Risk Factors for Prematurity and Congenital Malformations in Assisted Reproductive Technology Pregnancies—A Retrospective Study
Abstract
1. Introduction
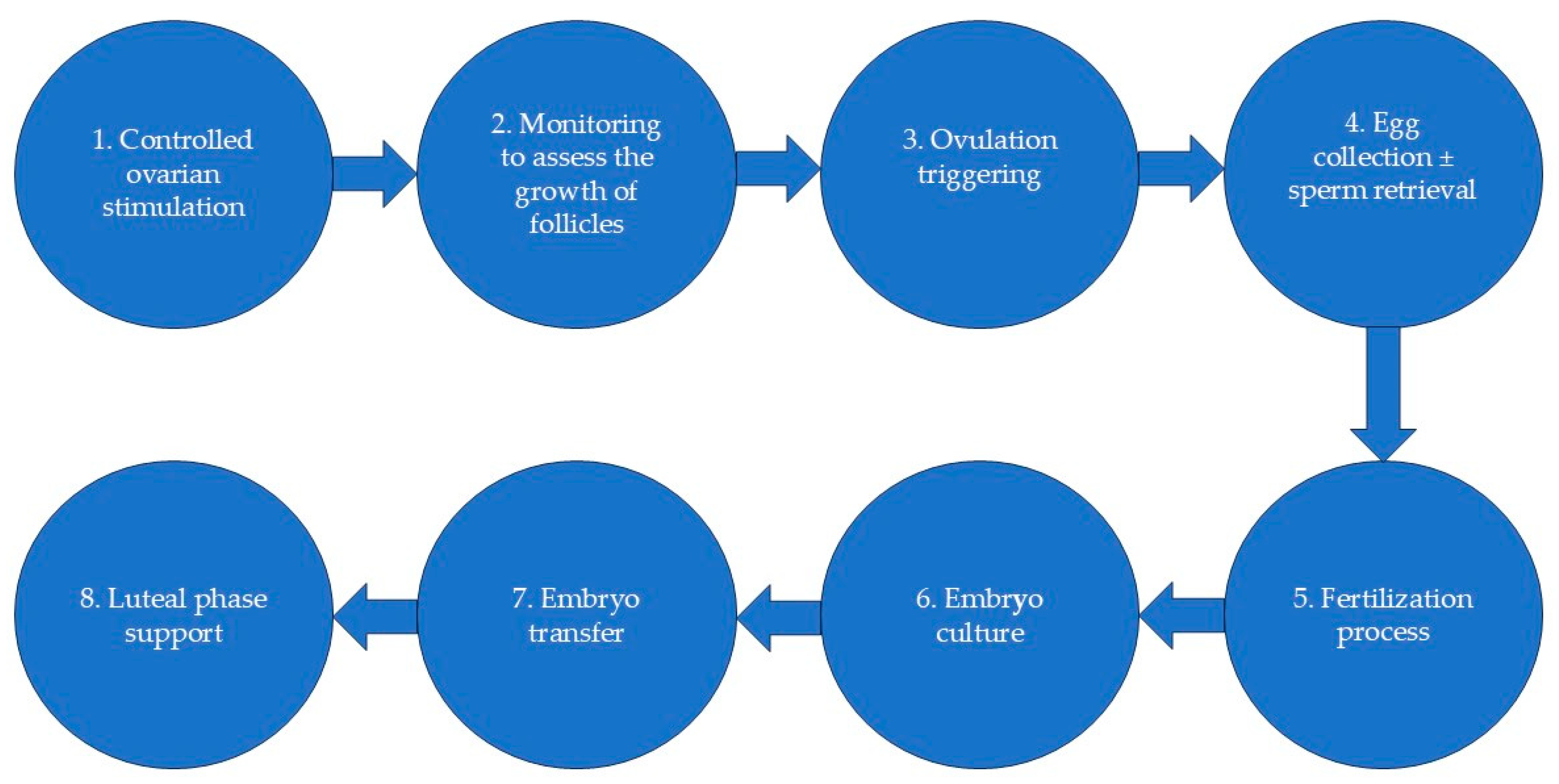
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Data Collection
2.3. Statistical Analyses
3. Results
3.1. Prematurity
- -
- Having a twin pregnancy increased the odds of prematurity by 1456% (95% CI: 968.3–2268%).
