Over-the-Wire Retrieval of Infectious Hemodialysis Catheter-Related Right Atrial Thrombus Causing Recurrent Pleural Empyema and Sepsis: A Case-Based Review
Abstract
1. Introduction
2. Literature Search
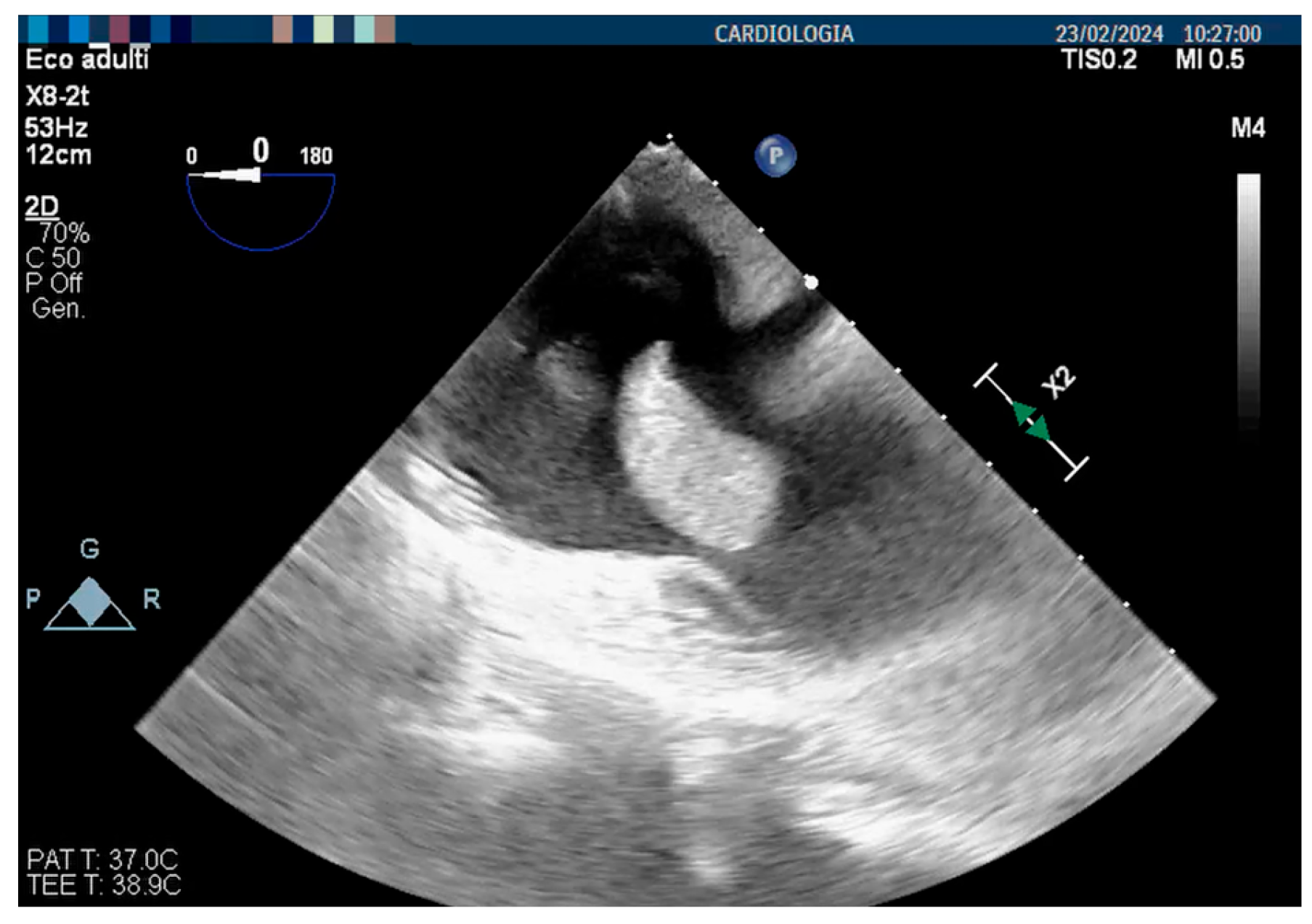
3. Case Report
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Momeni, A.; Mardani, S.; Kabiri, M.; Amiri, M. Comparison of Complications of Arteriovenous Fistula with Permanent Catheter in Hemodialysis Patients: A Six-Month Follow-Up. Adv. Biomed. Res. 2017, 6, 106. [Google Scholar] [CrossRef]
- Chiu, C.H.; Wang, C.Y.; Moi, S.H.; Wu, C.H.; Yang, C.H.; Chen, J.B. Comparison of Tunneled Central Venous Catheters and Native Arteriovenous Fistulae by Evaluating the Mortality and Morbidity of Patients with Prevalent Hemodialysis. J. Formos. Med. Assoc. 2019, 118, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Gedney, N. Arteriovenous Fistula or Dialysis Catheter: A Patient’s Perspective. Kidney360 2022, 3, 1109–1110. [Google Scholar] [CrossRef] [PubMed]
- Browne, L.D.; Dawood, M.; Leahy, F.; Ryan, M.C.; White, E.; O’Sullivan, A.; O’Sullivan, L.; Stack, A.G. Differential Impact of Central Venous Catheters versus Arteriovenous Fistulae on Quality of Life among Irish Haemodialysis Patients. Kidney360 2022, 3, 1065–1072. [Google Scholar] [CrossRef]
- Muralidharan, S.; Asokan, A.; Raja Rajan, N.; Mathew, G.G.; Jayaprakash, V. An Abysmal Conundrum Associated with a Misplaced Dialysis Catheter. J. Vasc. Access 2024, 11297298241262498. [Google Scholar] [CrossRef]
- Lenz, H.; Myre, K.; Draegni, T.; Dorph, E. A Five-Year Data Report of Long-Term Central Venous Catheters Focusing on Early Complications. Anesthesiol. Res. Pract. 2019, 2019, 6769506. [Google Scholar] [CrossRef]
- Otoya, D.; Simmonds, A.; Lavingia, K.; Amendola, M.F. Central Line Access for Hemodialysis Adversely Affects Ipsilateral Arteriovenous Graft Outcomes. Ann. Vasc. Surg. 2022, 86, 236–241. [Google Scholar] [CrossRef]
- Cardoso, I.; de Almeida, J.; Tsoumani, Z.; Alpendurada, F.; Mohiaddin, R.H. Central Venous Catheter–Related Right Atrial Thrombus in Oncology Patients: A Case Series of Cardiovascular Magnetic Resonance Studies. Eur. Heart J. Case Rep. 2024, 8, ytae296. [Google Scholar] [CrossRef]
- Tran, M.H.; Wilcox, T.; Tran, P.N. Catheter-Related Right Atrial Thrombosis. J. Vasc. Access 2020, 21, 300–307. [Google Scholar] [CrossRef]
- Shah, A.; Murray, M.; Nzerue, C.M. Right Atrial Thrombi Complicating Use of Central Venous Catheters in Hemodialysis. J. Vasc. Access 2005, 6, 18–24. [Google Scholar] [CrossRef]
