Effect of Vaginal Laser and Topical Therapies on Vulvovaginal Atrophy Symptoms in Breast Cancer Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
- P—women with breast cancer treatment;
- I—hormonal and non-hormonal treatment;
- C—placebo or sham treatment;
- O—Primary outcome: Vaginal Health Index (VHI), Female Sexual Function Index (FSFI)
2.1. Selection Process
2.2. Data Items
2.3. Study Risk of Bias Assessment
2.4. Synthesis Methods
3. Results
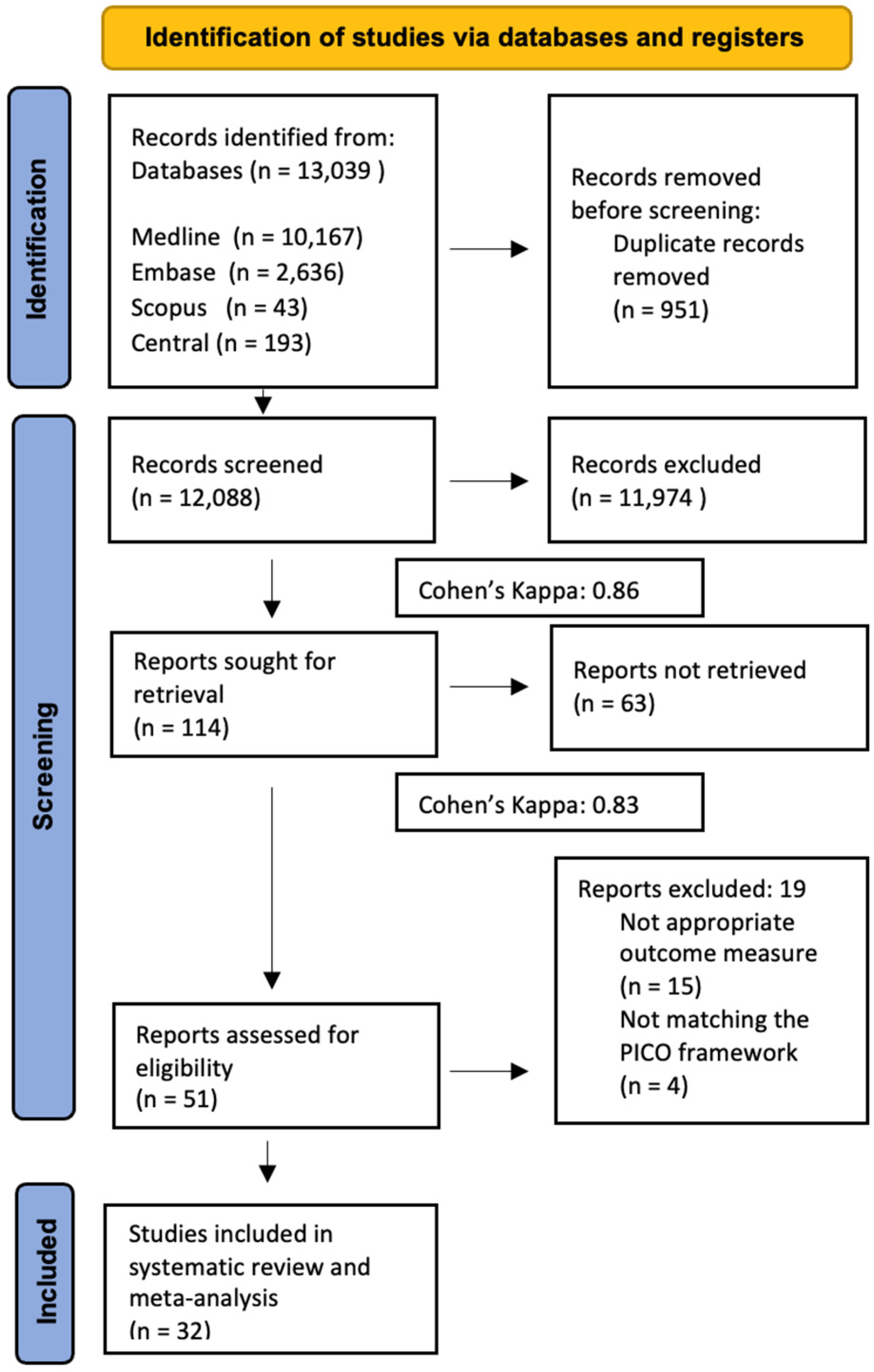
3.1. Search and Selection, Characteristics of the Included Studies
3.2. Basic Characteristics of Included Studies
3.3. Outcomes
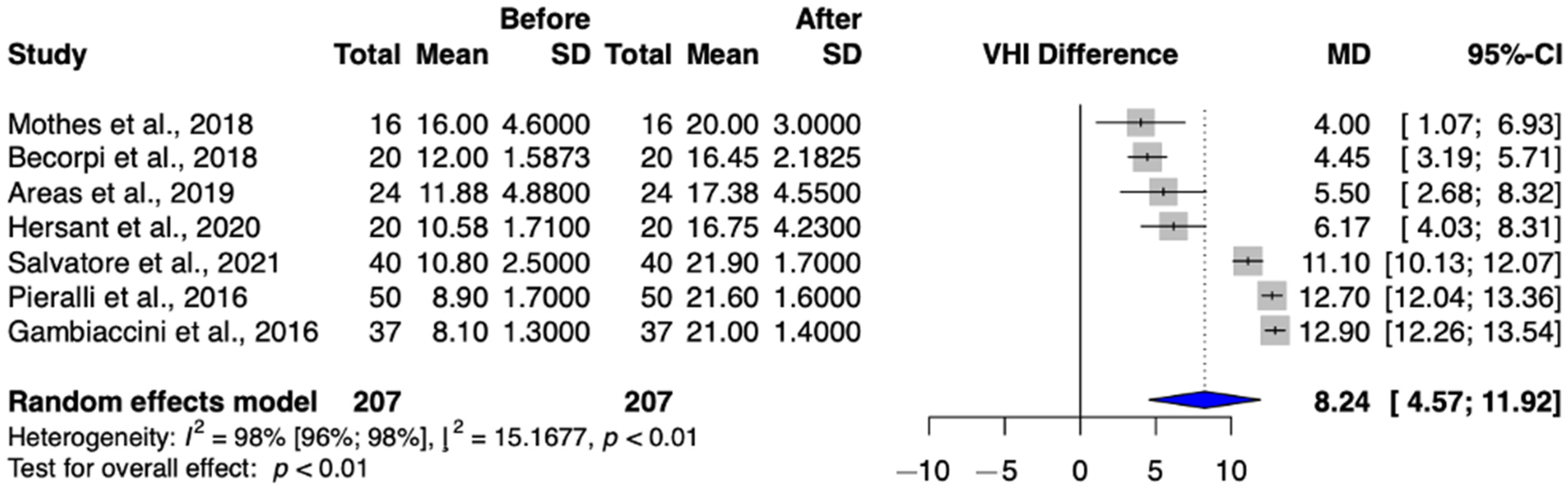
3.3.1. Primary Outcome: Vaginal Health Index
3.3.2. Primary Outcome: Female Sexual Function Index (FSFI)
3.4. Secondary Outcome
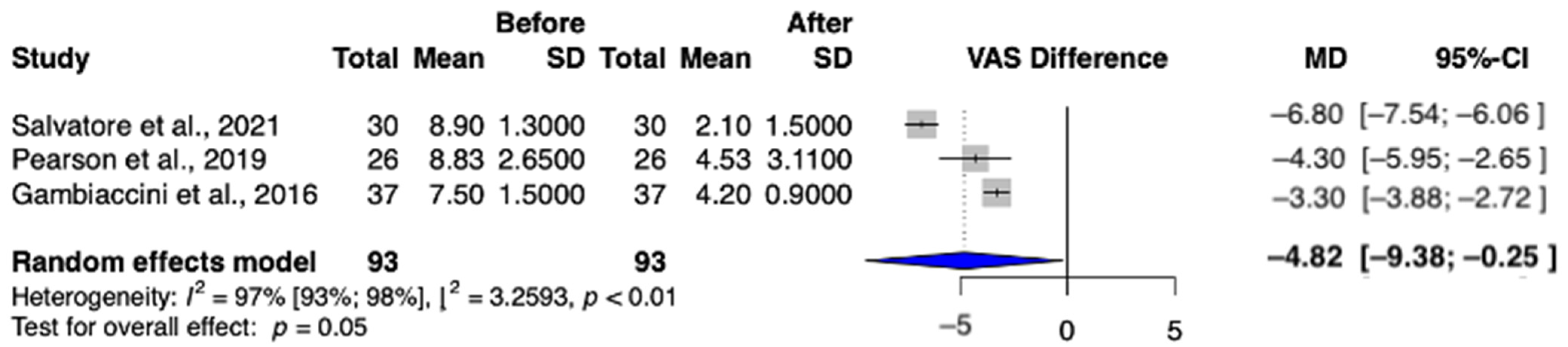
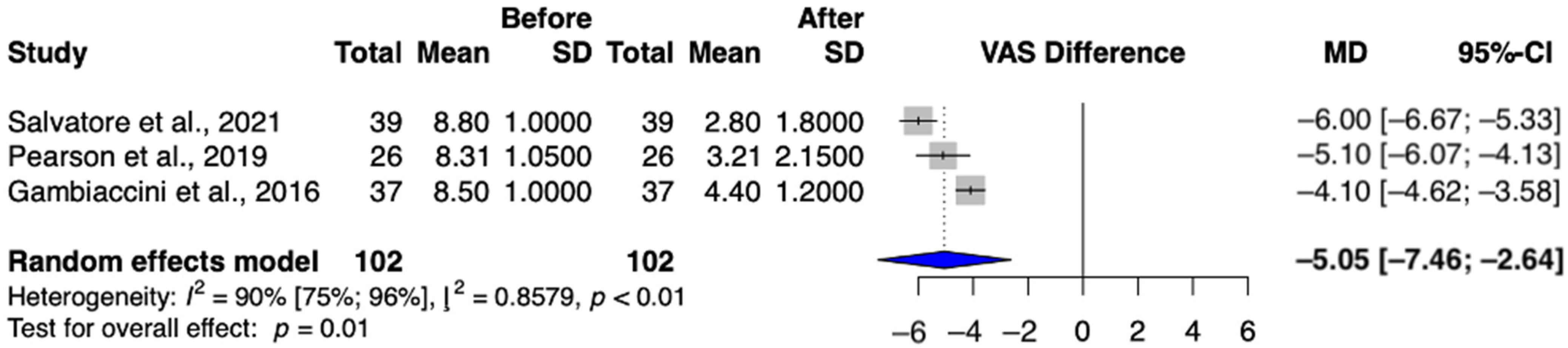
3.4.1. Secondary Outcome: Dyspareunia
3.4.2. Secondary Outcome: Dryness
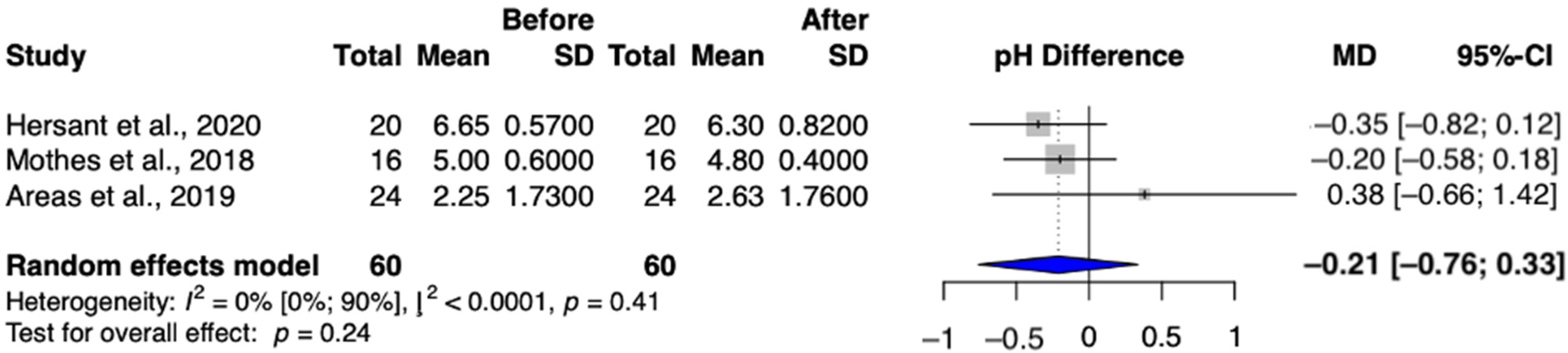
3.4.3. Secondary Outcome: Vaginal pH
3.5. Risk of Bias and Level of Evidence Certainty Assessments
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Practice
4.3. Implications for Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef] [PubMed]
- Brufsky, A.M.; Dickler, M.N. Estrogen Receptor-Positive Breast Cancer: Exploiting Signaling Pathways Implicated in Endocrine Resistance. Oncologist 2018, 23, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Nappi, R.E.; Palacios, S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric 2014, 17, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Lubián López, D.M. Management of genitourinary syndrome of menopause in breast cancer survivors: An update. World J. Clin. Oncol. 2022, 13, 71–100. [Google Scholar] [CrossRef]