- -
- Having a PIH pregnancy increased the odds of prematurity by 122.1% (95% CI: 44.1–242.2%).
- -
- Having an IFV pregnancy (vs. ICSI pregnancy) increased the odds of prematurity by 72.7% (95% CI: 25–138.5%).
- -
- Having a donor conception increased the odds of prematurity by 249.3% (95% CI: 114.4–469.1%).
- -
- Having a twin pregnancy increased the odds of prematurity by 1501% (95% CI: 974–2289%).
- -
- Having a PIH pregnancy increased the odds of prematurity by 167.6% (95% CI: 58.4–352%).
- -
- Having a donor conception increased the odds of prematurity by 80.4% (95% CI: 18.6–230.2%).
3.2. Congenital Malformations
- -
- Having a day 3 embryo increased the odds of congenital malformations by 228.1% (95% CI: 71.2–529%).
- -
- Having a fresh embryo increased the odds of congenital malformations by 122.5% (95% CI: 37.8–269%).
- -
- Having a donor conception increased the odds of congenital malformations by 142.1% (95% CI: 28.3–356.7%).
- -
- Having a day 3 embryo increased the odds of congenital malformations by 166.4% (95% CI: 36.2–420.8%).
- -
- Having a fresh embryo increased the odds of congenital malformations by 108.7% (95% CI: 25.9–246%).
- -
- Having a donor conception increased the odds of congenital malformations by 132.3% (95% CI: 21.2–345.4%).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eskew, A.M.; Jungheim, E.S. A History of Developments to Improve in Vitro Fertilization. Mo. Med. 2017, 114, 156–159. [Google Scholar] [PubMed]
- Szamatowicz, M. Assisted Reproductive Technology in Reproductive Medicine—Possibilities and Limitations. Ginekol. Pol. 2016, 87, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.E.; Jelin, A.; Hoon, A.H.; Wilms Floet, A.M.; Levey, E.; Graham, E.M. Assisted Reproductive Technology: Short- and Long-term Outcomes. Dev. Med. Child Neurol. 2023, 65, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Singh, M. Assisted Reproductive Technology (ART) Techniques; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Cox, C.M.; Thoma, M.E.; Tchangalova, N.; Mburu, G.; Bornstein, M.J.; Johnson, C.L.; Kiarie, J. Infertility Prevalence and the Methods of Estimation from 1990 to 2021: A Systematic Review and Meta-Analysis. Hum. Reprod. Open 2022, 2022, hoac051. [Google Scholar] [CrossRef]
- Allahbadia, G.N.; Morimoto, Y. (Eds.) Ovarian Stimulation Protocols; Springer: New Delhi, India, 2016; ISBN 978-81-322-1120-4. [Google Scholar]
- Bosch, E.; Broer, S.; Griesinger, G.; Grynberg, M.; Humaidan, P.; Kolibianakis, E.; Kunicki, M.; La Marca, A.; Lainas, G.; Le Clef, N.; et al. ESHRE Guideline: Ovarian Stimulation for IVF/ICSI †. Hum. Reprod. Open 2020, 2020, hoaa009. [Google Scholar] [CrossRef]
- Sunderam, S.; Kissin, D.M.; Zhang, Y.; Jewett, A.; Boulet, S.L.; Warner, L.; Kroelinger, C.D.; Barfield, W.D. Assisted Reproductive Technology Surveillance—United States, 2018. MMWR Surveill. Summ. 2022, 71, 1–19. [Google Scholar] [CrossRef]