- Bernardo, J.; Oliveira, J.; Gameiro, J.; Outerelo, C. Asymptomatic Pulmonary Thromboembolism Due to Hemodialisys Catheter Thrombosis: Case Series and Literature Review. CEN Case Rep. 2023, 12, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Pua, U.; Chia, P.L. Multidetector Row CT Diagnosis of an Infected Right Atrial Thrombus Following Repeated Dialysis Catheter Placement. Br. J. Radiol. 2009, 82, e240–e242. [Google Scholar] [CrossRef] [PubMed]
- Yew, M.S.; Leong, A.M.W.M. Contemporary Management and Outcomes of Infective Tunnelled Haemodialysis Catheter-Related Right Atrial Thrombi: A Case Series and Literature Review. Singap. Med. J. 2020, 61, 331. [Google Scholar] [CrossRef]
- Yang, H.; Chen, F.; Jiao, H.; Luo, H.; Yu, Y.; Hong, H.G.; Li, Y.; Fu, P.; Cui, T. Management of Tunneled-Cuffed Catheter-Related Right Atrial Thrombosis in Hemodialysis Patients. J. Vasc. Surg. 2018, 68, 1491–1498. [Google Scholar] [CrossRef]
- Rose, P.S.; Punjabi, N.M.; Pearse, D.B. Treatment of Right Heart Thromboemboli. Chest 2002, 121, 806–814. [Google Scholar] [CrossRef]
- Stavroulopoulos, A.; Aresti, V.; Zounis, C. Right Atrial Thrombi Complicating Haemodialysis Catheters. A Meta-Analysis of Reported Cases and a Proposal of a Management Algorithm. Nephrol. Dial. Transplant. 2012, 27, 2936–2944. [Google Scholar] [CrossRef]
- Gunawansa, N.; Sudusinghe, D.H.; Wijayaratne, D.R. Hemodialysis Catheter-Related Central Venous Thrombosis: Clinical Approach to Evaluation and Management. Ann. Vasc. Surg. 2018, 51, 298–305. [Google Scholar] [CrossRef]
- Balsorano, P.; Virgili, G.; Villa, G.; Pittiruti, M.; Romagnoli, S.; De Gaudio, A.R.; Pinelli, F. Peripherally Inserted Central Catheter–Related Thrombosis Rate in Modern Vascular Access Era—When Insertion Technique Matters: A Systematic Review and Meta-Analysis. J. Vasc. Access 2020, 21, 45–54. [Google Scholar] [CrossRef]
- Hammes, M.; Desai, A.; Pasupneti, S.; Kress, J.; Funaki, B.; Watson, S.; Herlitz, J.; Hines, J. Central Venous Catheters: Incidence and Predictive Factors of Venous Thrombosis. Clin. Nephrol. 2015, 84, 21. [Google Scholar] [CrossRef]
- Bichu, S.; Tilve, P.; Dhakate, T.; Kakde, P.; Bhasin, N.; Jawandhiya, P.; Dixit, A.; Jain, P.; Billa, V.; Kirpalani, A.; et al. Catheter Related Right Atrial Thrombus in Patients on Maintenance Hemodialysis: Results of a Single Centre Retrospective Study from a Tertiary Care Hospital. J. Assoc. Physicians India 2018, 66, 31–34. [Google Scholar]