- Biglia, N.; Bounous, V.E.; D’Alonzo, M.; Ottino, L.; Tuninetti, V.; Robba, E.; Perrone, T. Vaginal Atrophy in Breast Cancer Survivors: Attitude and Approaches Among Oncologists. Clin. Breast Cancer 2017, 17, 611–617. [Google Scholar] [CrossRef]
- Sarmento, A.C.A.; Costa, A.P.F.; Vieira-Baptista, P.; Giraldo, P.C.; Eleutério, J., Jr.; Gonçalves, A.K. Genitourinary Syndrome of Menopause: Epidemiology, Physiopathology, Clinical Manifestation and Diagnostic. Front. Reprod. Health 2021, 3, 779398. [Google Scholar] [CrossRef]
- Advani, P.; Brewster, A.M.; Baum, G.P.; Schover, L.R. A pilot randomized trial to prevent sexual dysfunction in postmenopausal breast cancer survivors starting adjuvant aromatase inhibitor therapy. J. Cancer Surviv. 2017, 11, 477–485. [Google Scholar] [CrossRef]
- Arêas, F.; Valadares, A.L.R.; Conde, D.M.; Costa-Paiva, L. The effect of vaginal erbium laser treatment on sexual function and vaginal health in women with a history of breast cancer and symptoms of the genitourinary syndrome of menopause: A prospective study. Menopause 2019, 26, 1052–1058. [Google Scholar] [CrossRef]
- Becorpi, A.; Campisciano, G.; Zanotta, N.; Tredici, Z.; Guaschino, S.; Petraglia, F.; Pieralli, A.; Sisti, G.; De Seta, F.; Comar, M. Fractional CO(2) laser for genitourinary syndrome of menopause in breast cancer survivors: Clinical, immunological, and microbiological aspects. Lasers Med. Sci. 2018, 33, 1047–1054. [Google Scholar] [CrossRef]
- Biglia, N.; Peano, E.; Sgandurra, P.; Moggio, G.; Panuccio, E.; Migliardi, M.; Ravarino, N.; Ponzone, R.; Sismondi, P. Low-dose vaginal estrogens or vaginal moisturizer in breast cancer survivors with urogenital atrophy: A preliminary study. Gynecol. Endocrinol. 2010, 26, 404–412. [Google Scholar] [CrossRef]