- Zargar, M.; Dehdashti, S.; Najafian, M.; Choghakabodi, P.M. Pregnancy Outcomes Following in Vitro Fertilization Using Fresh or Frozen Embryo Transfer. JBRA Assist. Reprod. 2021, 25, 570. [Google Scholar] [CrossRef]
- Bergenheim, S.J.; Saupstad, M.; Pistoljevic, N.; Andersen, A.N.; Forman, J.L.; Løssl, K.; Pinborg, A. Immediate versus Postponed Frozen Embryo Transfer after IVF/ICSI: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2021, 27, 623–642. [Google Scholar] [CrossRef]
- Vela, G.; Luna, M.; Sandler, B.; Copperman, A.B. Advances and Controversies in Assisted Reproductive Technology. Mt. Sinai J. Med. 2009, 76, 506–520. [Google Scholar] [CrossRef]
- De Geyter, C. Assisted Reproductive Technology: Impact on Society and Need for Surveillance. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 3–8. [Google Scholar] [CrossRef]
- Balli, M.; Cecchele, A.; Pisaturo, V.; Makieva, S.; Carullo, G.; Somigliana, E.; Paffoni, A.; Vigano’, P. Opportunities and Limits of Conventional IVF versus ICSI: It Is Time to Come off the Fence. J. Clin. Med. 2022, 11, 5722. [Google Scholar] [CrossRef] [PubMed]
- Matteo, M. Assisted Reproductive Technology. In Practical Clinical Andrology; Springer International Publishing: Cham, Germany, 2023; pp. 237–250. [Google Scholar]
- Bedoschi, G.; Roque, M.; Esteves, S.C. ICSI and Male Infertility: Consequences to Offspring. In Male Infertility; Springer International Publishing: Cham, Germany, 2020; pp. 767–775. [Google Scholar]
- Geng, T.; Cheng, L.; Ge, C.; Zhang, Y. The Effect of ICSI in Infertility Couples with Non-Male Factor: A Systematic Review and Meta-Analysis. J. Assist. Reprod. Genet. 2020, 37, 2929–2945. [Google Scholar] [CrossRef] [PubMed]
- Kawwass, J.F.; Badell, M.L. Maternal and Fetal Risk Associated with Assisted Reproductive Technology. Obstet. Gynecol. 2018, 132, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Rosca, I.; Turenschi, A.; Raris-Denisa, A.; Popescu, D.-E.; Stoica, C.; Carp-Veliscu, A.; Jura, A.-M.-C.; Teodora Constantin, A. Association of Assisted Reproductive Technology with the Risk of Congenital Heart Defects: A 5-Year Retrospective Study—Experience from a Tertiary Maternity Hospital in Bucharest. Obstet. Gynecol. Res. 2023, 6, 281–294. [Google Scholar] [CrossRef]
- Luke, B.; Brown, M.B.; Wantman, E.; Schymura, M.J.; Browne, M.L.; Fisher, S.C.; Forestieri, N.E.; Rao, C.; Nichols, H.B.; Yazdy, M.M.; et al. The Risks of Birth Defects and Childhood Cancer with Conception by Assisted Reproductive Technology. Hum. Reprod. 2022, 37, 2672–2689. [Google Scholar] [CrossRef]
- Metwally, M.; Ledger, W.L. Long-Term Complications of Assisted Reproductive Technologies. Hum. Fertil. 2011, 14, 77–87. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Preterm Birth; World Health Organization (WHO): Geneva, Switzerland, 2023. [Google Scholar]
- Ohuma, E.O.; Moller, A.-B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, Regional, and Global Estimates of Preterm Birth in 2020, with Trends from 2010: A Systematic Analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef]
