Mechanical Mapping of the Common Carotid Artery in Healthy Individuals Aged 2 to 40 Years
Abstract
1. Introduction
2. Materials
3. Methods
3.1. The Control Population Studied
3.2. Subjects in Pathological Conditions
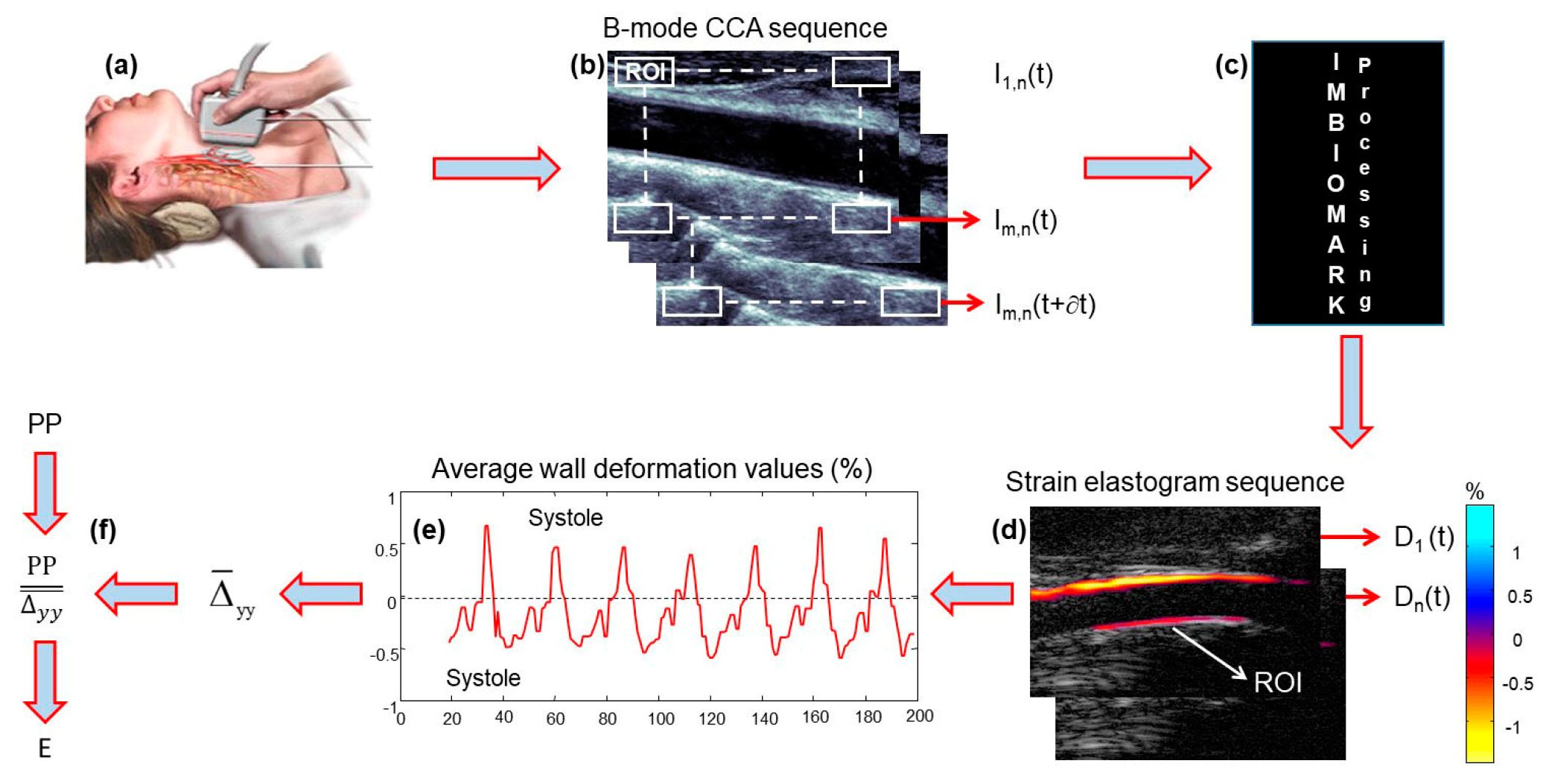
3.3. Data Acquisitions
3.4. ImBioMark and Elastic Modulus Calculation
4. Results
4.1. Somatic Data
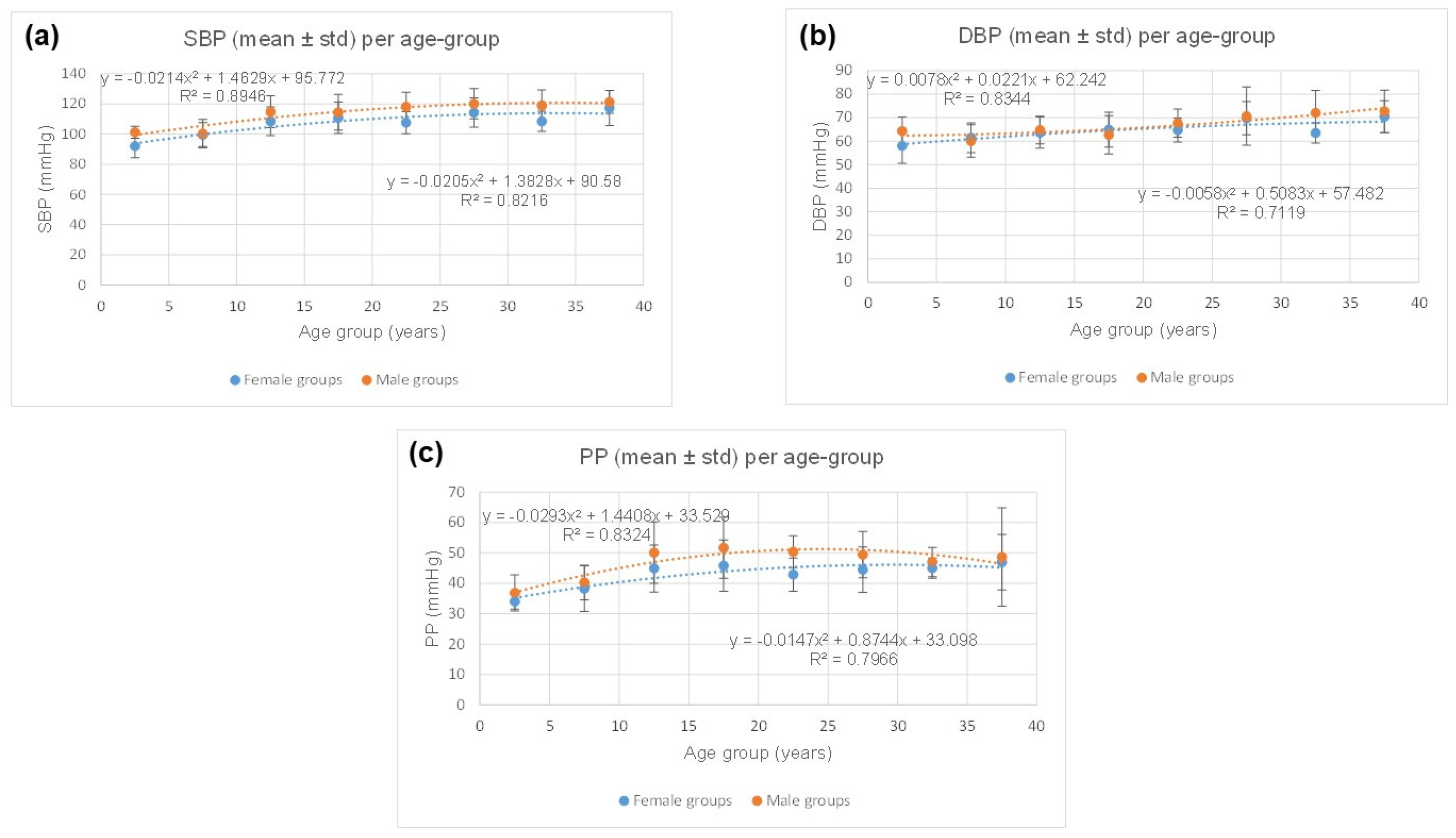
4.2. Physiological Data
4.3. CCA Stiffness According to Age
4.4. Contrasting Mechanical Data and Somatic Data
4.5. Comparison of CCA Stiffness of CTL Subjects with That of Subjects in Pathological Conditions
5. Discussion
5.1. Summary of Main Results
5.2. Advantages of ImBioMark
5.3. Comparisons Between Males’ and Females’ Data
5.4. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guzik, A.; Bushnell, C. Stroke Epidemiology and Risk Factor Management. Contin. Lifelong Learn. Neurol. 2017, 23, 15–39. [Google Scholar] [CrossRef] [PubMed]
- World Stroke Organization (WSO). Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Lecturio Medical. Lecturio Medical Knowledge Essentials—Atherosclerosis. Available online: https://www.lecturio.com/concepts/lecturio-medical-knowledge-essentials-atherosclerosis/ (accessed on 27 May 2024).
