Impact of Comorbidities and Skin Diseases on Post-Vaccination Reactions: A Study on COVID-19 Vaccinations in Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Population, and Sampling
2.2. Measures
2.3. Statistical Analysis
3. Results
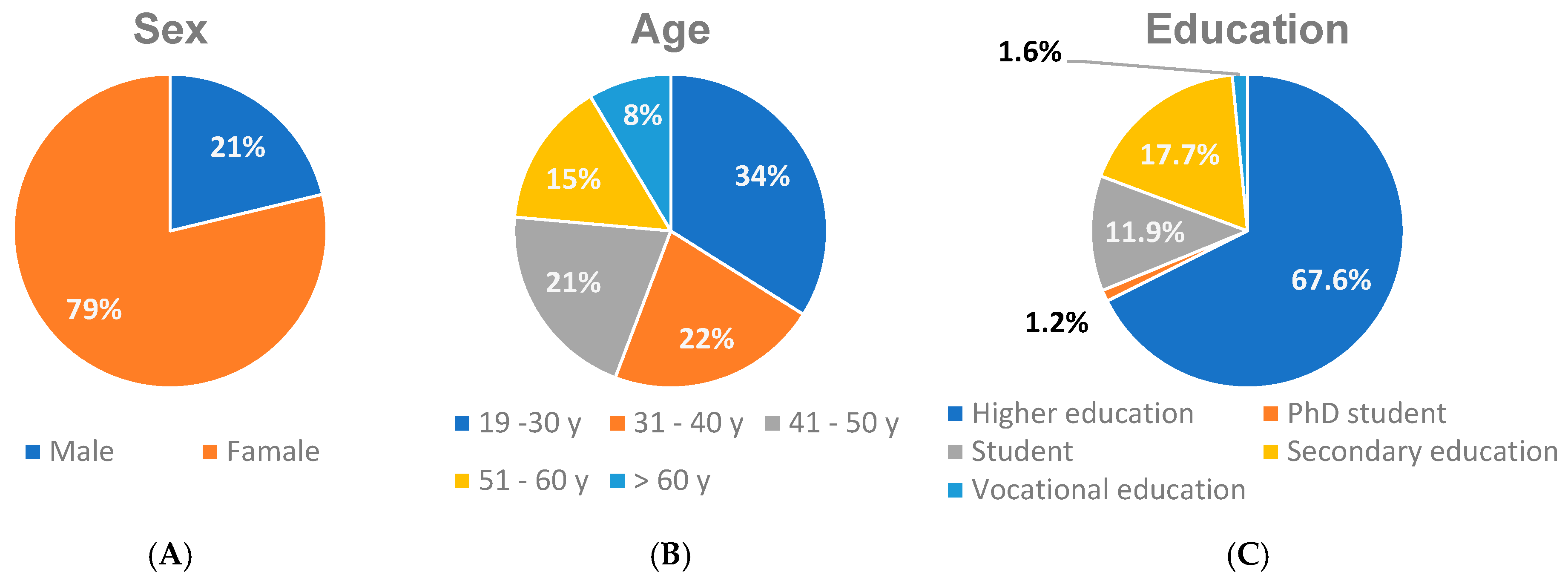
3.1. Study Sample Characteristics
3.2. Incident of COVID-19
3.2.1. Severity of Selected Skin Disease Following COVID-19 Infection
3.2.2. Severity of Selected Comorbid Disease after Contracting COVID-19
3.2.3. Increased Hair Loss after Contracting COVID-19 (Up to Approx. 3 to 4 Months after Infection)
3.3. COVID-19 Vaccine Preparations and Adverse Reaction after Vaccination
3.3.1. COVID-19 Vaccine Preparations
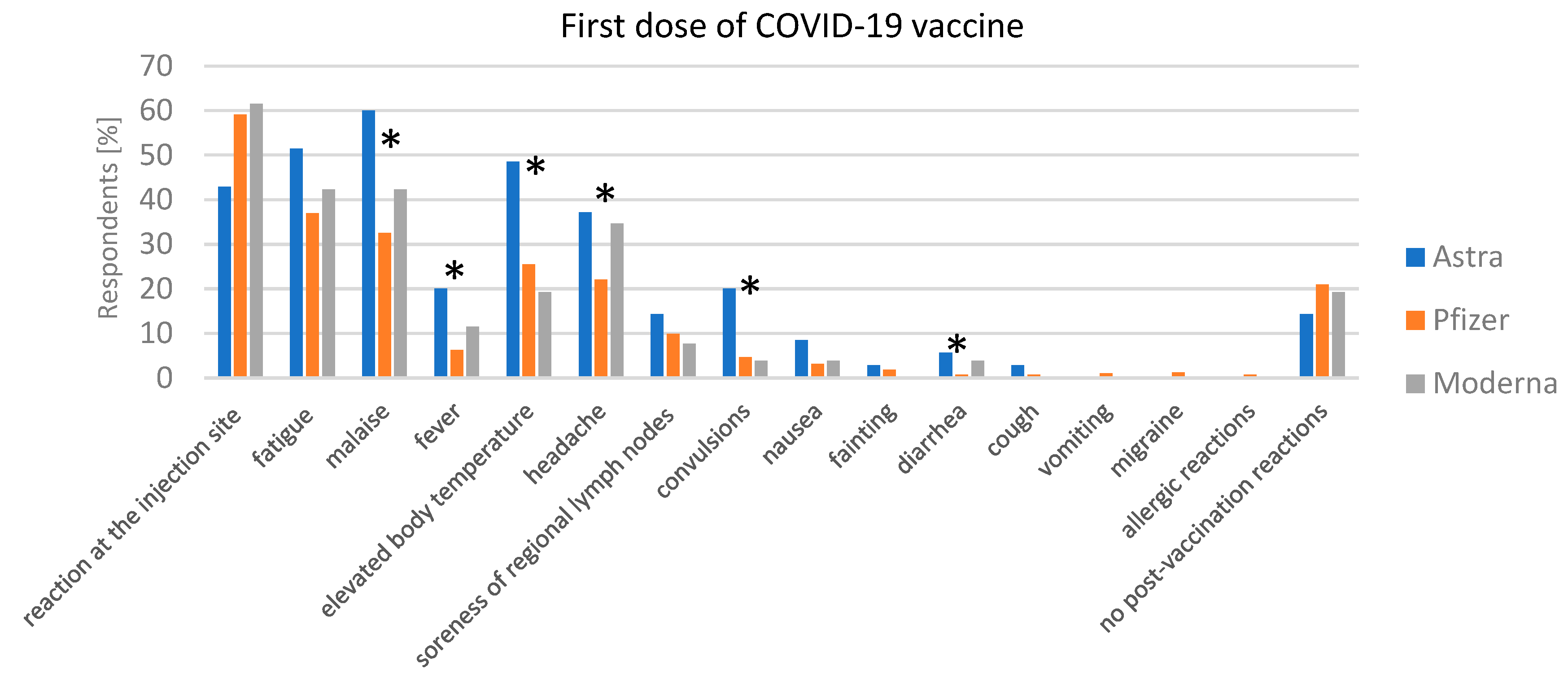
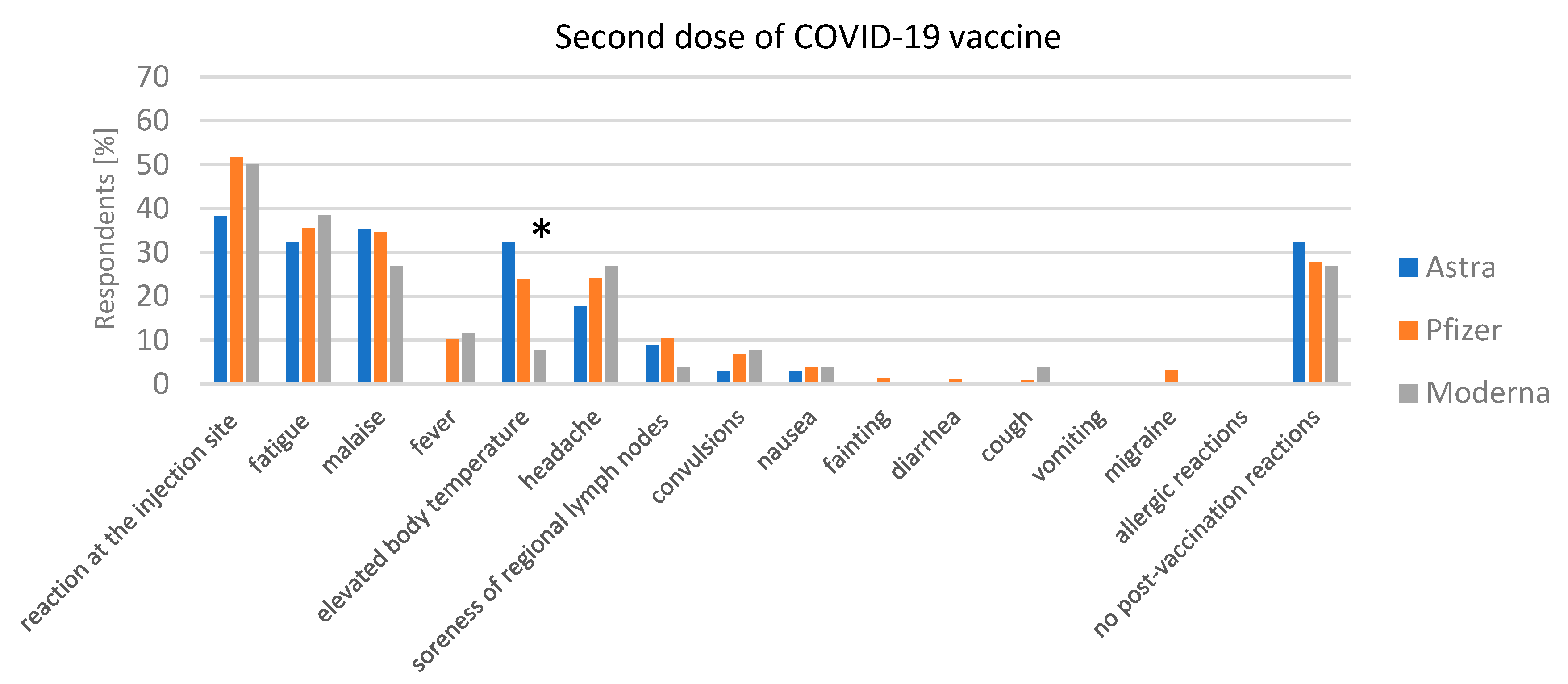
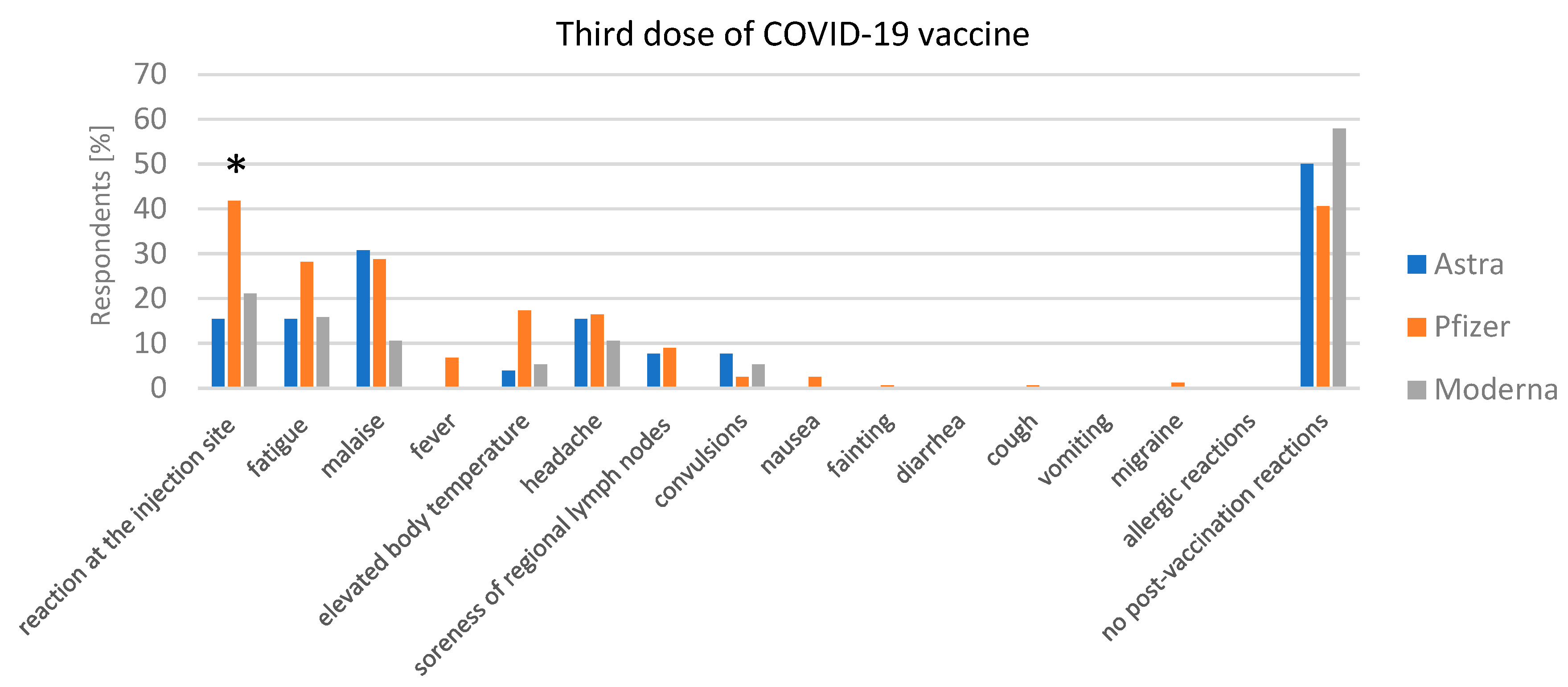
3.3.2. Adverse Reaction after Vaccination
Post-Vaccination Reactions after the First Dose of the COVID-19 Vaccine and COVID-19 Disease after the First Dose of the COVID-19 Vaccine
Post-Vaccination Reactions after the Second Dose of the COVID-19 Vaccine and COVID-19 Disease after the Second Dose of the COVID-19 Vaccine
Post-Vaccination Reactions after the Third Dose of the COVID-19 Vaccine and Incidence of COVID-19 after the Third Dose of the COVID-19 Vaccine
Comparison of the Severity of Post-Vaccination Symptoms after Administration of Different COVID-19 Vaccines
Comparison of the Severity of Post-Vaccination Symptoms after Receiving the First and Second Dose of the COVID-19 Vaccine
Comparison of the Severity of Post-Vaccination Symptoms after Receiving the Second and Third Dose of the COVID-19 Vaccine
Comparison of the Severity of Post-Vaccination Symptoms after Receiving the Subsequent Doses of the COVID-19 Vaccine
Reporting an Undesirable Post-Vaccination Reaction to the Sanitary Inspection
The Impact of Vaccination against COVID-19 on Selected Skin Diseases
The Impact of Vaccination against COVID-19 on Selected Comorbidities
Occurrence of Circulatory System Disorders, Kidney Dysfunction, and Uncontrolled Weight Gain in the Long Term after Vaccination against COVID-19 (At Least 3 Months)
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Awan, M.H.; Samreen, S.; Salim, B.; Gul, H.; Perveen, S.; Nasim, A. Corona Virus Disease-19 Vaccine-Associated Autoimmune Disorders. Rheumatol. Immunol. Res. 2022, 3, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Fadlyana, E.; Rusmil, K.; Tarigan, R.; Rahmadi, A.R.; Prodjosoewojo, S.; Sofiatin, Y.; Khrisna, C.V.; Sari, R.M.; Setyaningsih, L.; Surachman, F.; et al. A Phase III, Observer-Blind, Randomized, Placebo-Controlled Study of the Efficacy, Safety, and Immunogenicity of SARS-CoV-2 Inactivated Vaccine in Healthy Adults Aged 18–59 Years: An Interim Analysis in Indonesia. Vaccine 2021, 39, 6520–6528. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Qiao, S.; Zhang, R.; Yang, T.; Wang, Z.; Kong, Q.; Sun, M.; Geng, J.; Fang, C.; Chen, Y.; et al. Effects of Coronavirus Disease 2019 Vaccination on Seizures in Patients with Epilepsy. Chin. Med. J. 2023, 136, 571–577. [Google Scholar] [CrossRef]