- Ducatman, B.S.; McMichan, J.C.; Edwards, W.D. Catheter-Induced Lesions of the Right Side of the Heart: A One-Year Prospective Study of 141 Autopsies. JAMA J. Am. Med. Assoc. 1985, 253, 791–795. [Google Scholar] [CrossRef]
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.C.; Glickman, M.H.; et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am. J. Kidney Dis. 2020, 75, S1–S164. [Google Scholar] [CrossRef] [PubMed]
- Gilon, D.; Schechter, D.; Rein, A.J.J.T.; Gimmon, Z.; Or, R.; Rozenman, Y.; Slavin, S.; Gotsman, M.S.; Nagler, A. Right Atrial Thrombi Are Related to Indwelling Central Venous Catheter Position: Insights into Time Course and Possible Mechanism of Formation. Am. Heart J. 1998, 135, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Korones, D.N.; Buzzard, C.J.; Asselin, B.L.; Harris, J.P. Right Atrial Thrombi in Children with Cancer and Indwelling Catheters. J. Pediatr. 1996, 128, 841–846. [Google Scholar] [CrossRef]
- Napoli, M.; Guzzi, F.; Morale, W.; Lomonte, C.; Galli, F.; Lodi, M.; Bonforte, G.; Bonucchi, D.; Brunori, G.; Buzzi, L.; et al. Vascular Access for Hemodialysis in Italy: What a National Survey Reveals. J. Vasc. Access 2024, 11297298231217318. [Google Scholar] [CrossRef]
- Pisoni, R.L.; Zepel, L.; Fluck, R.; Lok, C.E.; Kawanishi, H.; Süleymanlar, G.; Wasse, H.; Tentori, F.; Zee, J.; Li, Y.; et al. International Differences in the Location and Use of Arteriovenous Accesses Created for Hemodialysis: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2018, 71, 469–478. [Google Scholar] [CrossRef]
- Ravani, P.; Palmer, S.C.; Oliver, M.J.; Quinn, R.R.; MacRae, J.M.; Tai, D.J.; Pannu, N.I.; Thomas, C.; Hemmelgarn, B.R.; Craig, J.C.; et al. Associations between Hemodialysis Access Type and Clinical Outcomes: A Systematic Review. J. Am. Soc. Nephrol. 2013, 24, 465–473. [Google Scholar] [CrossRef]
- Liu, R.; Lin, S.; Liu, L.; Xu, J.; Liu, L.; Pang, J.; An, H.; Yang, W.; Jian, J.; Wang, J.; et al. Vascular Access Type and Prognosis in Elderly Hemodialysis Patients: A Propensity-Score-Matched Study. Ren. Fail. 2024, 46, 2387205. [Google Scholar] [CrossRef]
- Buzzi, L.; Baragetti, I.; Barbagallo, M.M.; Marciello, A.; Lodi, M.; Morale, W.; Napoli, M.; Forneris, G. Insights into the Real-World Practice of Vascular Access Care Pathways in Italy: Data from a National Survey. J. Nephrol. 2024, 1–9. [Google Scholar] [CrossRef]
- Gover, A.; Sharif, D.; Yaniv, L.; Riskin, A. Intracardiac Thrombi in Preterm Infants—A Case Study and Review of the Literature. Diagnostics 2023, 13, 764. [Google Scholar] [CrossRef]