- Santi, E.; Nencini, G.; Cerni, A.; Greco, P.; Spelzini, F.; Tormettino, B.; Scioscia, M. The PLART Study: Incidence of Preterm Labor and Adverse Pregnancy Outcomes after Assisted Reproductive Techniques—A Retrospective Cohort Study. Arch. Gynecol. Obstet. 2019, 300, 911–916. [Google Scholar] [CrossRef]
- Cavoretto, P.; Candiani, M.; Giorgione, V.; Inversetti, A.; Abu-Saba, M.M.; Tiberio, F.; Sigismondi, C.; Farina, A. Risk of Spontaneous Preterm Birth in Singleton Pregnancies Conceived after IVF/ICSI Treatment: Meta-analysis of Cohort Studies. Ultrasound Obstet. Gynecol. 2018, 51, 43–53. [Google Scholar] [CrossRef]
- Bu, Z.; Zhang, J.; Hu, L.; Sun, Y. Preterm Birth in Assisted Reproductive Technology: An Analysis of More Than 20,000 Singleton Newborns. Front. Endocrinol. 2020, 11, 558819. [Google Scholar] [CrossRef]
- Mulualem, G.; Wondim, A.; Woretaw, A. The Effect of Pregnancy Induced Hypertension and Multiple Pregnancies on Preterm Birth in Ethiopia: A Systematic Review and Meta-Analysis. BMC Res. Notes 2019, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Ulfsdottir, H.; Grandahl, M.; Björk, J.; Karlemark, S.; Ekéus, C. The Association between Pre-eclampsia and Neonatal Complications in Relation to Gestational Age. Acta Paediatr. 2024, 113, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Tocariu, R.; Stan, D.; Mitroi, R.F.; Căldăraru, D.E.; Dinulescu, A.; Dobre, C.E.; Brătilă, E. Incidence of Complications among in Vitro Fertilization Pregnancies. J. Med. Life 2023, 16, 399–405. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Jin, M.; Li, Z.; Zhang, L.; Li, H.; Zhang, Y.; Ye, R.; Li, N. Impact of Gestational Hypertension and Pre-Eclampsia on Preterm Birth in China: A Large Prospective Cohort Study. BMJ Open 2022, 12, e058068. [Google Scholar] [CrossRef]
- Chih, H.J.; Elias, F.T.S.; Gaudet, L.; Velez, M.P. Assisted Reproductive Technology and Hypertensive Disorders of Pregnancy: Systematic Review and Meta-Analyses. BMC Pregnancy Childbirth 2021, 21, 449. [Google Scholar] [CrossRef]
- Almasi-Hashiani, A.; Omani-Samani, R.; Mohammadi, M.; Amini, P.; Navid, B.; Alizadeh, A.; Khedmati Morasae, E.; Maroufizadeh, S. Assisted Reproductive Technology and the Risk of Preeclampsia: An Updated Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth 2019, 19, 149. [Google Scholar] [CrossRef]
- Sullivan, E.A.; Wang, Y.A. Higher Prevalence of Pregnancy Induced Hypertension Following Assisted Reproductive Technology Treatment. Fertil. Steril. 2013, 100, S44. [Google Scholar] [CrossRef]
- Veeramani, M.; Balachandren, N.; Hong, Y.H.; Lee, J.; Corno, A.F.; Mavrelos, D.; Kastora, S.L. Assisted Reproduction and Congenital Malformations: A Systematic Review and Meta-analysis. Congenit. Anom. 2024, 64, 107–115. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Congenital Disorders; World Health Organization (WHO): Geneva, Switzerland, 2023. [Google Scholar]
