Particularities of a Cardiac Amorphous Left Ventricular Tumor in a Patient with Coronary Artery Disease—Diagnostic and Therapeutic Challenges: A Case Report and Literature Review
Abstract
1. Introduction
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reynolds, C.; Tazelaar, H.D.; Edwards, W.D. Calcified Amorphous Tumor of the Heart (Cardiac CAT). Hum. Pathol. 1997, 28, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Halder, V.; Ghosh, S.; Kumar, B.; Guha Neogi, S.; Thirunavukkarasu, B.; Bal, A. Calcified Amorphous Tumor of Left Ventricle: A Rare Cardiac Tumor. Cureus 2021, 13, e17908. [Google Scholar] [CrossRef] [PubMed]
- Ufuk, F.; Kilic, I.D. Case 315: Cardiac Calcified Amorphous Tumor. Radiology 2023, 308, e220881. [Google Scholar] [CrossRef] [PubMed]
- Kerndt, C.; Cameron, E.; Nowak, C.; Lesoski, C.; Lesoski, C.; Vishwanath, R. Echocardiography Findings in Cardiac Calcified Amorphous Tumor: A Systematic Review. J. Am. Coll. Cardiol. 2021, 77, 1339. [Google Scholar] [CrossRef]
- de Hemptinne, Q.; de Cannière, D.; Vandenbossche, J.-L.; Unger, P. Cardiac Calcified Amorphous Tumor: A Systematic Review of the Literature. Int. J. Cardiol. Heart Vasc. 2015, 7, 1–5. [Google Scholar] [CrossRef]
- Shah, A.C.; Marcoff, L.; Talati, S.; Donahue, J.; Uretsky, S.; Magovern, C.; Gillam, L.D. A Rare Beast: Cardiac Calcified Amorphous Tumor. CASE 2018, 2, 139–141. [Google Scholar] [CrossRef]
- Tsushima, S.; Maeda, T.; Nakashima, S.; Muraki, S.; Sakurada, T.; Sasaki, J.; Araki, E. Calcified Amorphous Tumor Diagnosed After Stroke:Report of a Case. Kyobu Geka 2024, 77, 316–318. [Google Scholar]
- Eizawa, S.; Morimoto, Y.; Yamada, A.; Sato, M. Multiple Calcified Amorphous Tumours of the Heart. BMJ Case Rep. 2023, 16, e254823. [Google Scholar] [CrossRef]
- Ushioda, R.; Shirasaka, T.; Kikuchi, S.; Kamiya, H.; Kanamori, T. Calcified Amorphous Tumor Located on a Severely Calcified Mitral Annulus in a Patient with Normal Renal Function. J. Surg. Case Rep. 2022, 2022, rjab608. [Google Scholar] [CrossRef]
- Endo, T.; Lei, K.; Ahsan, C. Cardiac Tumor in the Left Ventricular Outflow Tract: Atypical Presentation of Calcified Amorphous Tumor. J. Surg. Case Rep. 2022, 2022, rjac179. [Google Scholar] [CrossRef]
- Suzue, T.; Sawayama, Y.; Suzuki, T.; Nakagawa, Y. A Rapidly Growing Cardiac Calcified Amorphous Tumour Diagnosed after Coronary Artery Bypass Graft Surgery: A Case Report. Eur. Heart J. Case Rep. 2021, 5, ytab243. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, Y.; Matsuyama, H.; Shindo, A.; Matsuura, K.; Niwa, A.; Hirota, Y.; Fukuma, T.; Ito, H.; Kozuka, Y.; Tomimoto, H. Cerebral Embolism Associated with Calcified Amorphous Tumor: A Review of Cerebral Infarction Cases. Intern. Med. 2021, 60, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Hatori, K.; Mohara, J.; Shibata, S.; Murata, M.; Fukuda, N.; Hiroi, S.; Koyano, T. Cardiac Calcified Amorphous Tumor in a Patient with Lung Cancer. Gen. Thorac. Cardiovasc. Surg. Cases 2024, 3, 35. [Google Scholar] [CrossRef]
