Apical Sparing of Longitudinal Strain as a Specific Pattern of Myocardial Fibrosis in Patients with Severe Left Ventricular Hypertrophy: A Comparison between Deformation Imaging and Histological Findings
Abstract
1. Introduction
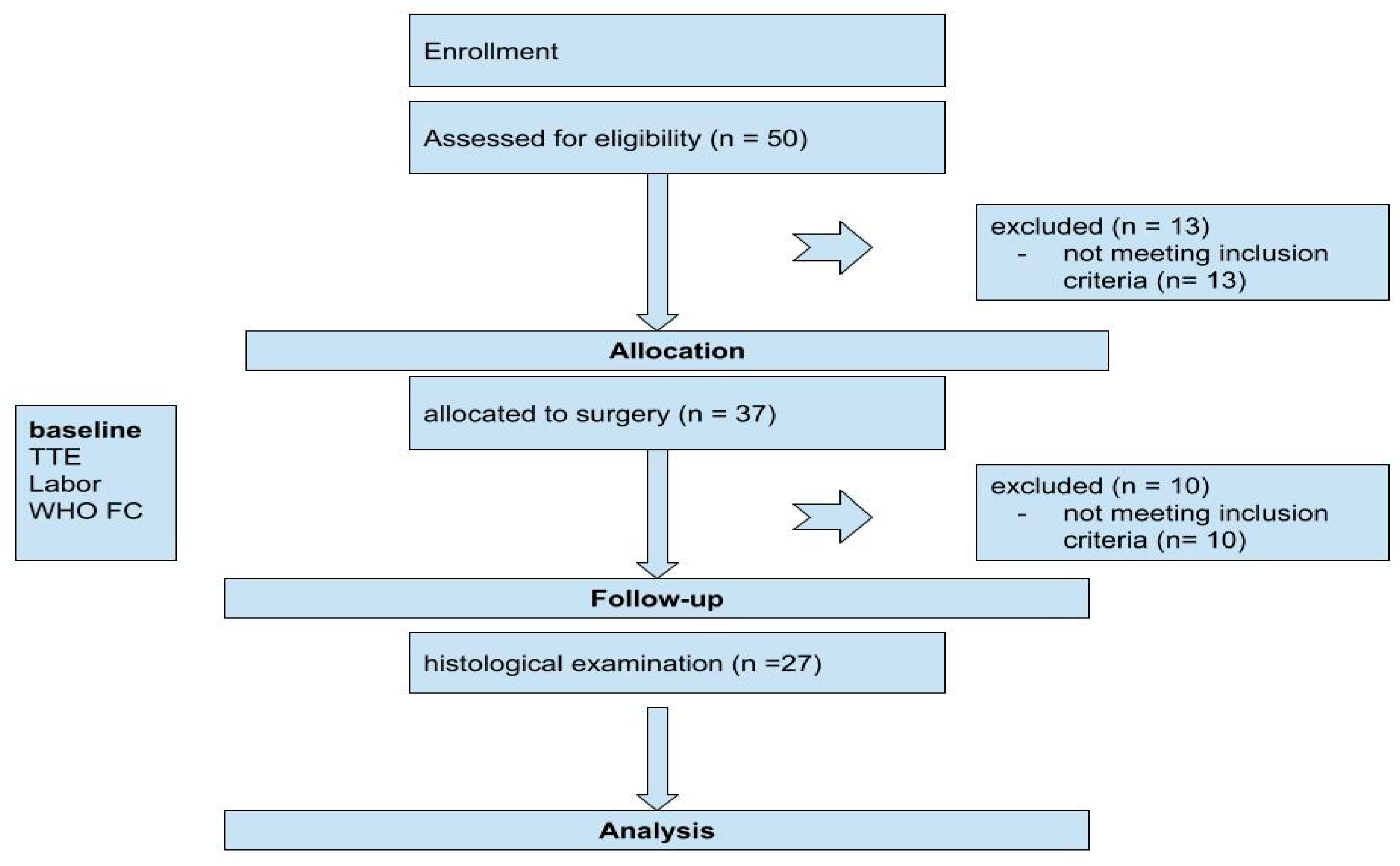
2. Materials and Methods
2.1. Patient Population
2.2. Echocardiography
2.3. Histology
2.4. Outcomes
2.5. Statistics
3. Results
3.1. Echocardiographic Results
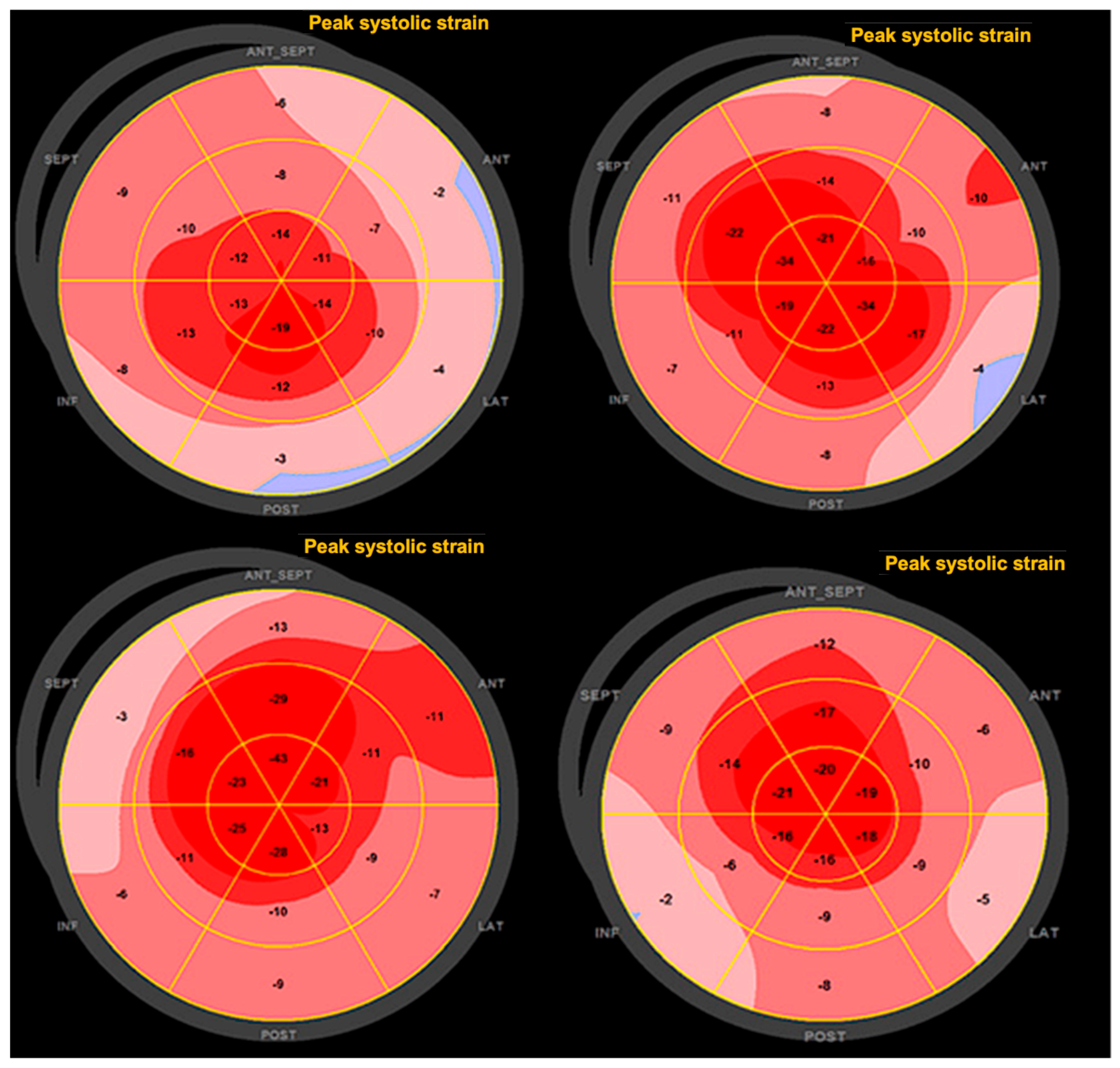
3.2. Two-Dimensional Speckle-Tracking Analysis
3.3. Histological Findings
Comparison between Patients with Identified Histological MF and Patients without MF
3.4. Correlation Analysis
4. Discussion
Limitations
- Sample size and selection bias: Our study included a relatively small sample size, which may limit the generalizability of our findings to broader populations of HOCM patients. Additionally, the patients included in our study were recruited from a single center, which may introduce selection bias and limit the extrapolation of our results to other settings or populations.
- Histological assessment: While histological analysis is considered the gold standard for assessing MF, our study relied on a single biopsy or imaging modality to evaluate fibrotic burden. This approach may not capture the full extent or distribution of fibrosis within the myocardium, potentially underestimating its prevalence or significance.
- Echocardiographic techniques: Although echocardiography is a widely used and valuable tool for assessing cardiac structure and function, it has inherent limitations, such as variability in image quality and operator dependency.