- Davis, S.R.; Robinson, P.J.; Jane, F.; White, S.; White, M.; Bell, R.J. Intravaginal Testosterone Improves Sexual Satisfaction and Vaginal Symptoms Associated with Aromatase Inhibitors. J. Clin. Endocrinol. Metab. 2018, 103, 4146–4154. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.; Neven, P.; Moegele, M.; Lintermans, A.; Bellen, G.; Prasauskas, V.; Grob, P.; Ortmann, O.; Buchholz, S. Ultra-low-dose estriol and Lactobacillus acidophilus vaginal tablets (Gynoflor®) for vaginal atrophy in postmenopausal breast cancer patients on aromatase inhibitors: Pharmacokinetic, safety, and efficacy phase I clinical study. Breast Cancer Res. Treat. 2014, 145, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Levancini, M. Vaginal erbium laser as second-generation thermotherapy for the genitourinary syndrome of menopause: A pilot study in breast cancer survivors. Menopause 2017, 24, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Levancini, M.; Cervigni, M. Vaginal erbium laser: The second-generation thermotherapy for the genitourinary syndrome of menopause. Climacteric 2015, 18, 757–763. [Google Scholar] [CrossRef]
- Gold, D.; Nicolay, L.; Avian, A.; Greimel, E.; Balic, M.; Pristauz-Telsnigg, G.; Tamussino, K.; Trutnovsky, G. Vaginal laser therapy versus hyaluronic acid suppositories for women with symptoms of urogenital atrophy after treatment for breast cancer: A randomized controlled trial. Maturitas 2023, 167, 1–7. [Google Scholar] [CrossRef]
- Hersant, B.; SidAhmed-Mezi, M.; Belkacemi, Y.; Darmon, F.; Bastuji-Garin, S.; Werkoff, G.; Bosc, R.; Niddam, J.; Hermeziu, O.; La Padula, S.; et al. Efficacy of injecting platelet concentrate combined with hyaluronic acid for the treatment of vulvovaginal atrophy in postmenopausal women with history of breast cancer: A phase 2 pilot study. Menopause 2018, 25, 1124–1130. [Google Scholar] [CrossRef]