- Uwamino, Y.; Kurafuji, T.; Sato, Y.; Tomita, Y.; Shibata, A.; Tanabe, A.; Yatabe, Y.; Noguchi, M.; Arai, T.; Ohno, A.; et al. Young Age, Female Sex, and Presence of Systemic Adverse Reactions Are Associated with High Post-Vaccination Antibody Titer after Two Doses of BNT162b2 MRNA SARS-CoV-2 Vaccination: An Observational Study of 646 Japanese Healthcare Workers and University Staff. Vaccine 2022, 40, 1019–1025. [Google Scholar] [CrossRef]
- Niebel, D.; Wenzel, J.; Wilsmann-Theis, D.; Ziob, J.; Wilhelmi, J.; Braegelmann, C. Single-Center Clinico-Pathological Case Study of 19 Patients with Cutaneous Adverse Reactions Following COVID-19 Vaccines. Dermatopathology 2021, 8, 463–476. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Bagher, A.M.; Binmahfouz, L.S.; Eid, B.G.; Almukadi, H.; Badr-Eldin, S.M.; El-Hamamsy, M.; Mohammedsaleh, Z.M.; Saleh, F.M.; Almuhayawi, M.S.; et al. The Adverse Reactions of Pfizer BioNTech COVID-19 Vaccine Booster Dose Are Mild and Similar to the Second Dose Responses: A Retrospective Cross-Sectional Study. Int. J. Gen. Med. 2022, 15, 6821–6836. [Google Scholar] [CrossRef]
- Cantisani, C.; Chello, C.; Grieco, T.; Ambrosio, L.; Kiss, N.; Tammaro, A.; Tosti, G.; Paolino, G.; Pellacani, G. Cutaneous Reactions to COVID-19 Vaccines in a Monocentric Study: A Case Series. J. Clin. Med. 2022, 11, 3811. [Google Scholar] [CrossRef]
- Sampath, V.; Rabinowitz, G.; Shah, M.; Jain, S.; Diamant, Z.; Jesenak, M.; Rabin, R.; Vieths, S.; Agache, I.; Akdis, M.; et al. Vaccines and Allergic Reactions: The Past, the Current COVID-19 Pandemic, and Future Perspectives. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 1640–1660. [Google Scholar] [CrossRef]
- Rogers, A.; Rooke, E.; Morant, S.; Guthrie, G.; Doney, A.; Duncan, A.; MacKenzie, I.; Barr, R.; Pigazzani, F.; Zutis, K.; et al. Adverse Events and Overall Health and Well-Being after COVID-19 Vaccination: Interim Results from the VAC4COVID Cohort Safety Study. BMJ Open 2022, 12, e060583. [Google Scholar] [CrossRef]
- Cai, C.; Peng, Y.; Shen, E.; Huang, Q.; Chen, Y.; Liu, P.; Guo, C.; Feng, Z.; Gao, L.; Zhang, X.; et al. A Comprehensive Analysis of the Efficacy and Safety of COVID-19 Vaccines. Mol. Ther. 2021, 29, 2794–2805. [Google Scholar] [CrossRef]