- van Popele, N.M.; Grobbee, D.E.; Bots, M.L.; Asmar, R.; Topouchian, J.; Reneman, R.S.; Hoeks, A.P.G.; van der Kuip, D.A.M.; Hofman, A.; Witteman, J.C.M. Association between Arterial Stiffness and Atherosclerosis. Stroke 2001, 32, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Waeber, B.; Izzo, J.; Mitchell, G.; Resnick, L.; Asmar, R.; Safar, M. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: Clinical applications. Am. J. Hypertens. 2002, 15, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Pizzino, F.; Paradossi, U.; Gueli, I.A.; Palazzini, M.; Gentile, P.; Di Spigno, F.; Ammirati, E.; Garascia, A.; Tedeschi, A.; et al. Charting the Unseen: How Non-Invasive Imaging Could Redefine Cardiovascular Prevention. J. Cardiovasc. Dev. Dis. 2024, 11, 245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, J.; Farmer, J. Arterial Stiffness as a Risk Factor for Coronary Artery Disease. Curr. Atheroscler. Rep. 2014, 16, 387. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.J.; Insana, M.F. Strain imaging of internal deformation. Ultrasound Med. Biol. 2002, 28, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Maurice, R.L.; Ohayon, J.; Frétigny, Y.; Bertrand, M.; Soulez, G.; Cloutier, G. Non-invasive Vascular Elastography: Theoretical Framework. IEEE–Trans. Med. Imaging 2004, 23, 164–180. [Google Scholar] [CrossRef] [PubMed]
- El Jalbout, R.; Cloutier, G.; Roy-Cardinal, M.-H.; Henderson, M.; Levy, E.; Lapierre, C.; Soulez, G.; Dubois, J. The value of non-invasive vascular elastography (NIVE) in detecting early vascular changes in overweight and obese children. Eur. Radiol. 2019, 29, 3854–3861. [Google Scholar] [CrossRef] [PubMed]
- Maurice, R.L.; Dahdah, N. Characterization of aortic remodeling following Kawasaki disease: Toward a fully-developed automatic bi-parametric model. Med. Phys. 2012, 30, 6104–6110. [Google Scholar] [CrossRef] [PubMed]
- Maurice, R.L.; Vaujois, L.; Dahdah, N.; Chibab, N.; Maurice, A.; Nuyt, A.-M.; Levy, E.; Bigras, J.-L. Carotid wall elastography to assess midterm vascular dysfunction secondary to intrauterine growth restriction: Feasibility and comparison with standardized intima-media thickness. Ultrasound Med. Biol. 2014, 40, 864–870. [Google Scholar] [CrossRef]
- Maurice, R.L.; Dahdah, N.; Tremblay, J. Imaging-Based Biomarkers: Characterization of Post-Kawasaki Vasculitis in Infants and Hypertension Phenotype in Rat Model. Int. J. Vasc. Med. 2012, 2012, 364145. [Google Scholar] [CrossRef] [PubMed]
- Vander, A.J.; Sherman, J.H.; Luciano, D.S. Physiologie Humaine, 2ème ed.; Mc-Graw-Hill: New York, NY, USA, 1989. [Google Scholar]
- Drižienė, Z.; Jakutienė, E.; Stakišaitis, D.; Pundzienė, B.; Sveikata, A. Characteristics of gender-related circadian arterial blood pressure in healthy adolescents. Medicina 2008, 44, 768. [Google Scholar] [CrossRef] [PubMed]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef] [PubMed]
- Ajmi, H.; Ghorbel, S.; Ezzi, O.; Mabrouk, S.; Mansour, K.; Kahloul, N.; Chemli, J.; Zouari, N.; Mejaouel, H.; Boughammoura, L.; et al. Coronary artery aneurysm in Kawasaki disease and its risk factors: A retrospective study about 65 Tunisian children. Ann. Cardiol. Angeiol. 2022, 71, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Mat Bah, M.N.; Alias, E.Y.; Razak, H.; Sapian, M.H.; Foo, F.H.; Abdullah, N. Epidemiology, clinical characteristics, and immediate outcome of Kawasaki disease: A population-based study from a tropical country. Eur. J. Pediatr. 2021, 180, 2599–2606. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yanagawa, H.; Harada, K.; Kato, H.; Kawasaki, T. Mortality among persons with a history of Kawasaki disease in Japan: Existence of cardiac sequelae elevated the mortality. J. Epidemiol. 2000, 10, 372–375. [Google Scholar] [CrossRef] [PubMed][Green Version]