- Hersant, B.; Werkoff, G.; Sawan, D.; Sidahmed-Mezi, M.; Bosc, R.; La Padula, S.; Kalsoum, S.; Ouidir, N.; Meningaud, J.P.; Belkacemi, Y. Carbon dioxide laser treatment for vulvovaginal atrophy in women treated for breast cancer: Preliminary results of the feasibility EPIONE trial. Ann. Chir. Plast. Esthet. 2020, 65, e23–e31. [Google Scholar] [CrossRef]
- Hirschberg, A.L.; Sánchez-Rovira, P.; Presa-Lorite, J.; Campos-Delgado, M.; Gil-Gil, M.; Lidbrink, E.; Suárez-Almarza, J.; Nieto-Magro, C. Efficacy and safety of ultra-low dose 0.005% estriol vaginal gel for the treatment of vulvovaginal atrophy in postmenopausal women with early breast cancer treated with nonsteroidal aromatase inhibitors: A phase II, randomized, double-blind, placebo-controlled trial. Menopause 2020, 27, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Juliato, P.T.; Rodrigues, A.T.; Stahlschmidt, R.; Juliato, C.R.; Mazzola, P.G. Can polyacrylic acid treat sexual dysfunction in women with breast cancer receiving tamoxifen? Climacteric 2017, 20, 62–66. [Google Scholar] [CrossRef]
- Keshavarzi, Z.; Janghorban, R.; Alipour, S.; Tahmasebi, S.; Jokar, A. The effect of vitamin D and E vaginal suppositories on tamoxifen-induced vaginal atrophy in women with breast cancer. Support. Care Cancer 2019, 27, 1325–1334. [Google Scholar] [CrossRef]
- Lee, Y.K.; Chung, H.H.; Kim, J.W.; Park, N.H.; Song, Y.S.; Kang, S.B. Vaginal pH-balanced gel for the control of atrophic vaginitis among breast cancer survivors: A randomized controlled trial. Obstet. Gynecol. 2011, 117, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Mothes, A.R.; Runnebaum, M.; Runnebaum, I.B. Ablative dual-phase Erbium:YAG laser treatment of atrophy-related vaginal symptoms in post-menopausal breast cancer survivors omitting hormonal treatment. J. Cancer Res. Clin. Oncol. 2018, 144, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.; Booker, A.; Tio, M.; Marx, G. Vaginal CO2 laser for the treatment of vulvovaginal atrophy in women with breast cancer: LAAVA pilot study. Breast Cancer Res. Treat. 