- Exclusion criteria: Our study may have excluded certain subgroups of HOCM patients, such as those with comorbidities or contraindications to invasive procedures like endomyocardial biopsy. This could potentially limit the generalizability of our findings to the broader HOCM population.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Baig, S.; Edward, N.C.; Kotecha, D.; Liu, B.; Nordin, S.; Kozor, R.; Moon, J.C.; Geberhiwot, T.; Steeds, R.P. Ventricular arrhythmia and sudden cardiac death in Fabry disease: A systematic review of risk factors in clinical practice. Europace 2018, 20, f153–f161. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Dearani, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Management of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 390–414. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef]
- Ommen, S.R.; Mital, S.; Burke, M.A.; Day, S.M.; Deswal, A.; Elliott, P.; Evanovich, L.L.; Hung, J.; Joglar, J.A.; Kantor, P.; et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020, 142, e533–e557. [Google Scholar] [CrossRef]
- Verheyen, N.; Batzner, A.; Zach, D.; Zirlik, A.; Gerull, B.; Frantz, S.; Maack, C.; Störk, S.; Seggewiss, H.; Morbach, C. Spatial relationship between mitral valve and ventricular septum assessed by resting echocardiography to diagnose left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 710–718. [Google Scholar] [CrossRef]
- Batzner, A.; Hahn, P.; Morbach, C.; Störk, S.; Maack, C.; Verheyen, N.; Gerull, B.; Frantz, S.; Seggewiss, H. Non-invasive estimation of left ventricular systolic peak pressure: A prerequisite to calculate myocardial work in hypertrophic obstructive cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 213–219. [Google Scholar] [CrossRef]
- Ommen, S.R.; Semsarian, C. Hypertrophic cardiomyopathy: A practical approach to guideline directed management. Lancet 2021, 398, 2102–2108. [Google Scholar] [CrossRef]
- Lisi, M.; Cameli, M.; Mandoli, G.E.; Pastore, M.C.; Righini, F.M.; D’Ascenzi, F.; Focardi, M.; Rubboli, A.; Mondillo, S.; Henein, M.Y. Detection of myocardial fibrosis by speckle-tracking echocardiography: From prediction to clinical applications. Heart Fail. Rev. 2022, 27, 1857–1867. [Google Scholar] [CrossRef]
- Raman, B.; Ariga, R.; Spartera, M.; Sivalokanathan, S.; Chan, K.; Dass, S.; Petersen, S.E.; Daniels, M.J.; Francis, J.; Smillie, R.; et al. Progression of myocardial fibrosis in hypertrophic cardiomyopathy: Mechanisms and clinical implications. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 157–167. [Google Scholar] [CrossRef]
- Habib, M.; Adler, A.; Fardfini, K.; Hoss, S.; Hanneman, K.; Rowin, E.J.; Maron, M.S.; Maron, B.J.; Rakowski, H.; Chan, R.H. Progression of Myocardial Fibrosis in Hypertrophic Cardiomyopathy: A Cardiac Magnetic Resonance Study. JACC Cardiovasc. Imaging 2021, 14, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Ajmone Marsan, N. Global and Regional Longitudinal Strain Assessment in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2019, 12, e009586. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Wells, S. Myocardial Strain in Hypertrophic Cardiomyopathy: A Force Worth Pursuing? JACC Cardiovasc. Imaging 2019, 12, 1943–1945. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, D.; Wang, H.; Wang, Y. Prognostic value of global longitudinal strain in hypertrophic cardiomyopathy: A systematic review and meta-analysis. Clin. Cardiol. 2022, 45, 1184–1191. [Google Scholar] [CrossRef]