- Chimah, O.U.; Emeagui, K.N.; Ajaegbu, O.C.; Anazor, C.V.; Ossai, C.A.; Fagbemi, A.J.; Emeagui, O.D. Congenital Malformations: Prevalence and Characteristics of Newborns Admitted into Federal Medical Center, Asaba. Heal. Sci. Rep. 2022, 5, e599. [Google Scholar] [CrossRef]
- Klonoff-Cohen, H.; Polavarapu, M. Assessing the Relationship Between Traditional In Vitro Fertilization and Birth Defects: A Systematic Review and Meta-Analysis. J. IVF-Worldw. 2023, 1, 1–19. [Google Scholar] [CrossRef]
- Deyhoul, N.; Mohamaddoost, T.; Hosseini, M. Infertility-Related Risk Factors: A Systematic Review. Int. J. Women’s Health Reprod. Sci. 2017, 5, 24–29. [Google Scholar] [CrossRef]
- European Society of Human Reproduction and Embryology. ART Fact Sheet; European Society of Human Reproduction and Embryology: Brussels, Belgium, 2023. [Google Scholar]
- Bhattacharya, S.; Hamilton, M.; Shaaban, M.; Khalaf, Y.; Seddler, M.; Ghobara, T.; Braude, P.; Kennedy, R.; Rutherford, A.; Hartshorne, G.; et al. Conventional In-Vitro Fertilisation versus Intracytoplasmic Sperm Injection for the Treatment of Non-Male-Factor Infertility: A Randomised Controlled Trial. Lancet 2001, 357, 2075–2079. [Google Scholar] [CrossRef] [PubMed]
- Gliozheni, O.; Hambartsoumian, E.; Strohmer, H.; Petrovskaya, E.; Tishkevich, O.; de Neubourg, D.; Bogaerts, K.; Balic, D.; Sibincic, S.; Antonova, I.; et al. ART in Europe, 2018: Results Generated from European Registries by ESHRE. Hum. Reprod. Open 2022, 2022, hoac022. [Google Scholar] [CrossRef]
- Haddad, M.; Stewart, J.; Xie, P.; Cheung, S.; Trout, A.; Keating, D.; Parrella, A.; Lawrence, S.; Rosenwaks, Z.; Palermo, G.D. Thoughts on the Popularity of ICSI. J. Assist. Reprod. Genet. 2021, 38, 101–123. [Google Scholar] [CrossRef]
- Gliozheni, O.; Hambartsoumian, E.; Strohmer, H.; Petrovskaya, E.; Tishkevich, O.; De Neubourg, D.; Bogaerts, K.; Balic, D.; Antonova, I.; Cvetkova, E.; et al. ART in Europe, 2019: Results Generated from European Registries by ESHRE. Hum. Reprod. 2023, 38, 2321–2338. [Google Scholar] [CrossRef]
- Pinborg, A.; Wennerholm, U.B.; Romundstad, L.B.; Loft, A.; Aittomaki, K.; Soderstrom-Anttila, V.; Nygren, K.G.; Hazekamp, J.; Bergh, C. Why Do Singletons Conceived after Assisted Reproduction Technology Have Adverse Perinatal Outcome? Systematic Review and Meta-Analysis. Hum. Reprod. Update 2013, 19, 87–104. [Google Scholar] [CrossRef]
- Zhang, N.; Tian, T.; Li, J.; Zhu, X.; Jiesisibieke, D.; Fang, S.; Liu, P.; Li, R.; Qiao, J.; Yang, R. A Comparison of Pregnancy Outcomes and Congenital Malformations in Offspring between Patients Undergoing Intracytoplasmic Sperm Injection and Conventional in Vitro Fertilization: A Retrospective Cohort Study. Fertil. Steril. 2024, 121, 982–990. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, J.; Ding, C.; Dai, J.; Liu, Y.; Xia, Y.; Liu, J.; Hu, Z. Birth Defects in Children Conceived by in Vitro Fertilization and Intracytoplasmic Sperm Injection: A Meta-Analysis. Fertil. Steril. 2012, 97, 1331–1337.e4. [Google Scholar] [CrossRef]