- Basman, C.; Rashid, U.; Parmar, Y.J.; Kliger, C.; Kronzon, I. The Role of Percutaneous Vacuum-Assisted Thrombectomy for Intracardiac and Intravascular Pathology. J. Card. Surg. 2018, 33, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Schaerf, R.H.M.; Najibi, S.; Conrad, J. Percutaneous Vacuum-Assisted Thrombectomy Device Used for Removal of Large Vegetations on Infected Pacemaker and Defibrillator Leads as an Adjunct to Lead Extraction. J. Atr. Fibrillation 2016, 9, 1455. [Google Scholar] [CrossRef]
- Jahangiri, Y.; Morrison, J.J.; Mowery, M.L.; Leach, A.J.; Musolf, R.L.; Knox, M.F. Effectiveness and Safety of Large-Bore Aspiration Thrombectomy for Intermediate- or High-Risk Pulmonary Embolism. J. Vasc. Interv. Radiol. 2024, 35, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.H.; Wong, A.; Shih, T.; Garg, A.; Elias, Y. Endovascular Aspiration of Native Tricuspid Valve Vegetation Using INARI Catheter in a Patient with Methicillin-Sensitive Staphylococcus aureus Endocarditis. Radiol. Case Rep. 2024, 19, 387–392. [Google Scholar] [CrossRef]
- Carroll, K.; Goncalves, J.; Kalimi, R.; Manvar-Singh, P. Hemodialysis Catheter-Related Right Atrial Thrombus Treated with the FlowTriever System. J. Vasc. Surg. Cases Innov. Tech. 2023, 9, 101318. [Google Scholar] [CrossRef]
- Oguslu, U.; Gümüş, B.; Yalçin, M.; Sahin, O.Z.; Yilmaz, G. Comparison of Supraclavicular Brachiocephalic and Femoral Vein Approaches for Tunneled Dialysis Catheter Placement in Patients with Thrombosed Internal Jugular Veins. Hemodial. Int. 2024, 28, 24–31. [Google Scholar] [CrossRef]
- Ngo Bell, E.C.; Chapon, V.; Bessede, E.; Meriglier, E.; Issa, N.; Domblides, C.; Bonnet, F.; Vandenhende, M.A. Central Venous Catheter-Related Bloodstream Infections: Epidemiology and Risk Factors for Hematogenous Complications. Infect. Dis. Now. 2024, 54, 104859. [Google Scholar] [CrossRef]
- El-Andari, R.; Nagendran, J.; Al Aklabi, M.; Wang, W. Persistent Methicillin-Resistant Staphylococcus Aureus Bacteremia from an Infected Superior Vena Cava Thrombus. Future Cardiol. 2023, 19, 87–90. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; De Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the Management of Endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Chen, J.W.; Hsu, C.C.; Su, C.C.; Hsu, R.B.; Chiu, Y.L.; Jung, C.J.; Chia, J.S. Transient Bacteremia Promotes Catheter-Related Central Venous Thrombosis through Neutrophil Extracellular Traps. Thromb. Haemost. 2022, 122, 1198–1208. [Google Scholar] [CrossRef]