- Tanaka, H. Efficacy of echocardiography for differential diagnosis of left ventricular hypertrophy: Special focus on speckle-tracking longitudinal strain. J. Echocardiogr. 2021, 19, 71–79. [Google Scholar] [CrossRef]
- Dahl, J.S.; Magne, J.; Pellikka, P.A.; Donal, E.; Marwick, T.H. Assessment of Subclinical Left Ventricular Dysfunction in Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 163–171. [Google Scholar] [CrossRef]
- Cikes, M.; Sutherland, G.R.; Anderson, L.J.; Bijnens, B.H. The role of echocardiographic deformation imaging in hypertrophic myopathies. Nat. Rev. Cardiol. 2010, 7, 384–396. [Google Scholar] [CrossRef]
- Suwalski, P.; Klingel, K.; Landmesser, U.; Heidecker, B. Apical sparing on speckle tracking in Morbus Fabry. Eur. Heart J. 2020, 41, 3486. [Google Scholar] [CrossRef]
- Phelan, D.; Collier, P.; Thavendiranathan, P.; Popović, Z.B.; Hanna, M.; Plana, J.C.; Marwick, T.H.; Thomas, J.D. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012, 98, 1442–1448. [Google Scholar] [CrossRef]
- Salvo, G.D.; Pergola, V.; Fadel, B.; Bulbul, Z.A.; Caso, P. Strain Echocardiography and Myocardial Mechanics: From Basics to Clinical Applications. J. Cardiovasc. Echogr. 2015, 25, 1–8. [Google Scholar] [CrossRef]
- Di Bella, G.; Pizzino, F.; Donato, R.; Di Nunzio, D.; de Gregorio, C. Advanced Non-invasive Imaging Techniques in Chronic Heart Failure and Cardiomyopathies: Focus on Cardiac Magnetic Resonance Imaging and Computed Tomographic. Adv. Exp. Med. Biol. 2018, 1067, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [PubMed]
- de Gregorio, C.; Trimarchi, G.; Faro, D.C.; De Gaetano, F.; Campisi, M.; Losi, V.; Zito, C.; Tamburino, C.; Di Bella, G.; Monte, I.P. Myocardial Work Appraisal in Transthyretin Cardiac Amyloidosis and Nonobstructive Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2023, 208, 173–179. [Google Scholar] [CrossRef] [PubMed]
- de Gregorio, C.; Trimarchi, G.; Faro, D.C.; Poleggi, C.; Teresi, L.; De Gaetano, F.; Zito, C.; Lofrumento, F.; Koniari, I.; Licordari, R.; et al. Systemic Vascular Resistance and Myocardial Work Analysis in Hypertrophic Cardiomyopathy and Transthyretin Cardiac Amyloidosis with Preserved Left Ventricular Ejection Fraction. J. Clin. Med. 2024, 13, 1671. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Mirea, O.; Pagourelias, E.D.; Duchenne, J.; Bogaert, J.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Variability and Reproducibility of Segmental Longitudinal Strain Measurement: A Report From the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc. Imaging 2018, 11, 15–24. [Google Scholar] [CrossRef]
- Pagourelias, E.D.; Mirea, O.; Duchenne, J.; Unlu, S.; Van Cleemput, J.; Papadopoulos, C.E.; Bogaert, J.; Vassilikos, V.P.; Voigt, J.U. Speckle tracking deformation imaging to detect regional fibrosis in hypertrophic cardiomyopathy: A comparison between 2D and 3D echo modalities. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 1262–1272. [Google Scholar] [CrossRef]
- Haland, T.F.; Almaas, V.M.; Hasselberg, N.E.; Saberniak, J.; Leren, I.S.; Hopp, E.; Edvardsen, T.; Haugaa, K.H. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 613–621. [Google Scholar] [CrossRef]
- Pagourelias, E.D.; Alexandridis, G.M.; Vassilikos, V.P. Fibrosis in hypertrophic cardiomyopathy: Role of novel echo techniques and multi-modality imaging assessment. Heart Fail. Rev. 2021, 26, 1297–1310. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, L.; Zhang, Z.; Lin, Y.; Ji, M.; He, Q.; Xie, M.; Li, Y. Clinical Utility of Strain Imaging in Assessment of Myocardial Fibrosis. J. Clin. Med. 2023, 12, 743. [Google Scholar] [CrossRef]
