Pulmonary Embolism Management Audit and Machine Learning Analysis of Delayed Anticoagulation in a Swiss Teaching Hospital
Abstract
1. Introduction
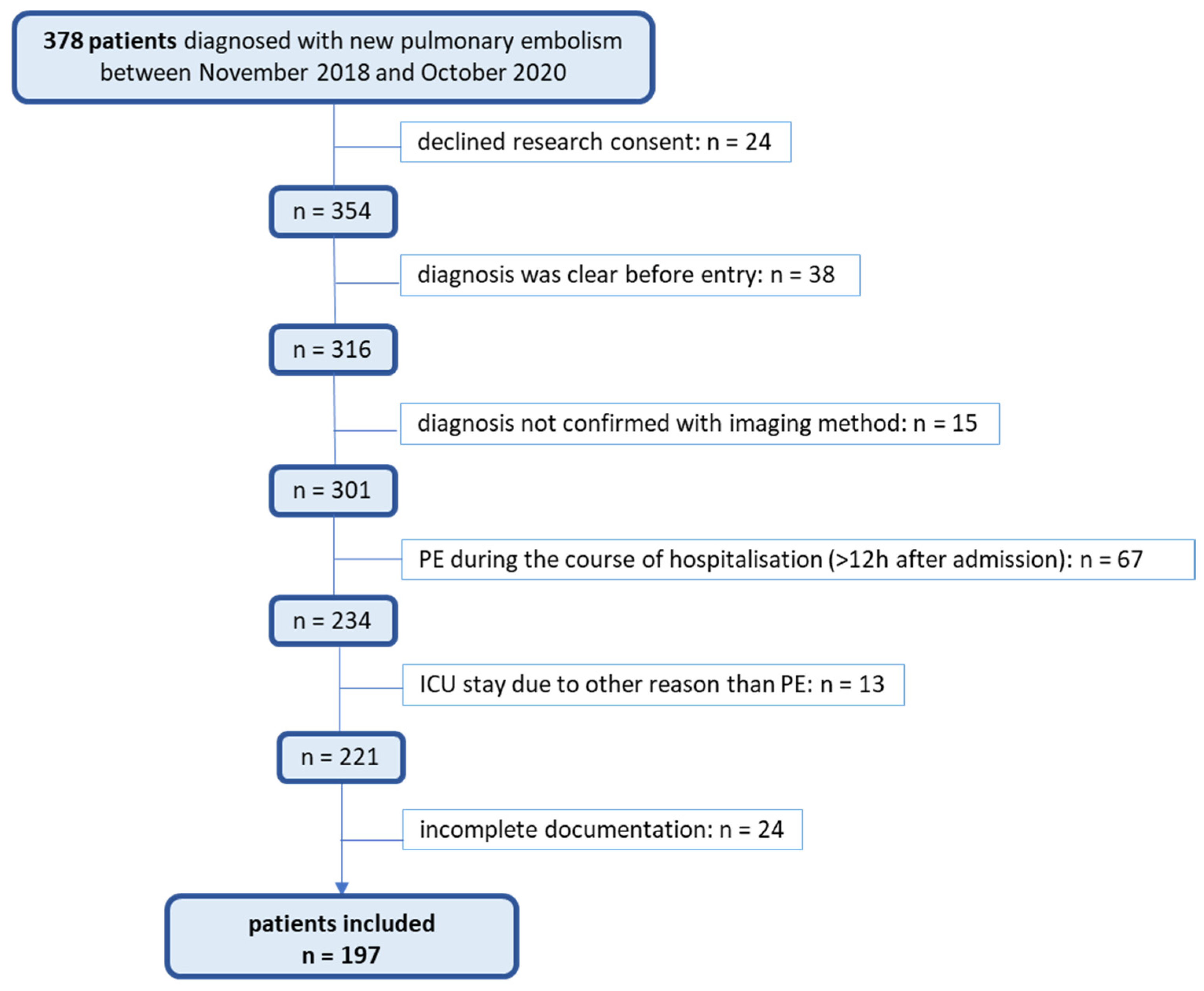
2. Materials and Methods
2.1. Design and Setting
2.2. Study Population
2.3. Data Collection and Analysis
Data Collection and Analysis
3. Results
3.1. Anamnesis, Clinical Presentation, and Examination
3.2. Pretest Probability and Risk Assessment
3.3. Diagnostic Workup
3.4. Treatments
3.5. Hospitalization and Follow Up
3.6. Factors Associated with Delayed Initiation of Anticoagulation (LASSO Analysis)
4. Discussion
4.1. Anamnesis, Clinical Presentation, and Examination
4.2. Pretest Probability and Risk Assessment
4.3. Diagnostic Workup
4.4. Treatments
4.5. Hospitalization and Follow-Up
4.6. Factors Associated with Delayed Initiation of Anticoagulation (LASSO Analysis)
4.7. Strength, Limitations, and Further Research
5. Conclusions
- Identifying and documenting risk factors, family history, and signs of DVT in medical records when PE is suspected.