- Bao, J.; Chen, L.; Hao, Y.; Wu, H.; He, X.; Lu, C.; Ji, X.; Qiao, J.; Wang, Y.; Chi, H. Prognosis of Congenital Anomalies in Conceptions Following In Vitro Fertilization: A Multicenter Retrospective Cohort Study in China. Front. Endocrinol. 2022, 13, 900499. [Google Scholar] [CrossRef]
- Henningsen, A.-K.A.; Opdahl, S.; Wennerholm, U.-B.; Tiitinen, A.; Rasmussen, S.; Romundstad, L.B.; Bergh, C.; Gissler, M.; Forman, J.L.; Pinborg, A. Risk of Congenital Malformations in Live-Born Singletons Conceived after Intracytoplasmic Sperm Injection: A Nordic Study from the CoNARTaS Group. Fertil. Steril. 2023, 120, 1033–1041. [Google Scholar] [CrossRef]
- Schwartz, K.M.; Boulet, S.L.; Kawwass, J.F.; Kissin, D.M. Perinatal Outcomes among Young Donor Oocyte Recipients. Hum. Reprod. 2019, 34, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Boulet, S.L.; Kawwass, J.F.; Crawford, S.; Davies, M.J.; Kissin, D.M. Preterm Birth and Small Size for Gestational Age in Singleton, In Vitro Fertilization Births Using Donor Oocytes. Am. J. Epidemiol. 2018, 187, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, S.; Larsen, E.C.; la Cour Freiesleben, N.; Pinborg, A. Pregnancy Outcomes Following Oocyte Donation. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 81–91. [Google Scholar] [CrossRef]
- Adams, D.H.; Clark, R.A.; Davies, M.J.; de Lacey, S. A Meta-Analysis of Neonatal Health Outcomes from Oocyte Donation. J. Dev. Orig. Health Dis. 2016, 7, 257–272. [Google Scholar] [CrossRef]
- Banker, M.; Arora, P.; Banker, J.; Benani, H.; Shah, S.; Lalitkumar, P.G.L. Prevalence of Structural Birth Defects in IVF-ICSI Pregnancies Resulting from Autologous and Donor Oocytes in Indian Sub-continent: Results from 2444 Births. Acta Obstet. Gynecol. Scand. 2019, 98, 715–721. [Google Scholar] [CrossRef]
- Tocariu, R.; Niculae, L.E.; Niculae, A.Ș.; Carp-Velișcu, A.; Brătilă, E. Fresh versus Frozen Embryo Transfer in In Vitro Fertilization/Intracytoplasmic Sperm Injection Cycles: A Systematic Review and Meta-Analysis of Neonatal Outcomes. Medicina 2024, 60, 1373. [Google Scholar] [CrossRef]
- Raja, E.-A.; Bhattacharya, S.; Maheshwari, A.; McLernon, D.J. Comparison of Perinatal Outcomes after Frozen or Fresh Embryo Transfer: Separate Analyses of Singleton, Twin, and Sibling Live Births from a Linked National in Vitro Fertilization Registry. Fertil. Steril. 2022, 118, 323–334. [Google Scholar] [CrossRef]
- Maheshwari, A.; Pandey, S.; Amalraj Raja, E.; Shetty, A.; Hamilton, M.; Bhattacharya, S. Is Frozen Embryo Transfer Better for Mothers and Babies? Can Cumulative Meta-Analysis Provide a Definitive Answer? Hum. Reprod. Update 2018, 24, 35–58. [Google Scholar] [CrossRef]
- Pelkonen, S.; Hartikainen, A.-L.; Ritvanen, A.; Koivunen, R.; Martikainen, H.; Gissler, M.; Tiitinen, A. Major Congenital Anomalies in Children Born after Frozen Embryo Transfer: A Cohort Study 1995–2006. Hum. Reprod. 2014, 29, 1552–1557. [Google Scholar] [CrossRef]