- Monte, I.P.; Faro, D.C.; Trimarchi, G.; de Gaetano, F.; Campisi, M.; Losi, V.; Teresi, L.; Di Bella, G.; Tamburino, C.; de Gregorio, C. Left Atrial Strain Imaging by Speckle Tracking Echocardiography: The Supportive Diagnostic Value in Cardiac Amyloidosis and Hypertrophic Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2023, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J.-Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Addetia, K.; Maffessanti, F.; Mor-Avi, V.; Lang, R.M. LA Strain for Categorization of LV Diastolic Dysfunction. JACC Cardiovasc. Imaging 2017, 10, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Urbano-Moral, J.A.; Rowin, E.J.; Maron, M.S.; Crean, A.; Pandian, N.G. Investigation of global and regional myocardial mechanics with 3-dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy. Circ. Cardiovasc. Imaging 2014, 7, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, M.; Fan, Y.; Wang, Y.; Song, Y.; Cui, X.; Fu, M.; Qi, B.; Han, X.; Zhou, J.; et al. Diffuse myocardial fibrosis and the prognosis of heart failure with reduced ejection fraction in Chinese patients: A cohort study. Int. J. Cardiovasc. Imaging 2020, 36, 671–689. [Google Scholar] [CrossRef]
- Galli, E.; Vitel, E.; Schnell, F.; Le Rolle, V.; Hubert, A.; Lederlin, M.; Donal, E. Myocardial constructive work is impaired in hypertrophic cardiomyopathy and predicts left ventricular fibrosis. Echocardiography 2019, 36, 74–82. [Google Scholar] [CrossRef]
- Williams, L.K.; Gruner, C.H.; Rakowski, H. The role of echocardiography in hypertrophic cardiomyopathy. Curr. Cardiol. Rep. 2015, 17, 6. [Google Scholar] [CrossRef]
- Popović, Z.B.; Kwon, D.H.; Mishra, M.; Buakhamsri, A.; Greenberg, N.L.; Thamilarasan, M.; Flamm, S.D.; Thomas, J.D.; Lever, H.M.; Desai, M.Y. Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhancement magnetic resonance imaging. J. Am. Soc. Echocardiogr. 2008, 21, 1299–1305. [Google Scholar] [CrossRef]
- Hu, X.; Bao, Y.; Zhu, Y.; Zheng, K.; Zhang, J.; Zhou, W.; Deng, Y.; Liu, Y. Predicting Left Ventricular Myocardial Fibrosis in Patients with Hypertrophic Cardiomyopathy by Speckle Tracking Automated Functional Imaging. Ultrasound Med. Biol. 2023, 49, 1309–1317. [Google Scholar] [CrossRef]
- Hinojar, R.; Fernández-Golfín, C.; González-Gómez, A.; Rincón, L.M.; Plaza-Martin, M.; Casas, E.; García-Martín, A.; Fernandez-Mendez, M.A.; Esteban, A.; Nacher, J.J.J.; et al. Prognostic implications of global myocardial mechanics in hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking. Relations to left ventricular hypertrophy and fibrosis. Int. J. Cardiol. 2017, 249, 467–472. [Google Scholar] [CrossRef]
- Saito, M.; Okayama, H.; Yoshii, T.; Higashi, H.; Morioka, H.; Hiasa, G.; Sumimoto, T.; Inaba, S.; Nishimura, K.; Inoue, K.; et al. Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Licordari, R.; Trimarchi, G.; Teresi, L.; Restelli, D.; Lofrumento, F.; Perna, A.; Campisi, M.; de Gregorio, C.; Grimaldi, P.; Calabrò, D.; et al. Cardiac Magnetic Resonance in HCM Phenocopies: From Diagnosis to Risk Stratification and Therapeutic Management. J. Clin. Med. 2023, 12, 3481. [Google Scholar] [CrossRef] [PubMed]