| Population Investigated (n = 95 Females + 107 Males) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age Group (years) | [0–5] | [5–10] | [10–15] | [15–20] | [20–25] | [25–30] | [30–35] | [35–40] |
| Nb of Females | 5 | 18 | 18 | 15 | 20 | 8 | 5 | 6 |
| Nb of Males | 7 | 30 | 29 | 10 | 11 | 9 | 5 | 6 |
| Females | Males | |||
|---|---|---|---|---|
| Age Group | Weight (kg) Mean ± Std | Height (cm) Mean ± Std | Weight (kg) Mean ± Std | Height (cm) Mean ± Std |
| [0–5] | 11 ± 3 | 88 ± 9 | 15 ± 5 | 97 ± 11 |
| [5–10] | 21 ± 6 | 116 ± 13 | 24 ± 7 | 120 ± 13 |
| [10–15] | 43 ± 10 | 150 ± 14 | 49 ± 15 | 156 ± 15 |
| [15–20] | 63 ± 10 | 167 ± 5 | 71 ± 15 | 177 ± 8 |
| [20–25] | 60 ± 9 | 163 ± 8 | 81 ± 11 | 181 ± 6 |
| [25–30] | 61 ± 8 | 161 ± 9 | 80 ± 14 | 178 ± 6 |
| [30–35] | 64 ± 10 | 164 ± 5 | 76 ± 8 | 174 ± 9 |
| [35–40] | 62 ± 5 | 170 ± 4 | 76 ± 3 | 178 ± 14 |
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Age Group | SBP (mmHg) Mean ± Std | DBP (mmHg) Mean ± Std | PP (mmHg) Mean ± Std | SBP (mmHg) Mean ± Std | DBP (mmHg) Mean ± Std | PP (mmHg) Mean ± Std |
| [0–5] | 92 ± 8 | 58 ± 7 | 34 ± 3 | 101 ± 4 | 64 ± 6 | 36 ± 6 |
| [5–10] | 99 ± 8 | 61 ± 6 | 38 ± 7 | 100 ± 9 | 60 ± 7 | 40 ± 6 |
| [10–15] | 108 ± 10 | 63 ± 7 | 44 ± 8 | 114 ± 11 | 64 ± 6 | 50 ± 10 |
| [15–20] | 110 ± 10 | 64 ± 7 | 45 ± 8 | 114 ± 12 | 62 ± 8 | 51 ± 10 |
| [20–25] | 107 ± 7 | 64 ± 5 | 42 ± 5 | 118 ± 10 | 67 ± 6 | 50 ± 5 |
| [25–30] | 114 ± 10 | 69 ± 7 | 44 ± 7 | 120 ± 10 | 70 ± 12 | 49 ± 8 |
| [30–35] | 108 ± 7 | 63 ± 4 | 45 ± 3 | 119 ± 10 | 72 ± 10 | 47 ± 5 |
| [35–40] | 117 ± 12 | 70 ± 7 | 46 ± 9 | 121 ± 8 | 72 ± 9 | 48 ± 16 |
| Population Investigated after Data Pooling (n = 132 Females + 130 Males) The Values of the Elastic Modulus (E) are Expressed in kPa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age-group (years) | [0–5] | [5–10] | [10–15] | [15–20] | [20–25] | [25–30] | [30–35] | [35–40] |
| Nb of Females | 12 | 48 | 18 | 15 | 20 | 8 | 5 | 6 |
| Nb of Males | 12 | 48 | 29 | 10 | 11 | 9 | 5 | 6 |
| E (mean ± std) Females | 39 ± 4 | 40 ± 10 | 43 ± 11 | 48 ± 13 | 54 ± 16 | 53 ± 17 | 54 ± 21 | 54 ± 17 |
| E (mean ± std) Males | 39 ± 4 | 40 ± 10 | 40 ± 11 | 44 ± 12 | 60 ± 17 | 68 ± 13 | 63 ± 22 | 64 ± 22 |
| Population of Individuals in Pathological Conditions Investigated (n = 38 Females + 39 Males) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age Group (years) | [0–5] | [5–10] | [10–15] | [15–20] | [20–25] | [25–30] | Total | |
| Prematurely Birth (PMB) | Nb females | 24 | 24 | |||||
| Nb males | 12 | 10 | 22 | |||||
| Kawasaki Disease (KD) | Nb females | 4 | 10 | 14 | ||||
| Nb males | 7 | 10 | 17 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurice, R.L.; Dahdah, N. Mechanical Mapping of the Common Carotid Artery in Healthy Individuals Aged 2 to 40 Years. J. Clin. Med. 2024, 13, 6220. https://doi.org/10.3390/jcm13206220
Maurice RL, Dahdah N. Mechanical Mapping of the Common Carotid Artery in Healthy Individuals Aged 2 to 40 Years. Journal of Clinical Medicine. 2024; 13(20):6220. https://doi.org/10.3390/jcm13206220
Chicago/Turabian StyleMaurice, Roch Listz, and Nagib Dahdah. 2024. "Mechanical Mapping of the Common Carotid Artery in Healthy Individuals Aged 2 to 40 Years" Journal of Clinical Medicine 13, no. 20: 6220. https://doi.org/10.3390/jcm13206220
APA StyleMaurice, R. L., & Dahdah, N. (2024). Mechanical Mapping of the Common Carotid Artery in Healthy Individuals Aged 2 to 40 Years. Journal of Clinical Medicine, 13(20), 6220. https://doi.org/10.3390/jcm13206220