- Yang, M.; Lin, L.; Sha, C.; Li, T.; Gao, W.; Chen, L.; Wu, Y.; Ma, Y.; Zhu, X. Which Is Better for Mothers and Babies: Fresh or Frozen-Thawed Blastocyst Transfer? BMC Pregnancy Childbirth 2020, 20, 559. [Google Scholar] [CrossRef]
- Hwang, S.S.; Dukhovny, D.; Gopal, D.; Cabral, H.; Diop, H.; Coddington, C.C.; Stern, J.E. Health Outcomes for Massachusetts Infants after Fresh versus Frozen Embryo Transfer. Fertil. Steril. 2019, 112, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.; Librach, C.L.; Gunby, J.; Bissonnette, F.; Cowan, L. Increased Risk of Preterm Birth in Singleton Pregnancies after Blastocyst versus Day 3 Embryo Transfer: Canadian ART Register (CARTR) Analysis. Hum. Reprod. 2013, 28, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Cai, L.; Liu, C.; Li, J.; Hu, X.; Lai, Y.; Shen, L.; Sui, C.; Zhang, H.; Qian, K. Nomogram for Predicting the Risk of Preterm Delivery after IVF/ICSI Treatment: An Analysis of 11,513 Singleton Births. Front. Endocrinol. 2023, 14, 1065291. [Google Scholar] [CrossRef] [PubMed]
- Jugulete, G.; Pacurar, D.; Pavelescu, M.L.; Safta, M.; Gheorghe, E.; Borcoș, B.; Pavelescu, C.; Oros, M.; Merișescu, M. Clinical and Evolutionary Features of SARS-CoV-2 Infection (COVID-19) in Children, a Romanian Perspective. Children 2022, 9, 1282. [Google Scholar] [CrossRef] [PubMed]
- Engels Calvo, V.; Cruz Melguizo, S.; Abascal-Saiz, A.; Forcén Acebal, L.; Sánchez-Migallón, A.; Pintado Recarte, P.; Cuenca Marín, C.; Marcos Puig, B.; Del Barrio Fernández, P.G.; Nieto Velasco, O.; et al. Perinatal Outcomes of Pregnancies Resulting from Assisted Reproduction Technology in SARS-CoV-2-Infected Women: A Prospective Observational Study. Fertil. Steril. 2021, 116, 731–740. [Google Scholar] [CrossRef]
- Pavelescu, M.L.; Dinulescu, A.; Păsărică, A.-S.; Dijmărescu, I.; Păcurar, D. Hematological Profile, Inflammatory Markers and Serum Liver Enzymes in COVID 19 Positive Children vs. COVID 19 Negative Ones—A Comparative Study. Front. Pediatr. 2024, 12, 1334591. [Google Scholar] [CrossRef]
- Popescu, D.E.; Roșca, I.; Jura, A.M.C.; Cioca, A.; Pop, O.; Lungu, N.; Popa, Z.-L.; Rațiu, A.; Boia, M. Prompt Placental Histopathological and Immunohistochemical Assessment after SARS-CoV-2 Infection during Pregnancy—Our Perspective of a Small Group. Int. J. Mol. Sci. 2024, 25, 1836. [Google Scholar] [CrossRef]
- Rjeily, W.A.; Alataş, C.; Alkon, T.; Luna, M.; Balic, D.; Barros, A.; Beckers, N.; Begum, R.; Boeykens, F.; Boleac, I.; et al. Outcomes of SARS-CoV-2 Infected Pregnancies after Medically Assisted Reproduction. Hum. Reprod. 2021, 36, 2883–2890. [Google Scholar] [CrossRef]
| Term Newborns | Preterm Newborns | Fisher’s Exact Test (p) | |
|---|---|---|---|
| Single pregnancy | 494 (83.2%) | 100 (16.8%) | <0.001 |
| Twin pregnancy | 53 (24.1%) | 167 (75.9%) |
| Term Newborns | Preterm Newborns | Fisher’s Exact Test (p) | |
|---|---|---|---|
| Non-hypertension pregnancy | 499 (69.4%) | 220 (30.6%) | <0.001 |
| PIH pregnancy | 48 (50.5%) | 47 (49.5%) |
| Term Newborns | Preterm Newborns | Fisher’s Exact Test (p) | |