- Horwood, L.; Attili, A.; Luba, F.; Ibrahim, E.H.; Parmar, H.; Stojanovska, J.; Gadoth-Goodman, S.; Fette, C.; Oral, H.; Bogun, F. Magnetic resonance imaging in patients with cardiac implanted electronic devices: Focus on contraindications to magnetic resonance imaging protocols. Europace 2017, 19, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Kolakalapudi, P.; Arora, G. Contrast-in cardiac magnetic resonance imaging. Echocardiography 2018, 35, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, J.; Sun, J.; Wang, Y.; Xia, R.; Yin, Q.; Chen, W.; Xu, Z.; Liao, J.; Zhang, B.; et al. Endogenous contrast T1rho cardiac magnetic resonance for myocardial fibrosis in hypertrophic cardiomyopathy patients. J. Cardiol. 2015, 66, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Rivers, B.J.; Carrick, R.T.; Muller, S.A.; Barth, A.S.; Madrazo, J.A.; James, C.A. How and Why to Organise Family-Based Screening Clinics for Hypertrophic Cardiomyopathy. Can. J. Cardiol. 2024, 40, 934–937. [Google Scholar] [CrossRef]
- Almaas, V.M.; Haugaa, K.H.; Strøm, E.H.; Scott, H.; Smith, H.J.; Dahl, C.P.; Geiran, O.R.; Endresen, K.; Aakhus, S.; Amlie, J.P.; et al. Noninvasive assessment of myocardial fibrosis in patients with obstructive hypertrophic cardiomyopathy. Heart 2014, 100, 631–638. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, S.; Yu, S.; Wu, G.; Wang, D.; Liu, L.; Song, J.; Zhu, Y.; Kang, L.; Wang, J.; et al. Patterns of Replacement Fibrosis in Hypertrophic Cardiomyopathy. Radiology 2022, 302, 298–306. [Google Scholar] [CrossRef]
- Maron, B.J.; Maron, M.S. Hypertrophic cardiomyopathy. Lancet 2013, 381, 242–255. [Google Scholar] [CrossRef]
- Weng, Z.; Yao, J.; Chan, R.H.; He, J.; Yang, X.; Zhou, Y.; He, Y. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1392–1402. [Google Scholar] [CrossRef]
- Gavina, C.; Falcão-Pires, I.; Santos-Faria, J.; Marinho, B.; Almeida, J.; Rodrigues, J.; Pinho, P.; Rocha-Gonçalves, F.; Leite-Moreira, A. Prognostic implications of fibrosis in low risk aortic stenosis patients. Rev. Port. Cardiol. 2022, 41, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Bing, R.; Cavalcante, J.L.; Everett, R.J.; Clavel, M.-A.; Newby, D.E.; Dweck, M.R. Imaging and Impact of Myocardial Fibrosis in Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Vahanian, A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011, 8, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Aluru, J.S.; Barsouk, A.; Saginala, K.; Rawla, P.; Barsouk, A. Valvular Heart Disease Epidemiology. Med. Sci. 2022, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Goody, P.R.; Hosen, M.R.; Christmann, D.; Niepmann, S.T.; Zietzer, A.; Adam, M.; Bönner, F.; Zimmer, S.; Nickenig, G.; Jansen, F. Aortic Valve Stenosis: From Basic Mechanisms to Novel Therapeutic Targets. Arter. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 885–900. [Google Scholar] [CrossRef]
- Dweck, M.R.; Boon, N.A.; Newby, D.E. Calcific aortic stenosis: A disease of the valve and the myocardium. J. Am. Coll. Cardiol. 2012, 60, 1854–1863. [Google Scholar] [CrossRef]
- Balčiūnaitė, G.; Palionis, D.; Žurauskas, E.; Skorniakov, V.; Janušauskas, V.; Zorinas, A.; Zaremba, T.; Valevičienė, N.; Aidietis, A.; Šerpytis, P.; et al. Prognostic value of myocardial fibrosis in severe aortic stenosis: Study protocol for a prospective observational multi-center study (FIB-AS). BMC Cardiovasc. Disord. 2020, 20, 275. [Google Scholar] [CrossRef]
- Azevedo, C.F.; Nigri, M.; Higuchi, M.L.; Pomerantzeff, P.M.; Spina, G.S.; Sampaio, R.O.; Tarasoutchi, F.; Grinberg, M.; Rochitte, C.E. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J. Am. Coll. Cardiol. 2010, 56, 278–287. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Everett, R.J.; Tastet, L.; Clavel, M.A.; Chin, C.W.L.; Capoulade, R.; Vassiliou, V.S.; Kwiecinski, J.; Gomez, M.; van Beek, E.J.R.; White, A.C.; et al. Progression of Hypertrophy and Myocardial Fibrosis in Aortic Stenosis: A Multicenter Cardiac Magnetic Resonance Study. Circ. Cardiovasc. Imaging 2018, 11, e007451. [Google Scholar] [CrossRef]