- Kim, M.A.; Lee, Y.W.; Kim, S.R.; Kim, J.H.; Min, T.K.; Park, H.S.; Shin, M.; Ye, Y.M.; Lee, S.; Lee, J.; et al. COVID-19 Vaccine-Associated Anaphylaxis and Allergic Reactions: Consensus Statements of the KAAACI Urticaria/Angioedema/Anaphylaxis Working Group. Allergy Asthma Immunol. Res. 2021, 13, 526–544. [Google Scholar] [CrossRef] [PubMed]
- Romantowski, J.; Kruszewski, J.; Solarski, O.; Bant, A.; Chciałowski, A.; Pietrzyk, I.; Sańpruch, P.; Górska, A.; Chełmińska, M.; Knurowska, A.; et al. Protocol of Safe Vaccination against COVID-19 in Patients with High Risk of Allergic Reactions. Clin. Transl. Allergy 2022, 12, e12152. [Google Scholar] [CrossRef] [PubMed]
- Jęśkowiak, I.; Wiatrak, B.; Grosman-Dziewiszek, P.; Szeląg, A. The Incidence and Severity of Post-Vaccination Reactions after Vaccination against COVID-19. Vaccines 2021, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, Z.; Qin, M.; Gao, Y.; Luo, N.; Xie, W.; Zou, Y.; Wang, J.; Ma, X. A Systematic Review and Meta-Analysis of the Effectiveness and Safety of COVID-19 Vaccination in Older Adults. Front. Immunol. 2023, 14, 1113156. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, L.; Shi, Y. Safety, Immunogenicity, and Efficacy of COVID-19 Vaccines in Adolescents, Children, and Infants: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 829176. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhang, M.; Zhang, X.; Li, H.; Wang, Y.; Wang, W.; Ji, J.; Wu, L.; Zheng, D. The Prevalence of Adverse Reactions among Individuals with Three-Dose COVID-19 Vaccination. J. Infect. Public Health 2023, 16, 125–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Lv, J.; Huang, T.; Zhang, R.; Zhang, D.; Luo, L.; Wei, S.; Liu, X.; Zhang, S.; et al. Evaluation of Immunogenicity and Safety of Vero Cell-Derived Inactivated COVID-19 Vaccine in Older Patients with Hypertension and Diabetes Mellitus. Vaccines 2022, 10, 1020. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Wieler, H.J.; Enders, P.; Buchholz, H.G.; Plachter, B. Age- and Sex-Graded Data Evaluation of Vaccination Reactions after Initial Injection of the Bnt162b2 Mrna Vaccine in a Local Vaccination Center in Germany. Vaccines 2021, 9, 911. [Google Scholar] [CrossRef]
- Granados Villalpando, J.M.; de Romero Tapia, S.J.; del Baeza Flores, G.C.; Ble Castillo, J.L.; Juarez Rojop, I.E.; Lopez Junco, F.I.; Olvera Hernández, V.; Quiroz Gomez, S.; Ruiz Quiñones, J.A.; Guzmán Priego, C.G. Prevalence and Risk Factors of Adverse Effects and Allergic Reactions after COVID-19 Vaccines in a Mexican Population: An Analytical Cross-Sectional Study. Vaccines 2022, 10, 2012. [Google Scholar] [CrossRef]
- Gianfredi, V.; Minerva, M.; Casu, G.; Capraro, M.; Chiecca, G.; Gaetti, G.; Mazzocchi, R.M.; Musarò, P.; Basteri, P.; Bertini, B.; et al. Immediate Adverse Events Following COVID-19 Immunization. A Cross-Sectional Study of 314,664 Italian Subjects. Acta Biomed. 2021, 92, e2021487. [Google Scholar] [CrossRef]