- Evaluating the pretest probability for suspected PE patients, measuring D-dimers only when the probability is low, and using age-adjusted cut-offs to improve the testing efficiency. For high-pretest-probability patients, direct CTPA should be considered.
- Documenting ECG findings comprehensively in medical records to maximize the benefit of ECG recordings.
- Establishing clear criteria for ABG testing in the ED, limiting its use to patients with respiratory symptoms to save resources.
- Using PESI or sPESI scores for risk assessments; measuring troponin and pro-BNP levels in intermediate-risk patients for further classification.
- Avoiding bed rest for patients with acute PE.
- Performing leg ultrasonography only in patients with leg symptoms, as additional asymptomatic DVT diagnoses have limited impact on treatment decisions.
- Identifying and documenting identifiable risk factors for PE, which is crucial for treatment planning.
- Defining the minimal duration of anticoagulation treatment in discharge reports to prevent under- or overtreatment.
- Planning follow-up examinations, such as cancer screening and thrombophilia testing, with careful consideration of their benefits.
- Encouraging physicians to use anticoagulation loading doses more readily in patients with a high suspicion of PE and a modest bleeding risk.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.E.; Angchaisuksiri, P.; Blanco, A.N.; Buller, H.; Gallus, A.; Hunt, B.J.; Hylek, E.M.; Kakkar, A.; Konstantinides, S.V.; McCumber, M.; et al. Thrombosis: A major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.T.; Agnelli, G.; Anderson, F.A.; Arcelus, J.I.; Bergqvist, D.; Brecht, J.G.; Greer, I.A.; Heit, J.A.; Hutchinson, J.L.; Kakkar, A.K.; et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. J. Thromb. Haemost. 2007, 98, 756–764. [Google Scholar]
- BFS Bundesamt für Statistik. Medizinische Statistik der Krankenhäuser: Anzahl Fälle und durchschnittliche Aufenthaltsdauer (DAD) nach Altersklasse und Diagnosekode. 2019 (su-d-14.04.01.02-ICD-zp-2018). Available online: https://www.bfs.admin.ch/bfs/de/home/statistiken/gesundheit/gesundheitswesen/spitaeler/patienten-hospitalisierungen.assetdetail.10787003.html (accessed on 1 September 2024).
- Silverstein, M.D.; Heit, J.A.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch. Intern. Med. 1998, 158, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Beckman, M.G.; Hooper, W.C.; Critchley, S.E.; Ortel, T.L. Venous thromboembolism: A public health concern. Am. J. Prev. Med. 2010, 38, S495–S501. [Google Scholar] [CrossRef]
- Giordano, N.J.; Jansson, P.S.; Young, M.N.; Hagan, K.A.; Kabrhel, C. Epidemiology, Pathophysiology, Stratification, and Natural History of Pulmonary Embolism. Tech. Vasc. Interv. Radiol. 2017, 20, 135–140. [Google Scholar] [CrossRef]
- Tapson, V.F. Acute pulmonary embolism. N. Engl. J. Med. 2008, 358, 1037–1052. [Google Scholar] [CrossRef]
- Righini, M.; Le Gal, G.; Aujesky, D.; Roy, P.-M.; Sanchez, O.; Verschuren, F.; Rutschmann, O.; Nonent, M.; Cornuz, J.; Thys, F.; et al. Diagnosis of pulmonary embolism by multidetector CT alone or combined with venous ultrasonography of the leg: A randomised non-inferiority trial. Lancet 2008, 371, 1343–1352. [Google Scholar] [CrossRef]
- Squizzato, A.; Luciani, D.; Rubboli, A.; Di Gennaro, L.; Landolfi, R.; de Luca, C.; Porro, F.; Moia, M.; Testa, S.; Imberti, D.; et al. Differential diagnosis of pulmonary embolism in outpatients with non-specific cardiopulmonary symptoms. Intern. Emerg. Med. 2013, 8, 695–702. [Google Scholar] [CrossRef]
- Reamy, B.V.; Williams, P.M.; Odom, M.R. Pleuritic Chest Pain: Sorting Through the Differential Diagnosis. Am. Fam. Physician 2017, 96, 306–312. [Google Scholar]
- Thomas, S.E.; Weinberg, I.; Schainfeld, R.M.; Rosenfield, K.; Parmar, G.M. Diagnosis of Pulmonary Embolism: A Review of Evidence-Based Approaches. J. Clin. Med. 2024, 13, 3722. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Geske, J.B.; Maguire, J.M.; Zane, N.A.; Carter, R.E.; Morgenthaler, T.I. Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest 2010, 137, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.-J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, J.S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Ortel, T.L.; Neumann, I.; Ageno, W.; Beyth, R.; Clark, N.P.; Cuker, A.; Hutten, B.A.; Jaff, M.R.; Manja, V.; Schulman, S.; et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020, 4, 4693–4738. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Venous thromboembolic diseases: Diagnosis, management and thrombophilia testing 2020, NG158. J. Thromb. Haemost. 2020, 120, 1143–1146. [Google Scholar]
- Deutsche Gesellschaft für Angiologie-Gesellschaft für Gefäßmedizin S2k-. Leitlinie zur Diagnostik und Therapie der Venenthrombose und der Lungenembolie. Verabschiedet am 2015, 10. Available online: https://register.awmf.org/assets/guidelines/065-002l_S2k_Venenthrombose-Lungenembolie_2023-09.pdf (accessed on 1 September 2024).
