circulating levels throughout pregnancy. J. Endocrinol. 1991, 128, 161–168. [Google Scholar] [CrossRef]
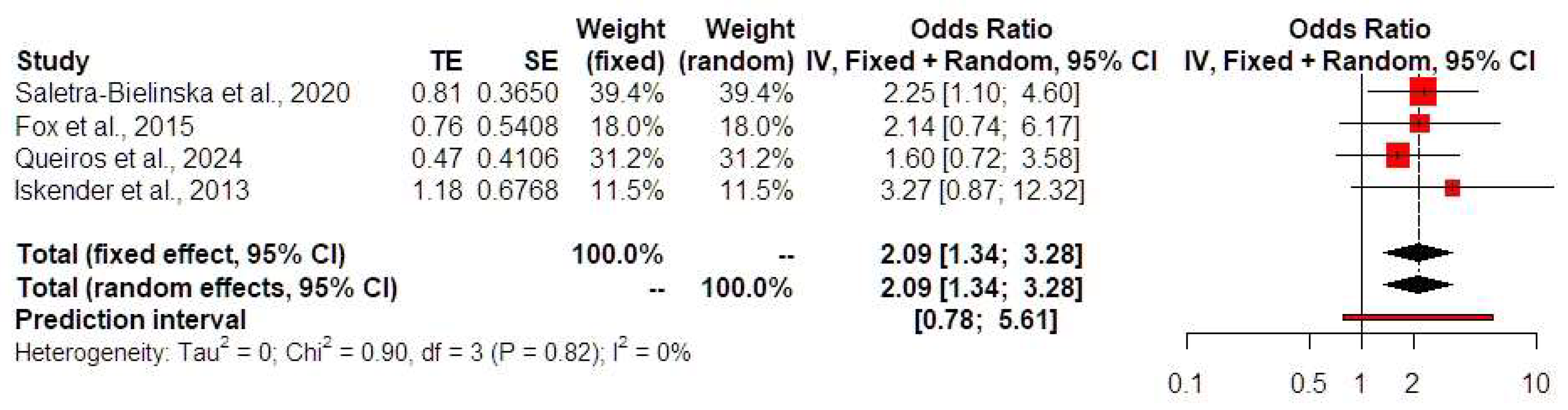
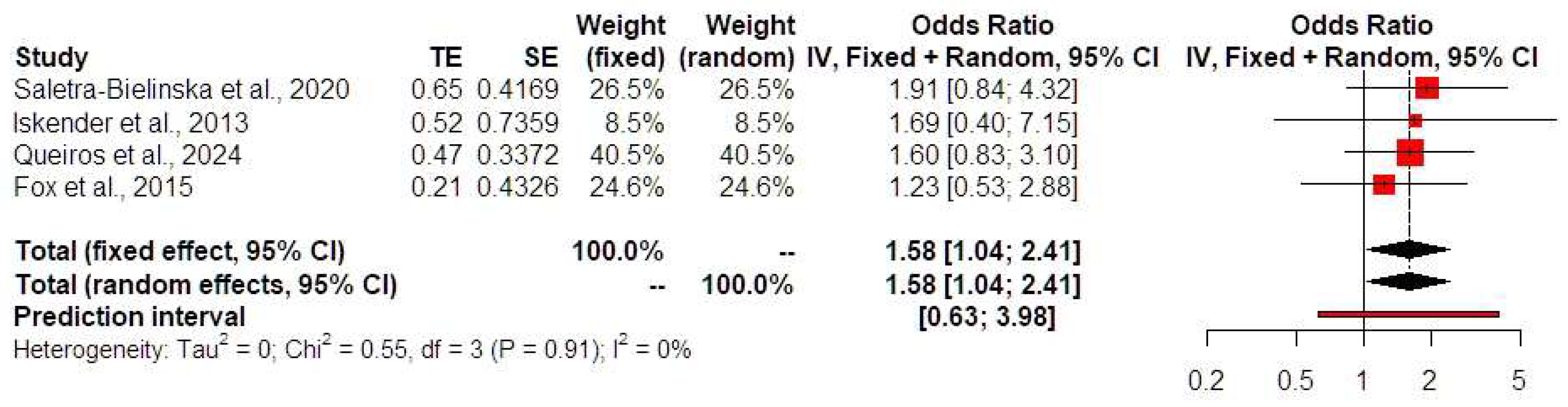
| Year; Author | N | Monochorionicity | Age | BMI at Testing | Gestation Age at Time of Testing | Race (%) (White/Black/Asian/Other) | Spontaneous Conception (%) | Preexisting DM (%) | Preexisting Chronic Hypertension (%) | Nulliparous (%) | Smoking (%) | Previous PE or GH (%) | Gestational Age at Delivery |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laughon et al., 2009 [11] | 70 | n/a | 36.1 | n/a | n/a | 72/4.2/6.9/6.9 | n/a | n/a | n/a | n/a | 4.3 | n/a | 36.4 |
| Iskender * et al., 2013 [12] | 10/94 | 0/4 | 29.8/30.3 | n/a | n/a | n/a | n/a | n/a | n/a | 100/90.4 | 0/4 | n/a | n/a |
| Fox et al., 2015 [13] | 340 | n/a | 36 | 23 | n/a | 80/n/a/n/a/n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
| Maymon ** et al., 2016 [14] | 9/96 | 13 | 36/31 | 72 kg/65 kg | 12.5(88d)/12.1(85d) | n/a | 33/66 | n/a | n/a | 56/38 | 11/20 | 11/8.3 | 35/36.6 |
| Svirsky et al., 2016 [7] | 144 | 18 | 31 | 23.5 | 12.1 | n/a | 48 | n/a | n/a | 25 | n/a | 11 | 36 + 3 |
| Maymon *** et al., 2018 [15] | 11/134 | 1/n/a | 32/31 | 82 kg/64 kg | 12/12.1 | n/a | 64/64 | n/a | n/a | 36/41 | 18/18 | n/a | 36.8/36.4 |
| Kim ** et al., 2019 [16] | 35/497 | n/a | 33.8/32.8 | 22.59/21.74 | n/a | n/a | 4/47 | n/a | n/a | n/a | n/a | n/a | 35.14/36.43 |
| Saletra-Bielinska * et al., 2020 [8] | 31/245 | 16/108 | 33.51/34.12 | 23.7/22.97 | n/a | n/a | 93.5/81.22 | n/a | n/a | n/a | 9.7/5.71 | n/a | 32.65/35.12 |
| Chen ** et al., 2020 [17] | 86/683 | 21/165 | 32.5/31.6 | 22.85/21.6 | 11.9/11.98 | n/a | 32.5/34.3 | n/a | n/a | 88.3/87.7 | n/a | n/a | n/a |
| Bang Loppke et al., 2023 [18] | 762 | 762 | n/a | n/a | n/a | n/a/1.6/2.8/4 | 93.3 | n/a | n/a | 45.2 | 8.1 | n/a | n/a |
| Queiros et al., 2024 [19] | 466 | 17.6 | 32.9 | <20:12.2% 20–35: 51.7% | n/a | 85.6/11.1/2.1/0.2 | 55.8 | 0.6 | 4,7 | 66.1 | 10.5 | n/a | 35.4 |