- Duijster, J.W.; Lieber, T.; Pacelli, S.; Van Balveren, L.; Ruijs, L.S.; Raethke, M.; Kant, A.; Van Hunsel, F. Sex-Disaggregated Outcomes of Adverse Events after COVID-19 Vaccination: A Dutch Cohort Study and Review of the Literature. Front. Immunol. 2023, 14, 1078736. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Saez, F.; Peiró, S.; Cuenca, L.; Vanaclocha, H.; Limón, R.; Salas, D.; Burgos, J.S.; Sánchez-Payá, J.; Meneu, R.; Díez, J.; et al. Side Effects during the Week after First Dose Vaccination with Four COVID-19 Vaccines. Results of the ProVaVac Survey Study with 13,837 People in Spain. Vaccine 2022, 40, 5942–5949. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; Bohlken, J.; Weber, K.; Konrad, M.; Luedde, T.; Roderburg, C.; Kostev, K. Factors Associated with Non-Severe Adverse Reactions after Vaccination against SARS-CoV-2: A Cohort Study of 908,869 Outpatient Vaccinations in Germany. Vaccines 2022, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central Obesity, Smoking Habit, and Hypertension Are Associated with Lower Antibody Titres in Response to COVID-19 MRNA Vaccine. Diabetes Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef]
- Chenchula, S.; Vidyasagar, K.; Pathan, S.; Sharma, S.; Chavan, M.R.; Bhagavathula, A.S.; Padmavathi, R.; Manjula, M.; Chhabra, M.; Gupta, R.; et al. Global Prevalence and Effect of Comorbidities and Smoking Status on Severity and Mortality of COVID-19 in Association with Age and Gender: A Systematic Review, Meta-Analysis and Meta-Regression. Sci. Rep. 2023, 13, 6415. [Google Scholar] [CrossRef]
- Bots, S.H.; Riera-Arnau, J.; Belitser, S.V.; Messina, D.; Aragón, M.; Alsina, E.; Douglas, I.J.; Durán, C.E.; García-Poza, P.; Gini, R.; et al. Myocarditis and Pericarditis Associated with SARS-CoV-2 Vaccines: A Population-Based Descriptive Cohort and a Nested Self-Controlled Risk Interval Study Using Electronic Health Care Data from Four European Countries. Front. Pharmacol. 2022, 13, 1038043. [Google Scholar] [CrossRef]
- Couderc, A.L.; Ninove, L.; Nouguerède, E.; Rey, D.; Rebroin, M.; Daumas, A.; Tomasini, P.; Greillier, L.; Salas, S.; Duffaud, F.; et al. Acceptance, Efficacy, and Safety of COVID-19 Vaccination in Older Patients with Cancer. J. Geriatr. Oncol. 2022, 13, 850–855. [Google Scholar] [CrossRef]
- Shulman, R.M.; Weinberg, D.S.; Ross, E.A.; Ruth, K.; Rall, G.F.; Olszanski, A.J.; Helstrom, J.; Hall, M.J.; Judd, J.; Chen, D.Y.T.; et al. Adverse Events Reported by Patients With Cancer After Administration of a 2-Dose MRNA COVID-19 Vaccine. JNCCN J. Natl. Compr. Cancer Netw. 2022, 20, 160–166. [Google Scholar] [CrossRef]