- Rosemann, A. MediX Guideline über die Thromboembolie; Verein MediX: Zürich, Switzerland, 2022. [Google Scholar]
- University Hospital Basel. MedStandards. Available online: https://www.medstandards.ch/ (accessed on 1 September 2024).
- Suchina, J.; Lüthi-Corridori, G.; Jaun, F.; Leuppi, J.D.; Boesing, M. Diagnosis and Treatment of Acute Heart Failure: A Retrospective Observational Study and Medical Audit. J. Clin. Med. 2024, 13, 5951. [Google Scholar] [CrossRef]
- Schnyder, D.; Lüthi-Corridori, G.; Leuppi-Taegtmeyer, A.B.; Boesing, M.; Geigy, N.; Leuppi, J.D. Audit of Asthma Exacerbation Management in a Swiss General Hospital. Respiration 2023, 102, 12–24. [Google Scholar] [CrossRef]
- Mangold, V.; Boesing, M.; Berset, C.; Bridevaux, P.-O.; Geiser, T.; Joos Zellweger, L.; Kohler, M.; Lüthi-Corridori, G.; Maier, S.; Miedinger, D.; et al. Adherence to the GOLD Guidelines in Primary Care: Data from the Swiss COPD Cohort. J. Clin. Med. 2023, 12, 6636. [Google Scholar] [CrossRef] [PubMed]
- Boesing, M.; Ottensarendt, N.; Lüthi-Corridori, G.; Leuppi, J.D. The Management of Acute Exacerbations in COPD: A Retrospective Observational Study and Clinical Audit. J. Clin. Med. 2023, 13, 19. [Google Scholar] [CrossRef]
- Isaak, J.; Boesing, M.; Potasso, L.; Lenherr, C.; Luethi-Corridori, G.; Leuppi, J.D.; Leuppi-Taegtmeyer, A.B. Diagnostic Workup and Outcome in Patients with Profound Hyponatremia. J. Clin. Med. 2023, 12, 3567. [Google Scholar] [CrossRef] [PubMed]
- Lüthi-Corridori, G.; Roth, A.I.; Boesing, M.; Jaun, F.; Tarr, P.E.; Leuppi-Taegtmeyer, A.B.; Leuppi, J.D. Diagnosis and Therapy of Community-Acquired Pneumonia in the Emergency Department: A Retrospective Observational Study and Medical Audit. J. Clin. Med. 2024, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Adelakun, G.; Boesing, M.; Mbata, M.K.; Pasha, Z.; Lüthi-Corridori, G.; Jaun, F.; Burkhalter, F.; Leuppi, J.D. Proteinuria Assessment and Therapeutic Implementation in Chronic Kidney Disease Patients-A Clinical Audit on KDIGO (“Kidney Disease: Improving Global Outcomes”) Guidelines. J. Clin. Med. 2024, 13, 5335. [Google Scholar] [CrossRef] [PubMed]
- Kanton Basellandschaft. Bevölkerungsstatistik 2020. 2021. Available online: https://www.baselland.ch/politik-und-behorden/direktionen/finanz-und-kirchendirektion/statistisches-amt/publikationen/bevoelkerung/webartikel_vom_31-03-2021_bevoelkerungsstatistik_2020 (accessed on 1 September 2024).
- Bundesamt für Gesundheit Schweiz. Kennzahlen der Schweizer Spitäler. 2019. Available online: https://spitalstatistik.bagapps.ch/data/download/kzp19_publication.pdf?v=1616491353 (accessed on 1 September 2024).