2019, 178, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, A.; Fallani, M.G.; Becorpi, A.; Bianchi, C.; Corioni, S.; Longinotti, M.; Tredici, Z.; Guaschino, S. Fractional CO2 laser for vulvovaginal atrophy (VVA) dyspareunia relief in breast cancer survivors. Arch. Gynecol. Obstet. 2016, 294, 841–846. [Google Scholar] [CrossRef]
- Quick, A.M.; Zvinovski, F.; Hudson, C.; Hundley, A.; Evans, C.; Suresh, A.; Stephens, J.A.; Arthur, E.; Ramaswamy, B.; Reinbolt, R.E.; et al. Fractional CO2 laser therapy for genitourinary syndrome of menopause for breast cancer survivors. Support. Care Cancer 2020, 28, 3669–3677. [Google Scholar] [CrossRef]
- Salvatore, S.; Nappi, R.E.; Casiraghi, A.; Ruffolo, A.F.; Degliuomini, R.; Parma, M.; Leone Roberti Maggiore, U.; Athanasiou, S.; Candiani, M. Microablative Fractional CO2 Laser for Vulvovaginal Atrophy in Women With a History of Breast Cancer: A Pilot Study at 4-week Follow-up. Clin. Breast Cancer 2021, 21, e539–e546. [Google Scholar] [CrossRef]
- Mension, E.; Alonso, I.; Anglès-Acedo, S.; Ros, C.; Otero, J.; Villarino, Á.; Farré, R.; Saco, A.; Vega, N.; Castrejón, N.; et al. Effect of Fractional Carbon Dioxide vs Sham Laser on Sexual Function in Survivors of Breast Cancer Receiving Aromatase Inhibitors for Genitourinary Syndrome of Menopause: The LIGHT Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2255697. [Google Scholar] [CrossRef]
- Löczi, L.; Keszthelyi, M.; Várbíró, S.; Turan, C. PROSPERO—Investigating the Effectiveness and the Safety of the Treatments of Vulvovaginal Atrophy in Women with Breast Cancer Treatments—A Systematic Review and Meta-Analysis of Randomized Control Trials. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=379866 (accessed on 9 December 2022).