- Weaver, K.N.; Zhang, X.; Dai, X.; Watkins, R.; Adler, J.; Dubinsky, M.C.; Kastl, A.; Bousvaros, A.; Strople, J.A.; Cross, R.K.; et al. Impact of SARS-CoV-2 Vaccination on Inflammatory Bowel Disease Activity and Development of Vaccine-Related Adverse Events: Results From PREVENT-COVID. Inflamm. Bowel Dis. 2022, 28, 1497–1505. [Google Scholar] [CrossRef]
- Miyazaki, H.; Watanabe, D.; Ito, Y.; Ikeda, S.; Okamoto, N.; Tokunaga, E.; Ku, Y.; Ooi, M.; Hoshi, N.; Kodama, Y. Differences in Coronavirus Disease—19 Vaccination Related Side Effects in Patients with Ulcerative Colitis and Crohn’s Disease in Japan. Indian J. Gastroenterol. 2023, 42, 701–707. [Google Scholar] [CrossRef]
- Wasserbauer, M.; Hlava, S.; Trojanek, M.; Stovicek, J.; Milota, T.; Drabek, J.; Koptová, P.; Cupkova, A.; Pichlerová, D.; Kucerova, B.; et al. Efficacy and Safety of SARS-CoV-2 Vaccination in Patients with Inflammatory Bowel Disease on Immunosuppressive and Biological Therapy: Prospective Observational Study. PLoS ONE 2022, 17, e0273612. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Figueroa-Parra, G.; Zhou, X.; Li, Y.; Jaquith, J.; McCarthy-Fruin, K.; Sletten, J.; Warrington, K.J.; Weyand, C.; Crowson, C.S.; et al. Immune Responses and Disease Biomarker Long-Term Changes Following COVID-19 MRNA Vaccination in a Cohort of Rheumatic Disease Patients. Front. Immunol. 2023, 14, 1224702. [Google Scholar] [CrossRef]
- Li, Y.K.; Lui, M.P.K.; Yam, L.L.; Cheng, C.S.; Tsang, T.H.T.; Kwok, W.S.; Chung, H.Y. COVID-19 Vaccination in Patients with Rheumatic Diseases: Vaccination Rates, Patient Perspectives, and Side Effects. Immun. Inflamm. Dis. 2022, 10, e589. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Liu, H.; Liu, Z.; Zhou, X.; Lu, X.; Yan, Z.; Zhou, Y.; Dai, L.; Chen, Y.; Yang, T.; et al. Safety and Immunogenicity of Inactivated COVID-19 Vaccination in Adult Rheumatic Patients in South China: A Prospective Study. Hum. Vaccin Immunother. 2022, 18, 2090176. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, S.; Geng, L.; Lai, P.; Dou, H.; Zhang, H.; Chen, H.; Liang, J.; Sun, L. COVID-19 Vaccination and Infection Status: A Cross-Sectional Survey of Patients with Rheumatic Diseases in China. Rheumatol. Int. 2024, 44, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Kridin, K.; Schonmann, Y.; Onn, E.; Bitan, D.T.; Weinstein, O.; Cohen, A.D. Determinants and Effectiveness of BNT162b2 MRNA Vaccination Among Patients with Atopic Dermatitis: A Population-Based Study. Am. J. Clin. Dermatol. 2022, 23, 385–392. [Google Scholar] [CrossRef]
- Yoon, D.; Jeon, H.L.; Noh, Y.; Choe, Y.J.; Choe, S.A.; Jung, J.; Shin, J.Y. A Nationwide Survey of MRNA COVID-19 Vaccinee’s Experiences on Adverse Events and Its Associated Factors. J. Korean Med. Sci. 2023, 38, e170. [Google Scholar] [CrossRef]