| Characteristics | |
|---|---|
| Age (years) | 66.37 ± 12.89 |
| male n (%) | 12 (44.4%) |
| BMI kg/m2 | 29.53 ± 5.55 |
| Aortic Valve Replacement n (%) | 9 (33.3%) |
| Coronary Artery Bypass Grafting n (%) | 6 (22.2%) |
| Atrial fibrillation n (%) | 6 (22.2%) |
| Chronic Obstructive Pulmonary Disease n (%) | 3 (11.1%) |
| Diabetes mellitus n (%) | 7 (25.9%) |
| Hypertension n (%) | 24 (88.9%) |
| Hyperlipidemia | 22 (81.5%) |
| NYHA II | 21 (77.8%) |
| NYHA III | 6 (22.2%) |
| WBC (109/L) | 9.09 ± 3.23 |
| Hb (g/dL) | 11.2 ± 1.91 |
| Platelets (109/L) | 314 ± 103 |
| LDH U/L | 332 ± 123 |
| Creatinine (mg/dL) | 0.969 ± 0.306 |
| GFR (mL/min) | 73.4 ± 24.1 |
| Potassium (mmol/L) | 4.38 ± 0.586 |
| Length of stay (days) | 16.31 ± 10.18 |
| ICU stay (days) | 6.41 ± 10.53 |
| Bleeding/Re-thoracotomy (%) | 4 (14.8%) |
| Stroke (%) | 2 (7.4%) |
| Cardiogenic shock n (%) | 2 (7.4%) |
| CVVH n (%) | 2 (7.4%) |
| Pneumonia n (%) | 8 (29.6%) |
| Delirium n (%) | 7 (25.9%) |
| Pacemaker n (%) | 2 (7.4%) |
| Parameters | |
|---|---|
| EF, % | 64.07 ± 6.72 |
| GLS, % | −15.39 ± 5.83 |
| Relative apical strain | 0.91 ± 0.30 |
| Base-to-apex gradient, mmHg | −12.69 ± 14.25 |
| IVSd, mm | 21.52 ± 4.30 |
| LVMI, g/m2 | 198.69 ± 58.54 |
| PWT, mm | 18.20 ± 4.41 |
| LVIDd, mm | 33.95 ± 7.58 |
| RWT | 1.22 ± 0.67 |
| E, m/s | 0.96 ± 0.31 |
| A, m/s | 1.02 ± 0.39 |
| E/A | 1.07 ± 0.54 |
| DT, ms | 250.16 ± 96.28 |
| E/e′ | 15.60 ± 5.63 |
| LAVI, mL/m2 | 49.57 ± 16.67 |
| MPG LVOT, mmHg | 8.56 ± 12.57 |
| PPG LVOT, mmHg | 15.85 ± 22.65 |
| Vmax LVOT, m/s | 1.75 ± 0.91 |
| MPG AV, mmHg | 41.19 ± 27.45 |
| PPG AV, mmHg | 75.72 ± 51.92 |
| Vmax AV, m/s | 4.05 ± 1.62 |
| Parameters | |
|---|---|
| Mean basal strain, (%) | −12.30 ± 4.35 |
| Mean mid-strain, (%) | −14.43 ± 3.29 |
| Mean apical strain, (%) | −20.45 ± 5.41 |
| Relative apical strain | 0.91 ± 0.30 |
| Base-to-apex gradient, mmHg | −12.69 ± 14.25 |
| No MF (n = 7) | MF (n = 20) | p | |
|---|---|---|---|
| Female n (%) | 6 (85.7) | 9 (45.0) | 0.154 |
| AVR n (%) | 2 (28.6) | 7 (35.0) | 1.000 |
| CABG n (%) | 0 (0.0) | 6 (30.0) | 0.265 |
| Age years | 66.3 ± 11.2 | 66.4 ± 13.7 | 0.984 |
| BSA m2 | 1.9 ± 0.2 | 1.9 ± 0.2 | 0.628 |
| BMI kg/m2 | 32.1 ± 5.5 | 28.6 ± 5.4 | 0.151 |
| Bleeding n (%) | 0 (0.0) | 4 (20.0) | 0.507 |
| Delirium n (%) | 0 (0.0) | 7 (35.0) | 0.188 |
| Pneumonia n (%) | 1 (14.3) | 7 (35.0) | 0.581 |
| Pacemaker n (%) | 1 (14.3) | 1 (5.0) | 1.000 |
| Stroke n (%) | 0 (0.0) | 2 (10.0) | 0.975 |
| Dialysis n (%) | 0 (0.0) | 2 (10.0) | 0.975 |
| Cardiogenic shock n (%) | 0 (0.0) | 2 (10.0) | 0.975 |
| CAD n (%) | 1 (14.3) | 11 (55.0) | 0.154 |
| AS n (%) | 2 (28.6) | 8 (40.0) | 0.933 |
| MI n (%) | 6 (85.7) | 8 (40.0) | 0.100 |
| AF n (%) | 2 (28.6) | 4 (20.0) | 1.000 |
| COPD n (%) | 0 (0.0) | 3 (15.0) | 0.698 |
| Diabetes mellitus n (%) | 3 (42.9) | 4 (20.0) | 0.492 |
| LOS days | 12.4 ± 3.4 | 17.7 ± 11.5 | 0.246 |
| ICU days | 2.1 ± 0.9 | 7.9 ± 11.9 | 0.220 |
| Wbc (109/L) | 10.5 ± 2.2 | 8.6 ± 3.4 | 0.184 |
| Hb (g/dL) | 11.5 ± 1.7 | 11.1 ± 2.0 | 0.606 |
| Platelets (109/L) | 346.9 ± 103.8 | 302.7 ± 103.0 | 0.339 |
| LDH U/L | 366.1 ± 157.2 | 319.8 ± 110.8 | 0.401 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 1.0 ± 0.3 | 0.398 |
| GFR (mL/min) | 72.7 ± 18.8 | 73.7 ± 26.2 | 0.926 |
| Potassium (mmol/L) | 4.5 ± 0.2 | 4.3 ± 0.7 | 0.485 |
| Arterial hypertension n (%) | 6 (85.7) | 18 (90.0) | 1.000 |