- Kowarik, A.; Templ, M. Imputation with the R Package VIM. J. Stat. Softw. 2016, 74, 1–16. [Google Scholar] [CrossRef]
- Aujesky, D.; Obrosky, D.S.; Stone, R.A.; Auble, T.E.; Perrier, A.; Cornuz, J.; Roy, P.-M.; Fine, M.J. Derivation and validation of a prognostic model for pulmonary embolism. Am. J. Respir. Crit. Care Med. 2005, 172, 1041–1046. [Google Scholar] [CrossRef]
- Vyas, V.; Sankari, A.; Goyal, A. Acute Pulmonary Embolism; StatPearls: St. Petersburg, FL, USA, 2024. [Google Scholar]
- Keller, K.; Hobohm, L.; Ebner, M.; Kresoja, K.-P.; Münzel, T.; Konstantinides, S.V.; Lankeit, M. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur. Heart J. 2020, 41, 522–529. [Google Scholar] [CrossRef]
- Jiménez, D.; de Miguel-Díez, J.; Guijarro, R.; Trujillo-Santos, J.; Otero, R.; Barba, R.; Muriel, A.; Meyer, G.; Yusen, R.D.; Monreal, M. Trends in the Management and Outcomes of Acute Pulmonary Embolism: Analysis From the RIETE Registry. J. Am. Coll. Cardiol. 2016, 67, 162–170. [Google Scholar] [CrossRef]
- Peterson, M.C.; Holbrook, J.H.; von Hales, D.; Smith, N.L.; Staker, L.V. Contributions of the history, physical examination, and laboratory investigation in making medical diagnoses. West. J. Med. 1992, 156, 163–165. [Google Scholar] [CrossRef]
- Matusov, Y.; Tapson, V. History and Physical Examination in Pulmonary Embolism. In PERT Consortium Handbook of Pulmonary Embolism: Research, Care, and Management; Grodzin, C.J., Merli, G.J., Ross, C.B., Rosovsky, R., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; pp. 1–10. ISBN 978-3-030-70904-4. [Google Scholar]
- Kline, J.A.; Mitchell, A.M.; Kabrhel, C.; Richman, P.B.; Courtney, D.M. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J. Thromb. Haemost. 2004, 2, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Penaloza, A.; Soulié, C.; Moumneh, T.; Delmez, Q.; Ghuysen, A.; El Kouri, D.; Brice, C.; Marjanovic, N.S.; Bouget, J.; Moustafa, F.; et al. Pulmonary embolism rule-out criteria (PERC) rule in European patients with low implicit clinical probability (PERCEPIC): A multicentre, prospective, observational study. Lancet Haematol. 2017, 4, e615–e621. [Google Scholar] [CrossRef] [PubMed]
- Hugli, O.; Righini, M.; Le Gal, G.; Roy, P.-M.; Sanchez, O.; Verschuren, F.; Meyer, G.; Bounameaux, H.; Aujesky, D. The pulmonary embolism rule-out criteria (PERC) rule does not safely exclude pulmonary embolism. J. Thromb. Haemost. 2011, 9, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J. Improving the diagnosis of pulmonary embolism in the emergency department. BMJ Qual. Improv. Programme 2015, 4, u208698–w4222. [Google Scholar] [CrossRef]
- Lüthi-Corridori, G.; Giezendanner, S.; Kueng, C.; Boesing, M.; Leuppi-Taegtmeyer, A.B.; Mbata, M.K.; Schuetz, P.; Leuppi, J.D. Risk factors for hospital outcomes in pulmonary embolism: A retrospective cohort study. Front. Med. 2023, 10, 1120977. [Google Scholar] [CrossRef]
- Crawford, F.; Andras, A.; Welch, K.; Sheares, K.; Keeling, D.; Chappell, F.M. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst. Rev. 2016, 2016, CD010864. [Google Scholar] [CrossRef]
- Righini, M.; van Es, J.; den Exter, P.L.; Roy, P.-M.; Verschuren, F.; Ghuysen, A.; Rutschmann, O.T.; Sanchez, O.; Jaffrelot, M.; Trinh-Duc, A.; et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: The ADJUST-PE study. JAMA 2014, 311, 1117–1124. [Google Scholar] [CrossRef]