| Author, Year | Timing of Assessment (Weeks) | Type of Study | Study Center | Recruitment Period | Inclusion Criteria | Exclusion Criteria | Low PAPP-A Definition | Investigated Outcome |
|---|---|---|---|---|---|---|---|---|
| Laughon et al., 2009 [11] | 11–14 | Retrospective study | New York University Hospital, USA | 2003–2004 |
|
| ≤25th centile |
|
| Iskender et al., 2013 [12] | 11–14 | Retrospective study | Ankara, Turkey | 2005–2011 |
|
| ≤10th centile |
|
| Fox et al., 2015 [13] | 10–14 | Retrospective study | New York, USA | 2005–2013 |
|
| <0.42 MoM |
|
| Maymon et al., 2016 [14] | 11–14 | Prospective observational study | Tel-Aviv, Israel | 2011–2013 |
|
| Not defined |
|
| Svirsky et al., 2016 [7] | 11–14 | Prospective observational study | Tel-Aviv, Israel | 2011–2013 |
|
| Not defined |
|
| Maymon et al., 2018 [15] | 11–14 | Prospective observational study | Zerifin, Israel | 2011–2013 |
|
| Not defined |
|
| Kim et al., 2019 [16] | 10–14 | Retrospective study | Seongnam, SouthKorea | 2005–2017 |
|
| Not defined |
|
| Saletra Bielinska et al., 2020 [8] | 10–14 | Retrospective study | Warsaw, Poland | 2013–2018 |
|
| ≤10th centile |
|
| Chen et al., 2020 [17] | 11–14 | Prospective observational study | Shanghai, China | 2014–2017 |
|
| Not defined |
|
| Bang Loppke et al., 2023 [18] | 11–14 | Register-based national cohort study | Denmark | 2008–2017 |
|
| Not defined |
|
| Queiros et al., 2024 [19] | 10–14 | Retrospective study | Lisbon, Portugal | 2010–2022 |
|
| ≤10th centile |
|
| Author, Year | GDM | IUD | Discordant Fetal Growth |
|---|---|---|---|
| Laughon et al., 2009 [11] | NS | NS | NS |
| Iskender et al., 2013 [12] | Low PAPP-A levels are not associated with GDM | NS | Low PAPP-A levels are not associated with discordant fetal growth |
| Fox et al., 2015 [13] | NS | Low PAPP-A levels are not associated with the IUD of at least one twin | NS |
| Maymon et al., 2016 [14] | NS | NS | NS |
| Svirsky et al., 2016 [7] | NS | NS | NS |
| Maymon et al., 2018 [15] | High levels of PAPP-A are associated with GDM | NS | NS |