- Català, A.; Muñoz-Santos, C.; Galván-Casas, C.; Roncero Riesco, M.; Revilla Nebreda, D.; Solá-Truyols, A.; Giavedoni, P.; Llamas-Velasco, M.; González-Cruz, C.; Cubiró, X.; et al. Cutaneous Reactions after SARS-CoV-2 Vaccination: A Cross-Sectional Spanish Nationwide Study of 405 Cases*. Br. J. Dermatol. 2022, 186, 142–152. [Google Scholar] [CrossRef]
- Morimoto, H.; Hayano, S.; Ozawa, N.; Ogura, Y.; Usui, H.; Usami, T.; Ohse, A.; Otsuka, M.; Miyachi, M.; Tokura, Y. Questionnaire Survey of Possible Association of Allergic Diseases with Adverse Reactions to Sars-Cov-2 Vaccination. Vaccines 2021, 9, 1421. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, J.; Wu, J.; Zhang, J.; Zhang, L.; Yuan, S.; Chen, J.; Tang, Q.; Zhang, A.; Cui, Y.; et al. Allergic Diseases Aggravate the Symptoms of SARS-CoV-2 Infection in China. Front. Immunol. 2023, 14, 1284047. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Murdock, D.J.; Marcus, A.; Hussein, M.; Jalbert, J.J.; Geba, G.P. Association between Allergic Conditions and COVID-19 Susceptibility and Outcomes. Ann. Allergy Asthma Immunol. 2024, 132, 637–645.e7. [Google Scholar] [CrossRef] [PubMed]
- Chirasuthat, S.; Ratanapokasatit, Y.; Thadanipon, K.; Chanprapaph, K. Immunogenicity, Effectiveness, and Safety of COVID-19 Vaccines among Patients with Immune-Mediated Dermatological Diseases: A Systematic Review and Meta-Analysis. Acta Derm. Venereol. 2024, 104, adv40009. [Google Scholar] [CrossRef] [PubMed]
- Hren, M.G.; Khattri, S. Low Rates of Vaccination among Atopic Dermatitis, Alopecia Areata, Psoriasis, and Psoriatic Arthritis Patients on Biologics. Arch. Dermatol. Res. 2024, 316, 285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lv, C.; Han, X.; Shen, M.; Kuang, Y. A Web-Based Survey on Factors for Unvaccination and Adverse Reactions of SARS-CoV-2 Vaccines in Chinese Patients with Psoriasis. J. Inflamm. Res. 2021, 14, 6265–6273. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Puig, L.; Di Lernia, V.; Graceea, D.; Bella, D.O. Immunogenicity of Three Doses of Anti-SARS-CoV-2 BNT162b2 Vaccine in Psoriasis Patients Treated with Biologics. Front. Med. 2022, 9, 961904. [Google Scholar]
- Kaya, O.; Keskinkaya, Z.; Mermutlu, S.I.; Oguz-Kilic, S.; Cakir, H. COVID-19 Among Patients with Psoriasis: A Single-Center Retrospective Cross-Sectional Study. Infect. Dis. Clin. Microbiol. 2023, 5, 127–135. [Google Scholar] [CrossRef]
- Shi, X.; Sun, Y.; Ding, X. Impact of COVID-19 Vaccine and COVID-19 Infection on Vitiligo Activity and Progression. Clin. Cosmet. Investig. Dermatol. 2023, 16, 3581–3587. [Google Scholar] [CrossRef]