- Roumen-Klappe, E.M.; den Heijer, M.; van Uum, S.H.M.; van der Ven-Jongekrijg, J.; van der Graaf, F.; Wollersheim, H. Inflammatory response in the acute phase of deep vein thrombosis. J. Vasc. Surg. 2002, 35, 701–706. [Google Scholar] [CrossRef]
- Kamphuisen, P.W.; Eikenboom, J.C.; Vos, H.L.; Pablo, R.; Sturk, A.; Bertina, R.M.; Rosendaal, F.R. Increased levels of factor VIII and fibrinogen in patients with venous thrombosis are not caused by acute phase reactions. Thromb. Haemost. 1999, 81, 680–683. [Google Scholar] [CrossRef]
- Steeghs, N.; Goekoop, R.J.; Niessen, R.W.L.M.; Jonkers, G.J.P.M.; Dik, H.; Huisman, M.V. C-reactive protein and D-dimer with clinical probability score in the exclusion of pulmonary embolism. Br. J. Haematol. 2005, 130, 614–619. [Google Scholar] [CrossRef]
- Aujesky, D.; Hayoz, D.; Yersin, B.; Perrier, A.; Barghouth, G.; Schnyder, P.; Bischof-Delaloye, A.; Cornuz, J. Exclusion of pulmonary embolism using C-reactive protein and D-dimer. A prospective comparison. J. Thromb. Haemost. 2003, 90, 1198–1203. [Google Scholar] [CrossRef]
- Grimnes, G.; Isaksen, T.; Tichelaar, Y.I.G.V.; Brox, J.; Brækkan, S.K.; Hansen, J.-B. C-reactive protein and risk of venous thromboembolism: Results from a population-based case-crossover study. Haematologica 2018, 103, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Geske, J.B.; Morgenthaler, T.I. Risk factors associated with delayed diagnosis of acute pulmonary embolism. J. Emerg. Med. 2012, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, L.; Adams, D.M.; Evans, R.S.; Lloyd, J.F.; Stevens, S.M.; Woller, S.C.; Bledsoe, J.R.; Aston, V.T.; Wilson, E.L.; Elliott, C.G. Preemptive Anticoagulation in Patients With a High Pretest Probability of Pulmonary Embolism: Are Guidelines Followed? Chest 2018, 153, 1153–1159. [Google Scholar] [CrossRef]
- Rodger, M.A.; Carrier, M.; Jones, G.N.; Rasuli, P.; Raymond, F.; Djunaedi, H.; Wells, P.S. Diagnostic value of arterial blood gas measurement in suspected pulmonary embolism. Am. J. Respir. Crit. Care Med. 2000, 162, 2105–2108. [Google Scholar] [CrossRef]
- Buess, M.; Schilter, D.; Schneider, T.; Maurer, M.; Borer, H.; Thurnheer, R.; Köhler, E.; Junker, L.; Jahn, K.; Grob, M.; et al. Treatment of COPD Exacerbation in Switzerland: Results and Recommendations of the European COPD Audit. Respiration 2017, 94, 355–365. [Google Scholar] [CrossRef]
- Hirmerova, J.; Seidlerova, J.; Chudacek, Z. The Prevalence of Concomitant Deep Vein Thrombosis, Symptomatic or Asymptomatic, Proximal or Distal, in Patients With Symptomatic Pulmonary Embolism. Clin. Appl. Thromb. Hemost. 2018, 24, 1352–1357. [Google Scholar] [CrossRef]
- Kahn, S.R.; Shapiro, S.; Wells, P.S.; Rodger, M.A.; Kovacs, M.J.; Anderson, D.R.; Tagalakis, V.; Houweling, A.H.; Ducruet, T.; Holcroft, C.; et al. Compression stockings to prevent post-thrombotic syndrome: A randomised placebo-controlled trial. Lancet 2014, 383, 880–888. [Google Scholar] [CrossRef]
- Goldhaber, S.Z.; Visani, L.; de Rosa, M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999, 353, 1386–1389. [Google Scholar] [CrossRef]
- Stein, P.D.; Beemath, A.; Matta, F.; Weg, J.G.; Yusen, R.D.; Hales, C.A.; Hull, R.D.; Leeper, K.V.; Sostman, H.D.; Tapson, V.F.; et al. Clinical characteristics of patients with acute pulmonary embolism: Data from PIOPED II. Am. J. Med. 2007, 120, 871–879. [Google Scholar] [CrossRef]