| Kim et al., 2019 [16] | NS | NS | NS |
| Saletra Bielinska et al., 2020 [8] | Low PAPP-A levels are associated with GDM | Low PAPP-A levels are not associated with the IUD of at least one twin | Low PAPP-A levels are not associated with discordant fetal growth |
| Chen et al., 2020 [17] | NS | NS | NS |
| Bang Loppke et al., 2023 [18] | NS | NS | Low PAPP-A levels are not associated with discordant fetal growth |
| Queiros et al., 2024 [19] | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapantzoglou, I.; Giourga, M.; Kontopoulou, A.M.; Pergialiotis, V.; Daskalaki, M.A.; Antsaklis, P.; Theodora, M.; Thomakos, N.; Daskalakis, G. Low PAPPA and Its Association with Adverse Pregnancy Outcomes in Twin Pregnancies: A Systematic Review of the Literature and Meta-Analysis. J. Clin. Med. 2024, 13, 6637. https://doi.org/10.3390/jcm13226637
Sapantzoglou I, Giourga M, Kontopoulou AM, Pergialiotis V, Daskalaki MA, Antsaklis P, Theodora M, Thomakos N, Daskalakis G. Low PAPPA and Its Association with Adverse Pregnancy Outcomes in Twin Pregnancies: A Systematic Review of the Literature and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(22):6637. https://doi.org/10.3390/jcm13226637
Chicago/Turabian StyleSapantzoglou, Ioakeim, Maria Giourga, Afroditi Maria Kontopoulou, Vasileios Pergialiotis, Maria Anastasia Daskalaki, Panagiotis Antsaklis, Marianna Theodora, Nikolaos Thomakos, and George Daskalakis. 2024. "Low PAPPA and Its Association with Adverse Pregnancy Outcomes in Twin Pregnancies: A Systematic Review of the Literature and Meta-Analysis" Journal of Clinical Medicine 13, no. 22: 6637. https://doi.org/10.3390/jcm13226637
APA StyleSapantzoglou, I., Giourga, M., Kontopoulou, A. M., Pergialiotis, V., Daskalaki, M. A., Antsaklis, P., Theodora, M., Thomakos, N., & Daskalakis, G. (2024). Low PAPPA and Its Association with Adverse Pregnancy Outcomes in Twin Pregnancies: A Systematic Review of the Literature and Meta-Analysis. Journal of Clinical Medicine, 13(22), 6637. https://doi.org/10.3390/jcm13226637