- Tsai, T.F.; Ng, C.Y. COVID-19 Vaccine–Associated Vitiligo: A Cross-Sectional Study in a Tertiary Referral Center and Systematic Review. J. Dermatol. 2023, 50, 982–989. [Google Scholar] [CrossRef]
- Hou, X.; Wu, N.; Xu, M.; Kharel, P.; Wu, F.; Wu, Y.; Wang, R.; Chen, J. Demographic and Clinical Feature Disparity between Progress and Non-Progress Patients with Vitiligo after COVID-19 Vaccination: A Cross-Sectional Study. Exp. Dermatol. 2023, 32, 1344–1349. [Google Scholar] [CrossRef]
- Siewert, B.; Szabat, A.; Chądzińska-Cebula, M.; Purpurowicz-Miękus, N.; Sujkowski, P.; Spachacz, R.; Dworacki, G.; Wysocki, J.; Januszkiewicz-Lewandowska, D.; Gowin, E. To vaccinate or not to vaccinate—bnt162b2 seroconversion rate and side effects among polish healthcare workers. Int. J. Occup. Med. Environ. Health 2022, 35, 761–766. [Google Scholar] [CrossRef]
- Fasano, G.; Bennardo, L.; Ruffolo, S.; Passante, M.; Ambrosio, A.G.; Napolitano, M.; Provenzano, E.; Nisticò, S.P.; Patruno, C. Erythema Migrans-like COVID Vaccine Arm: A Literature Review. J. Clin. Med. 2022, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Picone, V.; Martora, F.; Fabbrocini, G.; Marano, L. “Covid Arm”: Abnormal Side Effect after Moderna COVID-19 Vaccine. Dermatol. Ther. 2022, 35, e15197. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.L.; Austin, A.H.; Welsh, K.M. COVID Arm: Delayed Hypersensitivity Reactions to SARS-CoV-2 Vaccines Misdiagnosed as Cellulitis. J. Prim. Care Community Health 2021, 12, 21501327211024431. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.L.; Kelso, J.M. “COVID Arm”: Very Delayed Large Injection Site Reactions to MRNA COVID-19 Vaccines. J. Allergy Clin. Immunol. Pract. 2021, 9, 2480–2481. [Google Scholar] [CrossRef] [PubMed]
- Valera-Rubio, M.; Sierra-Torres, M.I.; Castillejo García, R.; Cordero-Ramos, J.; López-Márquez, M.R.; Cruz-Salgado, Ó.; Calleja-Hernández, M.Á. Adverse Events Reported after Administration of BNT162b2 and MRNA-1273 COVID-19 Vaccines among Hospital Workers: A Cross-Sectional Survey-Based Study in a Spanish Hospital. Expert Rev. Vaccines 2022, 21, 533–540. [Google Scholar] [CrossRef]
- Maruyama, A.; Sawa, T.; Teramukai, S.; Katoh, N. Adverse Reactions to the First and Second Doses of Pfizer-BioNTech COVID-19 Vaccine among Healthcare Workers. J. Infect. Chemother. 2022, 28, 934–942. [Google Scholar] [CrossRef]
- Bian, S.; Li, L.; Wang, Z.; Cui, L.; Xu, Y.; Guan, K.; Zhao, B. Allergic Reactions After the Administration of COVID-19 Vaccines. Front. Public Health 2022, 10, 878081. [Google Scholar] [CrossRef]
- Nassar, R.I.; Alnatour, D.; Thiab, S.; Nassar, A.; El-Hajji, F.; Basheti, I.A. Short-Term Side Effects of COVID-19 Vaccines: A Cross-Sectional Study in Jordan. Hum. Vaccin Immunother. 2022, 18, 2082792. [Google Scholar] [CrossRef]

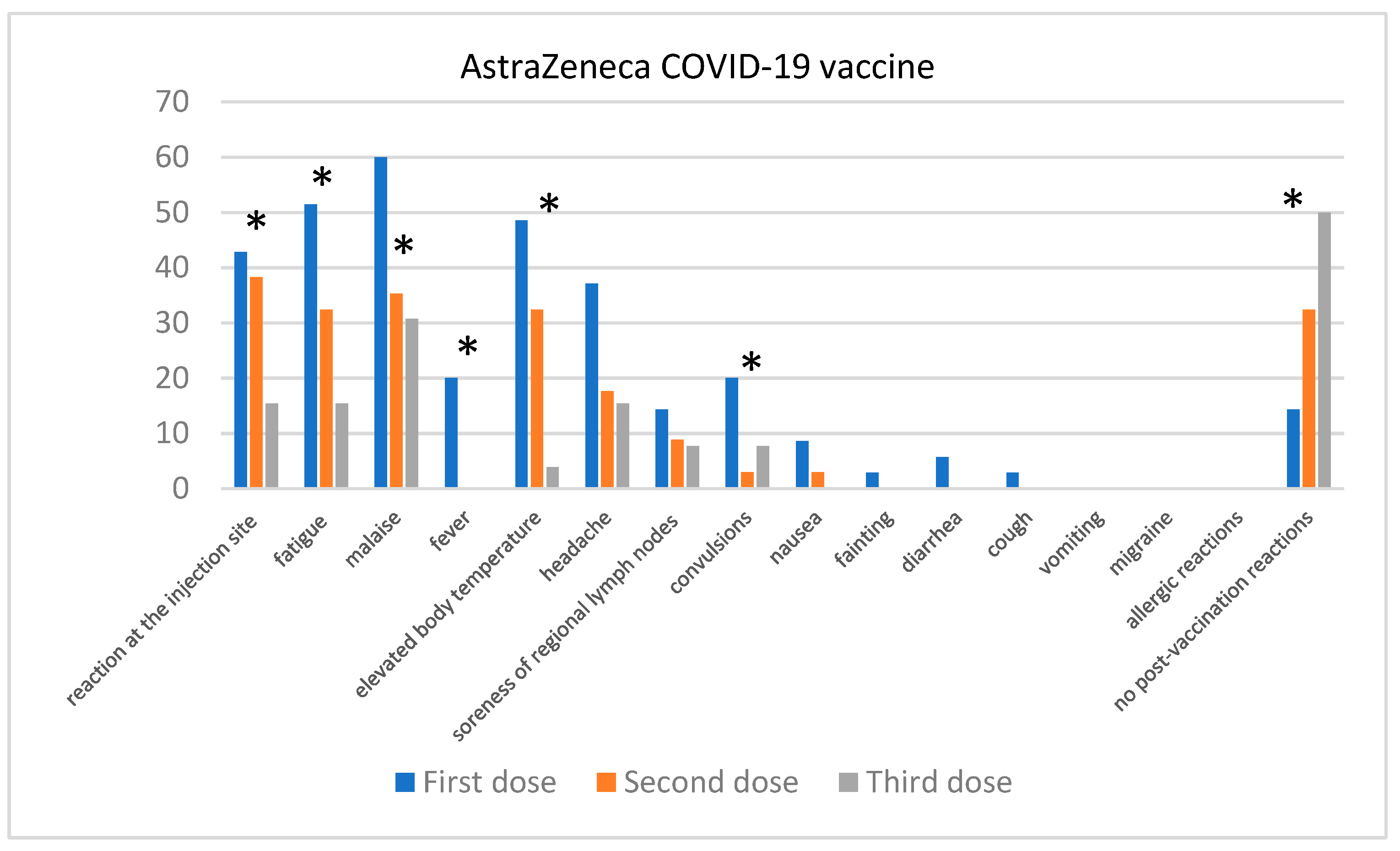