- Yoo, H.H.; Nunes-Nogueira, V.S.; Fortes Villas Boas, P.J. Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database Syst. Rev. 2020, 2, CD010222. [Google Scholar] [CrossRef] [PubMed]
- Harter, K.; Levine, M.; Henderson, S.O. Anticoagulation drug therapy: A review. West. J. Emerg. Med. 2015, 16, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, M. Warfarin: Almost 60 years old and still causing problems. Br. J. Clin. Pharmacol. 2006, 62, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Vanassche, T.; Verhamme, P. Rivaroxaban for the treatment of pulmonary embolism. Adv. Ther. 2013, 30, 589–606. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, N.; Martins, E.; Mouly, S.; Weber, S.; Meune, C. A meta-analysis of bed rest versus early ambulation in the management of pulmonary embolism, deep vein thrombosis, or both. Int. J. Cardiol. 2009, 137, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.; Yeoh, S.E.; Broderick, C.; Stansby, G.; Agarwal, R. Effect of testing for cancer on cancer- or venous thromboembolism (VTE)-related mortality and morbidity in people with unprovoked VTE. Cochrane Database Syst. Rev. 2018, 11, CD010837. [Google Scholar] [CrossRef]
- O’Rourke, R.W.; Diggs, B.S.; Spight, D.H.; Robinson, J.; Elder, K.A.; Andrus, J.; Thomas, C.R.; Hunter, J.G.; Jobe, B.A. Psychiatric illness delays diagnosis of esophageal cancer. Dis. Esophagus 2008, 21, 416–421. [Google Scholar] [CrossRef]
- Crea, F.; Battipaglia, I.; Andreotti, F. Sex differences in mechanisms, presentation and management of ischaemic heart disease. Atherosclerosis 2015, 241, 157–168. [Google Scholar] [CrossRef]
- Chapman, K.R.; Tashkin, D.P.; Pye, D.J. Gender bias in the diagnosis of COPD. Chest 2001, 119, 1691–1695. [Google Scholar] [CrossRef]
- Bach, A.G.; Bandzauner, R.; Nansalmaa, B.; Schurig, N.; Meyer, H.J.; Taute, B.-M.; Wienke, A.; Surov, A. Timing of pulmonary embolism diagnosis in the emergency department. Thromb. Res. 2016, 137, 53–57. [Google Scholar] [CrossRef]
- Al Dandan, O.; Hassan, A.; Alnasr, A.; Al Gadeeb, M.; AbuAlola, H.; Alshahwan, S.; Al Shammari, M.; Alzaki, A. The use of clinical decision rules for pulmonary embolism in the emergency department: A retrospective study. Int. J. Emerg. Med. 2020, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Caprini, J.A.; Tapson, V.F.; Hyers, T.M.; Waldo, A.L.; Wittkowsky, A.K.; Friedman, R.; Colgan, K.J.; Shillington, A.C. Treatment of venous thromboembolism: Adherence to guidelines and impact of physician knowledge, attitudes, and beliefs. J. Vasc. Surg. 2005, 42, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Barnett, M.; Zhou, L.; Woulfe, T.; Rolfe-Vyson, V.; Rowland, V.; Simpson, D.; Merriman, E. Management and outcomes of single subsegmental pulmonary embolus: A retrospective audit at North Shore Hospital, New Zealand. Intern. Med. J. 2014, 44, 872–876. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | All (n = 197) | Missing, n (%) |
|---|---|---|
| Age at diagnosis (years), mean (range) | 70 (19–96) | -- |
| Gender (male) | 90 (46%) | -- |
| Body mass index (BMI), mean (SD) (kg/m2) | 28.6 (±6.40) | 21 (10.6%) |
| Obesity (BMI ≥ 30) | 72 (38%) | 7 (3.5%) |
| Smoking Status | 111 (56.4%) | 86 (43.6%) |