- Bezerra, C.T.; Grande, A.J.; Galvão, V.K.; Santos, D.; Atallah Á., N.; Silva, V. Assessment of the strength of recommendation and quality of evidence: GRADE checklist. A descriptive study. Sao Paulo Med. J. 2022, 140, 829–836. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Knapp, G.; Hartung, J. Improved tests for a random effects meta-regression with a single covariate. Stat. Med. 2003, 22, 2693–2710. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Luo, D.; Weng, H.; Zeng, X.T.; Lin, L.; Chu, H.; Tong, T. Optimally estimating the sample standard deviation from the five-number summary. Res. Synth. Methods 2020, 11, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Baser, R.E.; Goldfrank, D.J.; Seidel, B.; Milli, L.; Stabile, C.; Canty, J.; Saban, S.; Goldfarb, S.; Dickler, M.N.; et al. A single-arm, prospective trial investigating the effectiveness of a non-hormonal vaginal moisturizer containing hyaluronic acid in postmenopausal cancer survivors. Support. Care Cancer 2021, 29, 311–322. [Google Scholar] [CrossRef]
- Chatsiproios, D.; Schmidts-Winkler, I.M.; König, L.; Masur, C.; Abels, C. Topical treatment of vaginal dryness with a non-hormonal cream in women undergoing breast cancer treatment—An open prospective multicenter study. PLoS ONE 2019, 14, e0210967. [Google Scholar] [CrossRef] [PubMed]
- Dahir, M.; Travers-Gustafson, D. Breast cancer, aromatase inhibitor therapy, and sexual functioning: A pilot study of the effects of vaginal testosterone therapy. Sex. Med. 2014, 2, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Dew, J.E.; Wren, B.G.; Eden, J.A. A cohort study of topical vaginal estrogen therapy in women previously treated for breast cancer. Climacteric 2003, 6, 45–52. [Google Scholar] [CrossRef]
- Goetsch, M.F.; Lim, J.Y.; Caughey, A.B. Locating pain in breast cancer survivors experiencing dyspareunia: A randomized controlled trial. Obstet. Gynecol. 2014, 123, 1231–1236. [Google Scholar] [CrossRef]
- Hickey, M.; Marino, J.L.; Braat, S.; Wong, S. A randomized, double-blind, crossover trial comparing a silicone- versus water-based lubricant for sexual discomfort after breast cancer. Breast Cancer Res. Treat. 2016, 158, 79–90. [Google Scholar] [CrossRef]
- Juraskova, I.; Jarvis, S.; Mok, K.; Peate, M.; Meiser, B.; Cheah, B.C.; Mireskandari, S.; Friedlander, M. The acceptability, feasibility, and efficacy (phase I/II study) of the OVERcome (Olive Oil, Vaginal Exercise, and MoisturizeR) intervention to improve dyspareunia and alleviate sexual problems in women with breast cancer. J. Sex. Med. 2013, 10, 2549–2558. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Abu-Ghazaleh, S.; Sloan, J.A.; vanHaelst-Pisani, C.; Hammer, A.M.; Rowland, K.M., Jr.; Law, M.; Windschitl, H.E.; Kaur, J.S.; Ellison, N. Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J. Clin. Oncol. 1997, 15, 969–973. [Google Scholar] [CrossRef]
- Melisko, M.E.; Goldman, M.E.; Hwang, J.; De Luca, A.; Fang, S.; Esserman, L.J.; Chien, A.J.; Park, J.W.; Rugo, H.S. Vaginal Testosterone Cream vs Estradiol Vaginal Ring for Vaginal Dryness or Decreased Libido in Women Receiving Aromatase Inhibitors for Early-Stage Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2017, 3, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Pfeiler, G.; Glatz, C.; Königsberg, R.; Geisendorfer, T.; Fink-Retter, A.; Kubista, E.; Singer, C.F.; Seifert, M. Vaginal estriol to overcome side-effects of aromatase inhibitors in breast cancer patients. Climacteric 2011, 14, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Witherby, S.; Johnson, J.; Demers, L.; Mount, S.; Littenberg, B.; Maclean, C.D.; Wood, M.; Muss, H. Topical testosterone for breast cancer patients with vaginal atrophy related to aromatase inhibitors: A phase I/II study. Oncologist 2011, 16, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.; Larkin, L.C.; Stuenkel, C.A.; Bachmann, G.A.; Chism, L.A.; Kagan, R.; Kaunitz, A.M.; Krychman, M.L.; Parish, S.J.; Partridge, A.H.; et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: Consensus recommendations from The North American Menopause Society and The International Society for the Study of Women’s Sexual Health. Menopause 2018, 25, 596–608. [Google Scholar] [CrossRef]