|---|---|---|---|
| IVF pregnancy | 339 (63.2%) | 197 (36.8%) | =0.001 |
| ICSI pregnancy | 208 (74.8%) | 70 (25.2%) |
| Term Newborns | Preterm Newborns | Fisher’s Exact Test (p) | |
|---|---|---|---|
| No | 517 (70%) | 222 (30%) | <0.001 |
| Yes | 30 (40%) | 45 (60%) |
| Parameter | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Twin pregnancy | 15.556 (10.683–22.680) | <0.001 | 16.018 (10.74–23.889) | <0.001 |
| PIH pregnancy | 2.221 (1.441–3.422) | <0.001 | 2.676 (1.584–4.52) | <0.001 |
| IVF pregnancy | 1.727 (1.25–2.385) | =0.001 | 0.881 (0.595–1.306) | =0.528 |
| Donor conception | 3.493 (2.144–5.691) | <0.001 | 1.804 (1.186–3.302) | =0.044 |
| Day 3 Embryo | Day 5 Embryo | Fisher’s Exact Test (p) | |
|---|---|---|---|
| Normal newborn | 46 (76.7%) | 690 (91.5%) | =0.001 |
| Congenital malformations | 14 (23.3%) | 64 (8.5%) |
| Fresh Embryo | Frozen Embryo | Fisher’s Exact Test (p) | |
|---|---|---|---|
| Normal newborn | 153 (84.1%) | 583 (92.2%) | =0.002 |
| Congenital malformations | 29 (15.9%) | 49(7.8%) |
| Own Conception | Donor Conception | Fisher’s Exact Test (p) | |
|---|---|---|---|
| Normal newborn | 675 (91.3%) | 61 (81.3%) | =0.011 |
| Congenital malformations | 64 (8.7%) | 14 (18.7%) |
| Parameter | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Stage of embryo (day 3) | 3.281 (1.712–6.29) | <0.001 | 2.664 (1.362–5.208) | 0.004 |
| Protocol for embryo transfer (fresh) | 2.255 (1.378–3.69) | <0.001 | 2.087 (1.259–3.46) | 0.004 |
| Donor conception | 2.421 (1.283–4.567) | =0.006 | 2.323 (1.212–4.454) | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tocariu, R.; Dinulescu, A.; Prejmereanu, A.; Maier, C.; Coricovac, A.-M.; Archir, E.-D.; Niculae, L.E.; Brătilă, E. Risk Factors for Prematurity and Congenital Malformations in Assisted Reproductive Technology Pregnancies—A Retrospective Study. J. Clin. Med. 2024, 13, 6470. https://doi.org/10.3390/jcm13216470
Tocariu R, Dinulescu A, Prejmereanu A, Maier C, Coricovac A-M, Archir E-D, Niculae LE, Brătilă E. Risk Factors for Prematurity and Congenital Malformations in Assisted Reproductive Technology Pregnancies—A Retrospective Study. Journal of Clinical Medicine. 2024; 13(21):6470. https://doi.org/10.3390/jcm13216470
Chicago/Turabian StyleTocariu, Raluca, Alexandru Dinulescu, Ana Prejmereanu, Călina Maier, Anca-Magdalena Coricovac, Evelyn-Denise Archir, Lucia Elena Niculae, and Elvira Brătilă. 2024. "Risk Factors for Prematurity and Congenital Malformations in Assisted Reproductive Technology Pregnancies—A Retrospective Study" Journal of Clinical Medicine 13, no. 21: 6470. https://doi.org/10.3390/jcm13216470
APA StyleTocariu, R., Dinulescu, A., Prejmereanu, A., Maier, C., Coricovac, A.-M., Archir, E.-D., Niculae, L. E., & Brătilă, E. (2024). Risk Factors for Prematurity and Congenital Malformations in Assisted Reproductive Technology Pregnancies—A Retrospective Study. Journal of Clinical Medicine, 13(21), 6470. https://doi.org/10.3390/jcm13216470