| Lifelong non-smoker | 48 (43%) | -- |
| Current smoker | 28 (25%) | -- |
| Former smoker | 35 (32%) | -- |
| Comorbidities | 178 (90%) | -- |
| Active cancer | 12 (6%) | -- |
| Cardiovascular disease | 69 (35%) | -- |
| Chronic lung disease | 35 (18%) | -- |
| Hypertension | 98 (50%) | -- |
| Mental health disorder | 49 (25%) | -- |
| Severe renal insufficiency | 21 (11%) | -- |
| Medical History-Taking | All (n = 197) | Missing/Not Performed, n (%) |
|---|---|---|
| Risk factor anamnesis asked | 115 (58%) | -- |
| Family history for VTE asked | 85 (43%) | -- |
| Smoking status cleared | 111 (56%) | -- |
| B-symptoms asked | 41 (21%) | -- |
| Clinical examination | ||
| Respiratory rate measured | 169 (85.7%) | 28 (14.2%) |
| DVT symptoms checked | 105 (53%) | 92 (46.7%) |
| Pressure pain in the calf | 20 (20%) | 98 (49.7%) |
| Circumference difference | 31 (30%) | 92 (46.7%) |
| Jugular vein assessed | 159 (81%) | 38 (19.3) |
| Jugular vein distention | 16 (10%) | 38 (19.3) |
| Lung auscultation | 197 (100%) | -- |
| Rales | 77 (39%) | -- |
| Initial suspicion diagnosis PE | 107 (54%) | -- |
| Electrocardiography assessments | ||
| ECG performed | 193 (98%) | 4 (2%) |
| ECG analysed in discharge report | 146 (76%) | 4 (2%) |
| PE specific abnormalities described | 25 (17%) |
| Pretest Probability | All (n = 197) |
|---|---|
| Pretest probability calculated (Geneva or Wells score) | 5 (3%) |
| Geneva score calculated retrospectively: | |
| Low pretest probability (0–2 P) | 101 (51%) |
| High pretest probability (≥3 P) | 96 (49%) |
| Risk assessment with PESI | |
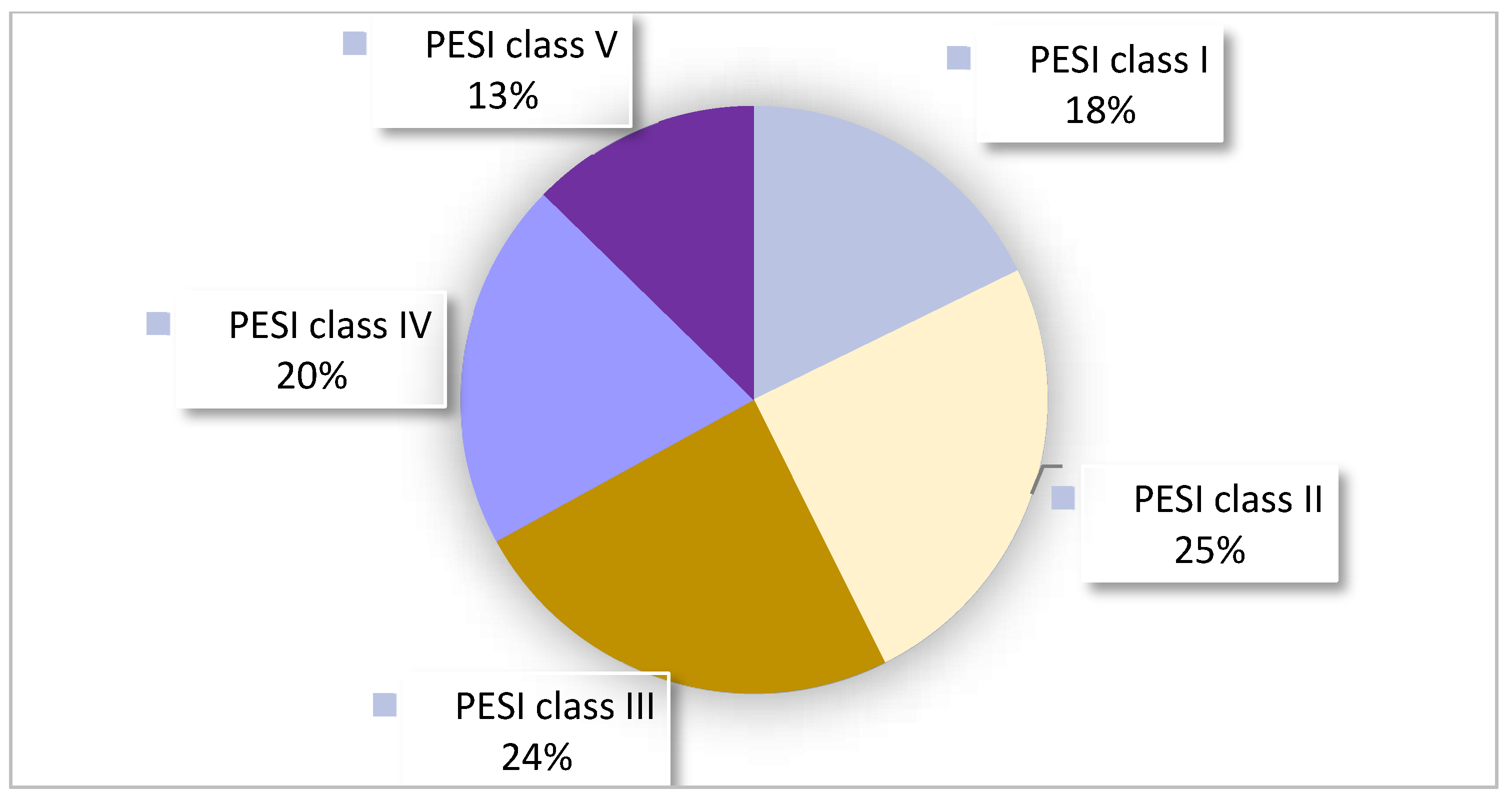
| PESI score calculated and documented | 41 (21%) |
| Retrospectively differently calculated than in the report | 19 (46%) |
| PE-Specific Laboratory | All (n = 197) |
|---|---|
| Diagnostic according to guidelines | 113 (57%) |
| D-dimers measured | 138 (70%) |
| D-dimers unnecessarily measured | 59 (70%) |
| D-dimers wrongly not measured | 21 (25%) |
| Troponin T hs measured | 129 (65%) |
| NT-proBNP measured | 150 (76%) |
| Arterial blood gas analysis performed in the ED | 68 (35%) |
| No ABG despite respiratory problems | 97 (62%) |
| Performed diagnostic imaging | |
| Chest radiography | 46 (23%) |
| Computed tomography pulmonary angiography | 189 (96%) |
| Right ventricular stress signs | 46 (24%) |
| Time between admission and CT, mean (SD) in minutes | 162 (±110) |
| Scintigraphy | 6 (3%) |
| Diagnosis only by compression ultrasound | 4 (2%) |
| Transthoracic echocardiogram | 98 (50%) |
| Ultrasound leg | |
| Ultrasound leg performed | 113 (57%) |
| Thrombosis found | 83 (73%) |
| CUS performed | 113 (57%) |
| CUS because of symptoms | 51 (45%) |
| CUS despite legs not examined | 43 (38%) |
| No CUS despite symptoms or signs | 10 (12%) |
| Anticoagulant Treatment | All (n = 197) |
|---|---|
| Time between entry and anticoagulation, mean (SD) in minutes | 237 (±150) |
| Loading before CT | 31 (16%) |
| Lysis therapy | 13 (7%) |
| Systemic lysis | 3 (23%) |
| Local lysis (EKOS®) | 10 (77%) |
| Parenteral anticoagulation | 120 (61%) |
| Oral anticoagulation right from the beginning | 77 (39%) |
| Oral anticoagulation before discharge | 186 (94%) |
| Maximum duration anticoagulation: | (n = 187) * |
| 3 months | 42 (21%) |
| 6 months | 45 (23%) |
| 12 months | 7 (4%) |