- Nappi, R.E.; Palacios, S.; Bruyniks, N.; Particco, M.; Panay, N. The European Vulvovaginal Epidemiological Survey (EVES). Impact of history of breast cancer on prevalence, symptoms, sexual function and quality of life related to vulvovaginal atrophy. Gynecol. Endocrinol. 2021, 37, 78–82. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, B.; Navarro-Brazález, B.; Arranz-Martín, B.; Sánchez-Méndez, Ó.; de la Rosa-Díaz, I.; Torres-Lacomba, M. The Female Sexual Function Index: Transculturally Adaptation and Psychometric Validation in Spanish Women. Int. J. Environ. Res. Public Health 2020, 17, 994. [Google Scholar] [CrossRef]
- Kingsberg, S.A. The impact of aging on sexual function in women and their partners. Arch. Sex. Behav. 2002, 31, 431–437. [Google Scholar] [CrossRef]
- Heinemann, J.; Atallah, S.; Rosenbaum, T. The Impact of Culture and Ethnicity on Sexuality and Sexual Function. Curr. Sex. Health Rep. 2016, 8, 144–150. [Google Scholar] [CrossRef]
- Bober, S.L.; Chevalier, L.L. Culture and Sexual Medicine: A Road Map for Clinical Inquiry and Practice. J. Sex. Med. 2021, 18, 1475–1478. [Google Scholar] [CrossRef]
- Atallah, S.; Johnson-Agbakwu, C.; Rosenbaum, T.; Abdo, C.; Byers, E.S.; Graham, C.; Nobre, P.; Wylie, K.; Brotto, L. Ethical and Sociocultural Aspects of Sexual Function and Dysfunction in Both Sexes. J. Sex. Med. 2016, 13, 591–606. [Google Scholar] [CrossRef]
- Mercier, J.; Morin, M.; Lemieux, M.C.; Reichetzer, B.; Khalifé, S.; Dumoulin, C. Pelvic floor muscles training to reduce symptoms and signs of vulvovaginal atrophy: A case study. Menopause 2016, 23, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.; Sood, R.; Kapoor, E. Genitourinary Syndrome of Menopause: Management Strategies for the Clinician. Mayo Clin. Proc. 2017, 92, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Kagan, R.; Kellogg-Spadt, S.; Parish, S.J. Practical Treatment Considerations in the Management of Genitourinary Syndrome of Menopause. Drugs Aging 2019, 36, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, P.; Erőss, B.; Izbéki, F.; Párniczky, A.; Szentesi, A. Accelerating the translational medicine cycle: The Academia Europaea pilot. Nat. Med. 2021, 27, 1317–1319. [Google Scholar] [CrossRef]
- Hegyi, P.; Petersen, O.H.; Holgate, S.; Erőss, B.; Garami, A.; Szakács, Z.; Dobszai, D.; Balaskó, M.; Kemény, L.; Peng, S.; et al. Academia Europaea Position Paper on Translational Medicine: The Cycle Model for Translating Scientific Results into Community Benefits. J. Clin. Med. 2020, 9, 1532. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Author (Year) | Country | Study Type | Included in the Meta-Analysis | Number of Patients | Age (Year, Mean) | Intervention | Control | Main Outcomes |
|---|---|---|---|---|---|---|---|---|
| Advani (2017) [7] | USA | randomized controlled trial | No | 57 | 59 | vaginal moisturizer | no therapy | FSFI, MSIQ, FSDS-R, menopausal symptom scale |
| Areas (2019) [8] | Brazil | open, prospective, therapeutic intervention study | Yes | 24 | 53.7 | Er:YAG laser | - | VHI |
| Becorpi (2018) [9] | Italy | clinical prospective study | Yes | 20 | 58.2 | CO2 laser | - | VHI, FSFI, FSDS-R |
| Biglia (2010) [10] | Italy | randomized double-blind study | No | 26 | NR | Estriol cream or Estadiol tablets | Moisturizer | VSS, PFSF, VHI, KI |
| Carter (2020) [34] | USA | single-arm, prospective longitudinal trial | No | 101 | 55 | HLA-based vaginal moisturizing | - | VAS, VuAS, FSFI, MSCL |
| Chatsiproios (2019) [35] | Germany | prospective, multicenter, observational study | No | 117 | 52 | Oil-in-water emulsion | - | Subjective symptoms |
| Dahir (2014) [36] | USA | single-arm interventional study | No | 12 | 59.67 | Testosterone vaginal cream | - | FSFI |
| Davis (2018) [11] | Australia | Double-blind, randomized, placebo-controlled trial | No | 44 | 56.4 | Testosterone vaginal cream | Placebo cream | FSFI, FSDS-R |
| Dew (2003) [37] | Australia | Cohort study | No | 1472 | NR | Estriol cream or estradiol tablets | Other subjects | Menopausal symptoms |
| Donders (2014) [12] | Belgium, Germany | Open-label bicentric phase I pharmacokinetic study | No | 16 | 57 | Estriol and Lactobacillus vaginal tablet | - | Hormone level, Vaginal pH, vaginal symptoms |
| Gambacciani (2015) [14] | Italy | prospective, cohort study | No | 45 | NR | Er:YAG or Vaginal Erbium laser | - | VHI, Subjective symptoms |