- Vorla, M.; Kalra, D.K. Meta-Analysis of the Safety and Efficacy of Direct Oral Anticoagulants for the Treatment of Left Ventricular Thrombus. Pharmaceuticals 2024, 17, 708. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, A.; Zulty, M.; Rao, S.; Young, R. The Prevalence and Safety of Direct Oral Anticoagulants in Patients with Non-Atrial Fibrillation Associated Intra-Cardiac Thrombus. Eur. Heart J. 2021, 42, ehab724.2970. [Google Scholar] [CrossRef]
- Levine, G.N.; McEvoy, J.W.; Fang, J.C.; Ibeh, C.; McCarthy, C.P.; Misra, A.; Shah, Z.I.; Shenoy, C.; Spinler, S.A.; Vallurupalli, S.; et al. Management of Patients at Risk for and With Left Ventricular Thrombus: A Scientific Statement from the American Heart Association. Circulation 2022, 146, e205–e223. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Giannakoulas, G.; Theofilis, P.; Patoulias, D.; Fragakis, N. Direct Oral Anticoagulants or Vitamin K Antagonists in Adult Patients with Congenital Heart Disease? Eur. J. Intern. Med. 2024, 129, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Covella, B.; Libutti, P.; Teutonico, A.; Casucci, F.; Lomonte, C. How to Manage Catheter-Related Right Atrial Thrombosis: Our Conservative Approach. J. Vasc. Access 2021, 22, 480–484. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barilaro, G.; Galassi, A.; Gatto, M.C.; Ciocci, G.; Fabrizio, F.P.; Cappelli, A. Over-the-Wire Retrieval of Infectious Hemodialysis Catheter-Related Right Atrial Thrombus Causing Recurrent Pleural Empyema and Sepsis: A Case-Based Review. J. Clin. Med. 2024, 13, 6630. https://doi.org/10.3390/jcm13226630
Barilaro G, Galassi A, Gatto MC, Ciocci G, Fabrizio FP, Cappelli A. Over-the-Wire Retrieval of Infectious Hemodialysis Catheter-Related Right Atrial Thrombus Causing Recurrent Pleural Empyema and Sepsis: A Case-Based Review. Journal of Clinical Medicine. 2024; 13(22):6630. https://doi.org/10.3390/jcm13226630
Chicago/Turabian StyleBarilaro, Giuseppe, Amedeo Galassi, Maria Chiara Gatto, Giulia Ciocci, Fabrizia Paola Fabrizio, and Alessandra Cappelli. 2024. "Over-the-Wire Retrieval of Infectious Hemodialysis Catheter-Related Right Atrial Thrombus Causing Recurrent Pleural Empyema and Sepsis: A Case-Based Review" Journal of Clinical Medicine 13, no. 22: 6630. https://doi.org/10.3390/jcm13226630
APA StyleBarilaro, G., Galassi, A., Gatto, M. C., Ciocci, G., Fabrizio, F. P., & Cappelli, A. (2024). Over-the-Wire Retrieval of Infectious Hemodialysis Catheter-Related Right Atrial Thrombus Causing Recurrent Pleural Empyema and Sepsis: A Case-Based Review. Journal of Clinical Medicine, 13(22), 6630. https://doi.org/10.3390/jcm13226630