| Lifelong | 56 (28%) |
| As long as a risk factor is present | 3 (1.5%) |
| No timeframe in the discharge report | 34 (17%) |
| Non-anticogulant treatment | (n = 197) |
| Physiotherapy | 114 (58%) |
| New inhalation therapy | 32 (16%) |
| Antibiotic therapy during hospitalisation | 67 (34%) |
| Hospitalization | All (n = 197) |
|---|---|
| Length of stay, median (range) | 6.5 (1–32) |
| Non-stop regular ward | 138 (70%) |
| Surveillance at IMC or ICU | 59 (30%) |
| IMC stay | 23 (12%) * |
| Length of IMC stay, mean (SD) in nights | 1.4 (±0.6) |
| ICU stay | 37 (19%) * |
| Length of ICU stay, mean (SD) in nights | 2.0 (±1.1) |
| Initial bed rest | 36 (18%) |
| In-hospital death | 10 (5.1%) |
| Cancer search | |
| Cancer search performed at all (recommended and planned) | 94 (48%) |
| Cancer search despite provoked PE | 19 (10%) |
| No consciously performed cancer search | 30 (29%) |
| Incidental finding of initial PE-CT control | 16 (8%) |
| Follow up | (n = 187) |
| Suggested follow-up inspection | 140 (74.9%) |
| Coagulation assessment recommended | 30 (16%) |
| Cancer search recommended | 56 (29.9%) |
| Variable | Estimate (in Min) |
|---|---|
| Time between presentation and CT scan | +88.85 |
| Jugular vein distention present in examination in the ED | +37.04 |
| Weakness or tiredness as a symptom | +23.24 |
| Entry time, morning | +9.07 |
| Rheumatic disease in medical history | +0.85 |
| Entry time, evening | +0.30 |
| BMI | −0.54 |
| Tachypnea (>20/min) as a symptom | −1.36 |
| Dyspnea as a symptom | −1.47 |
| ABG performed in the ED | −1.63 |
| Hypertension in the ED | −2.69 |
| Risk factors anamnesis performed | −5.61 |
| DVT symptoms checked in examination in the ED | −5.78 |
| New leg swelling as a symptom | −10.67 |
| Diagnostic approach was according to internal guidelines | −14.18 |
| Constant monitoring in the ED (except the imaging process) | −15.46 |
| Female sex | −15.56 |
| ICU status positive (patient did not decline an ICU stay) | −21.85 |
| PE was initially suspected as a diagnosis | −29.44 |
| Patient received loading dose before CT scan | −31.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kueng, C.; Boesing, M.; Giezendanner, S.; Leuppi, J.D.; Lüthi-Corridori, G. Pulmonary Embolism Management Audit and Machine Learning Analysis of Delayed Anticoagulation in a Swiss Teaching Hospital. J. Clin. Med. 2024, 13, 6103. https://doi.org/10.3390/jcm13206103
Kueng C, Boesing M, Giezendanner S, Leuppi JD, Lüthi-Corridori G. Pulmonary Embolism Management Audit and Machine Learning Analysis of Delayed Anticoagulation in a Swiss Teaching Hospital. Journal of Clinical Medicine. 2024; 13(20):6103. https://doi.org/10.3390/jcm13206103
Chicago/Turabian StyleKueng, Cedrine, Maria Boesing, Stéphanie Giezendanner, Jörg Daniel Leuppi, and Giorgia Lüthi-Corridori. 2024. "Pulmonary Embolism Management Audit and Machine Learning Analysis of Delayed Anticoagulation in a Swiss Teaching Hospital" Journal of Clinical Medicine 13, no. 20: 6103. https://doi.org/10.3390/jcm13206103
APA StyleKueng, C., Boesing, M., Giezendanner, S., Leuppi, J. D., & Lüthi-Corridori, G. (2024). Pulmonary Embolism Management Audit and Machine Learning Analysis of Delayed Anticoagulation in a Swiss Teaching Hospital. Journal of Clinical Medicine, 13(20), 6103. https://doi.org/10.3390/jcm13206103