| Gambacciani (2016) [13] | Italy | prospective, interventional study | Yes | 37 | 50.8 | Er:YAG laser | - | VHI |
| Goetsch (2014) [38] | USA | randomized, double-blind, controlled study | No | 49 | 55.6 | Topical lidocaine | normal saline | Subjective symptoms |
| Gold (2023) [15] | Austria | randomized, controlled trial | No | 43 | 54 | Er:YAG laser | hyaluronic acid suppositories | VHI, quality of life, sexual health |
| Hersant (2018) [16] | France | phase II clinical trial | No | 20 | 60.8 | platelet concentrate combined with hyaluronic acid | - | VHI, FSD |
| Hersant (2020) [17] | France | prospective monocentric study | Yes | 20 | 56.1 | CO2 laser | - | VHI, FSD |
| Hickey (2016) [39] | Australia | randomized, double-blind, crossover trial | No | 38 | 53.1 | silicone-based gel | water-based gel | sexual discomfort |
| Hirschberg (2020) [18] | Spain | phase II, randomized, double-blind, placebo-controlled trial | No | 69 | 59 | Estriol vaginal gel | Placebo | FSFI |
| Juliato (2016) [19] | Brazil | randomized clinical trial | No | 52 | NR | polyacrylic acid | lubricant | FSFI |
| Juraskova (2013) [40] | Australia | Phase I/II Study | No | 25 | 51 | Olive Oil, Vaginal Exercise, and Moisturizer | - | Sexual Activity Questionnaire Satisfaction subscale of the Female Sexual Function Index |
| Keshavarzi (2019) [20] | Iran | triple-blind, controlled, randomized clinical trial | No | 32 | NR | D and E vitamin suppositories | Placebo | VMI, vaginal pH |
| Lee (2011) [21] | South Korea | randomized, double-blind, placebo-controlled study | No | 86 | NR | pH balanced gel | placebo | subjective symptoms, VHI, VMI |
| Loprinzi (1997) [41] | USA | double-blind, crossover, randomized clinical trial | No | 45 | NR | vaginal moisturizer | placebo | VHI, subjective symptoms |
| Melisko (2016) [42] | USA | randomized clinical trial | No | 76 | 56 | Intravaginal testosterone cream | Estradiol vaginal ring | Sexual Satisfaction |
| Mension (2023) [27] | Spain | randomized clinical trial | No | 72 | 52.6 | CO2 laser therapy | Sham laser therapy | FSFI, vaginal pH, Vaginal Health Index, quality of life, body image |
| Mothes (2018) [22] | Germany | retrospective, single-center cohort study | Yes | 16 | 71 | Er:YAG | - | VHI |
| Pearson (2019) [23] | Australia | prospective interventional study | Yes | 26 | 56 | CO2 laser | - | FSFI, VVA symptoms |
| Pfeiler (2011) [43] | Austria | prospective study | No | 10 | 65 | Vaginal estriol | - | Vaginal symptoms |
| Pieralli (2016) [24] | Italy | prospective descriptive cohort study | Yes | 50 | 53.3 | CO2 laser | - | VHI, symptoms |
| Quick (2021) [25] | USA | multicenter randomized controlled trial (RCT) | No | 18 | 56.3 | CO2 laser | - | symptoms, FSFI, UD6 score |
| Salvatore (2021) [26] | Italy, Greece | prospective cohort study | Yes | 40 | 57.6 | CO2 laser | - | VHI, VAS, FSFI |
| Witherby (2011) [44] | USA | phase I/II study | No | 21 | NR | Testosterone cream | - | Symptoms, VMI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lőczi, L.L.; Vleskó, G.; Éliás, M.; Turan, C.; Kajtár, P.; Tóth, R.; Sipos, M.; Nagy, R.; Hegyi, P.; Ács, N.; et al. Effect of Vaginal Laser and Topical Therapies on Vulvovaginal Atrophy Symptoms in Breast Cancer Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 6131. https://doi.org/10.3390/jcm13206131
Lőczi LL, Vleskó G, Éliás M, Turan C, Kajtár P, Tóth R, Sipos M, Nagy R, Hegyi P, Ács N, et al. Effect of Vaginal Laser and Topical Therapies on Vulvovaginal Atrophy Symptoms in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(20):6131. https://doi.org/10.3390/jcm13206131
Chicago/Turabian StyleLőczi, Lotti Lúcia, Gábor Vleskó, Máté Éliás, Caner Turan, Panna Kajtár, Réka Tóth, Miklós Sipos, Rita Nagy, Péter Hegyi, Nándor Ács, and et al. 2024. "Effect of Vaginal Laser and Topical Therapies on Vulvovaginal Atrophy Symptoms in Breast Cancer Patients: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 20: 6131. https://doi.org/10.3390/jcm13206131
APA StyleLőczi, L. L., Vleskó, G., Éliás, M., Turan, C., Kajtár, P., Tóth, R., Sipos, M., Nagy, R., Hegyi, P., Ács, N., Várbíró, S., & Keszthelyi, M. (2024). Effect of Vaginal Laser and Topical Therapies on Vulvovaginal Atrophy Symptoms in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(20), 6131. https://doi.org/10.3390/jcm13206131