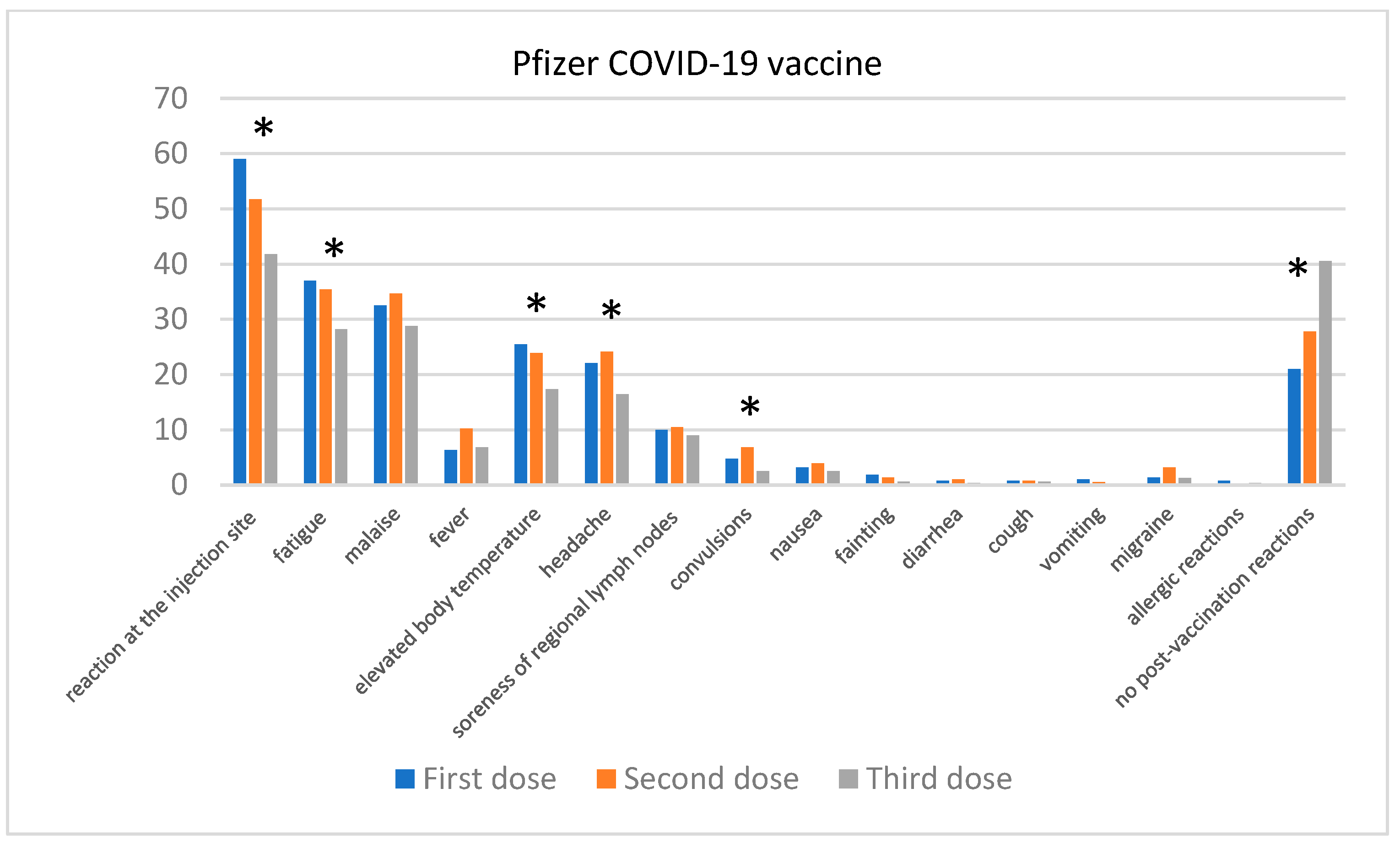
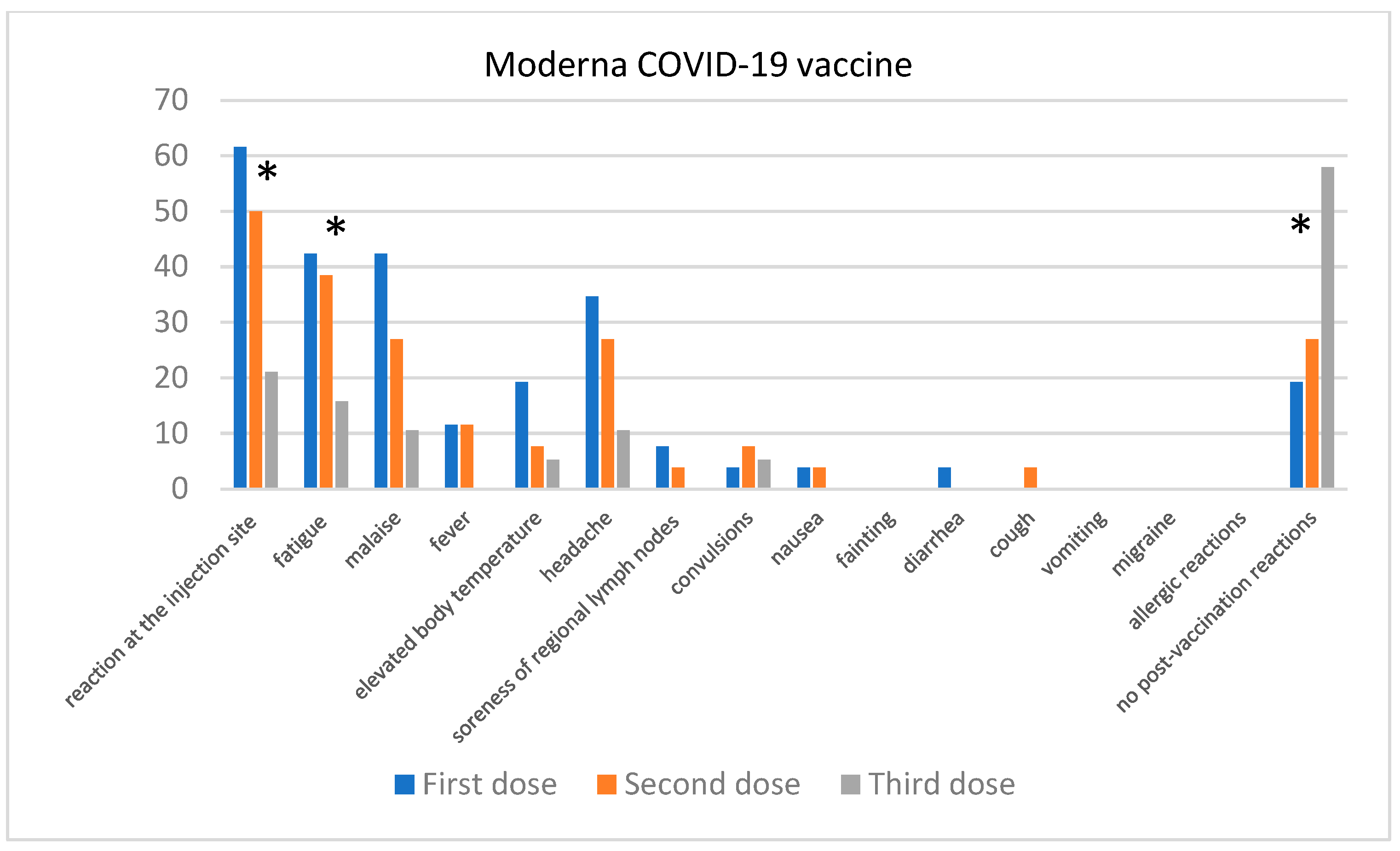
| Skin Disease | Number of Participants | Participants Who Contracted COVID-19 |
|---|---|---|
| Atopic dermatitis | 47 | 32 |
| Psoriasis | 16 | 13 |
| Acne vulgaris | 54 | 38 |
| Rosacea | 16 | 6 |
| Vitiligo | 8 | 6 |
| Alopecia areata | 2 | 1 |
| Comorbidities | Number of Participants | Participants Contracted COVID-19 | Worsening of the Disease after Contracting COVID-19 |
|---|---|---|---|
| Hashimoto’s disease | 49 | 29 | 3 |
| Heart disease/hypertension | 50 | 29 | 5 |
| Rheumatoid arthritis | 7 | 3 | 2 |
| Diabetes | 12 | 3 | 1 |
| Cancer | 6 | 3 | 0 |
| Atherosclerosis | 6 | 4 | 0 |
| Multiple sclerosis | 1 | 0 | 0 |
| Digestive disorders | 2 | 2 | 1 |
| Skin Disease | Number of Participants | Vaccinated against COVID-19 | Worsening of the Disease after Vaccination |
|---|---|---|---|
| Atopic dermatitis | 47 | 41 | 8 |
| Psoriasis | 16 | 13 | 3 |
| Acne vulgaris | 54 | 47 | 5 |
| Rosacea | 16 | 15 | 0 |
| Vitiligo | 8 | 6 | 1 |
| Alopecia areata | 2 | 2 | 0 |
| Comorbidities | Number of Participants | Vaccinated against COVID-19 | Worsening of the Disease after Vaccination |
|---|---|---|---|
| Hashimoto’s disease | 49 | 45 | 4 |
| Heart disease/hypertension | 50 | 48 | 7 |
| Rheumatoid arthritis | 7 | 7 | 2 |
| Diabetes | 12 | 12 | 1 |
| Cancer | 6 | 5 | 1 |
| Atherosclerosis | 6 | 5 | 0 |
| Multiple sclerosis | 1 | 1 | 0 |
| Digestive disorders | 2 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jęśkowiak-Kossakowska, I.; Nowotarska, P.; Grosman-Dziewiszek, P.; Szeląg, A.; Wiatrak, B. Impact of Comorbidities and Skin Diseases on Post-Vaccination Reactions: A Study on COVID-19 Vaccinations in Poland. J. Clin. Med. 2024, 13, 6173. https://doi.org/10.3390/jcm13206173
Jęśkowiak-Kossakowska I, Nowotarska P, Grosman-Dziewiszek P, Szeląg A, Wiatrak B. Impact of Comorbidities and Skin Diseases on Post-Vaccination Reactions: A Study on COVID-19 Vaccinations in Poland. Journal of Clinical Medicine. 2024; 13(20):6173. https://doi.org/10.3390/jcm13206173
Chicago/Turabian StyleJęśkowiak-Kossakowska, Izabela, Paulina Nowotarska, Patrycja Grosman-Dziewiszek, Adam Szeląg, and Benita Wiatrak. 2024. "Impact of Comorbidities and Skin Diseases on Post-Vaccination Reactions: A Study on COVID-19 Vaccinations in Poland" Journal of Clinical Medicine 13, no. 20: 6173. https://doi.org/10.3390/jcm13206173
APA StyleJęśkowiak-Kossakowska, I., Nowotarska, P., Grosman-Dziewiszek, P., Szeląg, A., & Wiatrak, B. (2024). Impact of Comorbidities and Skin Diseases on Post-Vaccination Reactions: A Study on COVID-19 Vaccinations in Poland. Journal of Clinical Medicine, 13(20), 6173. https://doi.org/10.3390/jcm13206173