| Hyperlipidemia n (%) | 2 (28.6) | 3 (15.0) | 0.818 |
| NYHA >II | 1 (14.3) | 5 (25.0) | 0.953 |
| EF % | 60.9 ± 8.3 | 65.2 ± 5.9 | 0.144 |
| GLS % | −23.0 ± 5.7 | −12.7 ± 2.7 | <0.001 |
| Mean basal-strain % | −17.3 ± 4.6 | −10.6 ± 2.6 | <0.001 |
| Mean mid-strain % | −15.1 ± 4.0 | −14.2 ± 3.1 | 0.548 |
| Mean apical strain % | −16.7 ± 5.6 | −21.8 ± 4.8 | 0.032 |
| Relative apical strain | 0.6 ± 0.1 | 1.0 ± 0.3 | 0.003 |
| Base-to-apex gradient mmHg | −4.7 ± 5.5 | −15.5 ± 15.4 | 0.085 |
| IVSd mm | 20.7 ± 3.4 | 21.8 ± 4.6 | 0.576 |
| LVMI g/m² | 165.5 ± 54.0 | 210.3 ± 56.8 | 0.081 |
| PWT mm | 17.6 ± 3.9 | 18.4 ± 4.7 | 0.668 |
| LVIDd mm | 32.3 ± 8.5 | 34.5 ± 7.4 | 0.510 |
| RWT mm | 1.3 ± 0.7 | 1.2 ± 0.7 | 0.656 |
| E m/s | 0.9 ± 0.3 | 1.0 ± 0.3 | 0.433 |
| E/A | 0.9 ± 0.2 | 1.1 ± 0.4 | 0.637 |
| DT ms | 247.6 ± 61.6 | 251.1 ± 107.2 | 0.905 |
| E/e′ | 14.9 ± 5.4 | 15.8 ± 5.8 | 0.736 |
| LAVI mL/m² | 44.5 ± 10.0 | 51.4 ± 18.3 | 0.316 |
| MPG LVOT mmHg | 4.4 ± 1.2 | 10.0 ± 14.4 | 0.356 |
| PPG LVOT mmHg | 9.1 ± 2.5 | 18.2 ± 26.0 | 0.316 |
| Vmax LVOT m/s | 1.5 ± 0.2 | 1.8 ± 1.0 | 0.368 |
| MPG AV mmHg | 21.9 ± 19.6 | 47.9 ± 26.9 | 0.387 |
| PPG AV mmHg | 39.5 ± 33.1 | 88.4 ± 51.9 | 0.027 |
| Vmax AV m/s | 2.9 ± 1.3 | 4.5 ± 1.5 | 0.029 |
| RVSD mmHg | 27.0 ± 5.5 | 26.7 ± 6.1 | 0.944 |
| 95% Confidence Interval | ||||||
|---|---|---|---|---|---|---|
| Names | SE | Odds Ratio | Lower | Upper | z | p |
| GLS % | 0.01 | 1.07 | 1.05 | 1.09 | 6.42 | <0.001 |
| Mean apical strain % | 0.01 | 1.00 | 0.98 | 1.03 | 0.09 | 0.929 |
| Relative apical strain | 0.24 | 1.30 | 0.81 | 2.08 | 1.10 | 0.285 |
| Base-to-apex gradient | 0.00 | 1.00 | 0.99 | 1.01 | 0.25 | 0.804 |
| PPG AV mmHg | 0.00 | 1.00 | 0.99 | 1.01 | −0.71 | 0.488 |
| Vmax_AV m/s | 0.15 | 1.09 | 0.81 | 1.45 | 0.56 | 0.585 |
| Mean basal-strain % | 0.02 | 1.00 | 0.97 | 1.03 | −0.10 | 0.920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yelenski, S.; Zayat, R.; Spillner, J.; Donuru, S.R.; Kolashov, A.; Khattab, M.A.; Hatam, N.; Kalverkamp, S. Apical Sparing of Longitudinal Strain as a Specific Pattern of Myocardial Fibrosis in Patients with Severe Left Ventricular Hypertrophy: A Comparison between Deformation Imaging and Histological Findings. J. Clin. Med. 2024, 13, 6141. https://doi.org/10.3390/jcm13206141
Yelenski S, Zayat R, Spillner J, Donuru SR, Kolashov A, Khattab MA, Hatam N, Kalverkamp S. Apical Sparing of Longitudinal Strain as a Specific Pattern of Myocardial Fibrosis in Patients with Severe Left Ventricular Hypertrophy: A Comparison between Deformation Imaging and Histological Findings. Journal of Clinical Medicine. 2024; 13(20):6141. https://doi.org/10.3390/jcm13206141
Chicago/Turabian StyleYelenski, Siarhei, Rashad Zayat, Jan Spillner, Srinath Reddy Donuru, Alish Kolashov, Mohammad Amen Khattab, Nima Hatam, and Sebastian Kalverkamp. 2024. "Apical Sparing of Longitudinal Strain as a Specific Pattern of Myocardial Fibrosis in Patients with Severe Left Ventricular Hypertrophy: A Comparison between Deformation Imaging and Histological Findings" Journal of Clinical Medicine 13, no. 20: 6141. https://doi.org/10.3390/jcm13206141
APA StyleYelenski, S., Zayat, R., Spillner, J., Donuru, S. R., Kolashov, A., Khattab, M. A., Hatam, N., & Kalverkamp, S. (2024). Apical Sparing of Longitudinal Strain as a Specific Pattern of Myocardial Fibrosis in Patients with Severe Left Ventricular Hypertrophy: A Comparison between Deformation Imaging and Histological Findings. Journal of Clinical Medicine, 13(20), 6141. https://doi.org/10.3390/jcm13206141