- Ghabally, M.; Shebli, B.; Alafandi, B.Z.; Allouzi, S.; Markaby, J.; Malhis, M.; Babi, A. An Ambiguous Presentation of Cardiac Calcified Amorphous Tumor in a 37-Year-Old Male. J. Surg. Case Rep. 2023, 2023, rjad397. [Google Scholar] [CrossRef]
- Odujoko, O.; Asad, M.; Bal, S.; Dodge, J.; Janjua, J. A Rare Case of Cardiac Calcified Amorphous Tumor in a 34-Year-Old Woman Diagnosed at Autopsy. Am. J. Clin. Pathol. 2023, 160, S3–S4. [Google Scholar] [CrossRef]
- Kimura, M.; Niwa, J.-I.; Ito, H.; Matsuyama, K.; Doyu, M. Multiple Cerebral Infarctions Due to Calcified Amorphous Tumor Growing Rapidly from an Antecedent Infection and Decreasing Rapidly by Detachment of Fibrin and Antithrombotic Drugs: A Case Report. BMC Neurol. 2022, 22, 391. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Tsujimoto, S.; Yamamoto, M. Calcified Amorphous Tumor in the Aortic Valve Identified by Computed Tomography. J. Thorac. Cardiovasc. Surg. 2020, 159, e161–e163. [Google Scholar] [CrossRef]
- Yamanaka, T.; Fukatsu, T.; Uchimuro, T.; Takanashi, S. Cardiac calcified amorphous tumour associated with multiple myeloma. BMJ Case Rep. CP 2020, 13, e233679. [Google Scholar] [CrossRef]
- Okazaki, A.; Oyama, Y.; Hosokawa, N.; Ban, H.; Miyaji, Y.; Moody, S. The first report of calcified amorphous tumor associated with infective endocarditis: A case report and review of literature. Am. J. Case Rep. 2020, 21, e922960-1. [Google Scholar] [CrossRef]
- Formelli, B.; Farina, A.; Pescini, F.; Palumbo, V.; Grazia D’Alfonso, M.; Oddo, A.; Mori, F.; Poggesi, A. Cardiac Calcified Amorphous Tumor as a Rare Cause of Ischemic Stroke: Clinical Case. Circ. Cardiovasc. Imaging 2020, 13, e009623. [Google Scholar] [CrossRef]
- Tudoran, M.; Tudoran, C.; Ciocarlie, T.; Pop, G.N.; Berceanu-Vaduva, M.M.; Velimirovici, D.E.; Ahmed, A.A.; Berceanu-Vaduva, D.M. Aspects of Heart Failure in Patients with Ischemic Heart Disease after Percutaneous Coronary Revascularization with Polymer-Coated Drug-Eluting Stents versus Bare-Metal Stents. Mater. Plast. 2019, 56, 37–40. [Google Scholar] [CrossRef]
- Takahashi, Y.; Inaba, Y.; Tsuchiya, H.; Minegishi, S.; Niino, T.; Endo, H.; Kubota, H. Clinical Features of Cardiac Calcified Amorphous Tumor: A Narrative Review. Cardiol. Rev. 2024. [Google Scholar] [CrossRef] [PubMed]
- Perone, F.; Bernardi, M.; Redheuil, A.; Mafrica, D.; Conte, E.; Spadafora, L.; Ecarnot, F.; Tokgozoglu, L.; Santos-Gallego, C.G.; Kaiser, S.E.; et al. Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. J. Clin. Med. 2023, 12, 5563. [Google Scholar] [CrossRef] [PubMed]
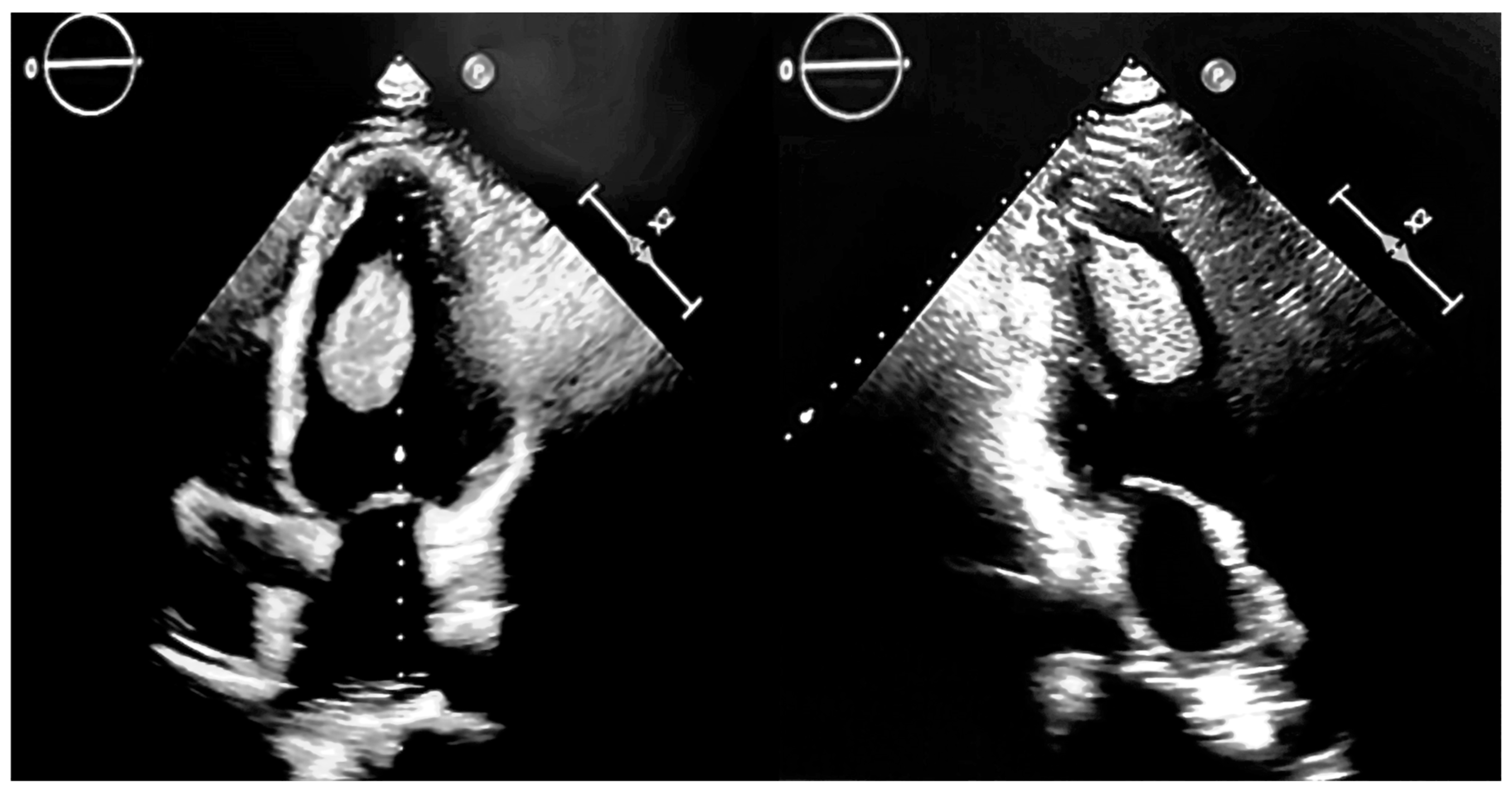
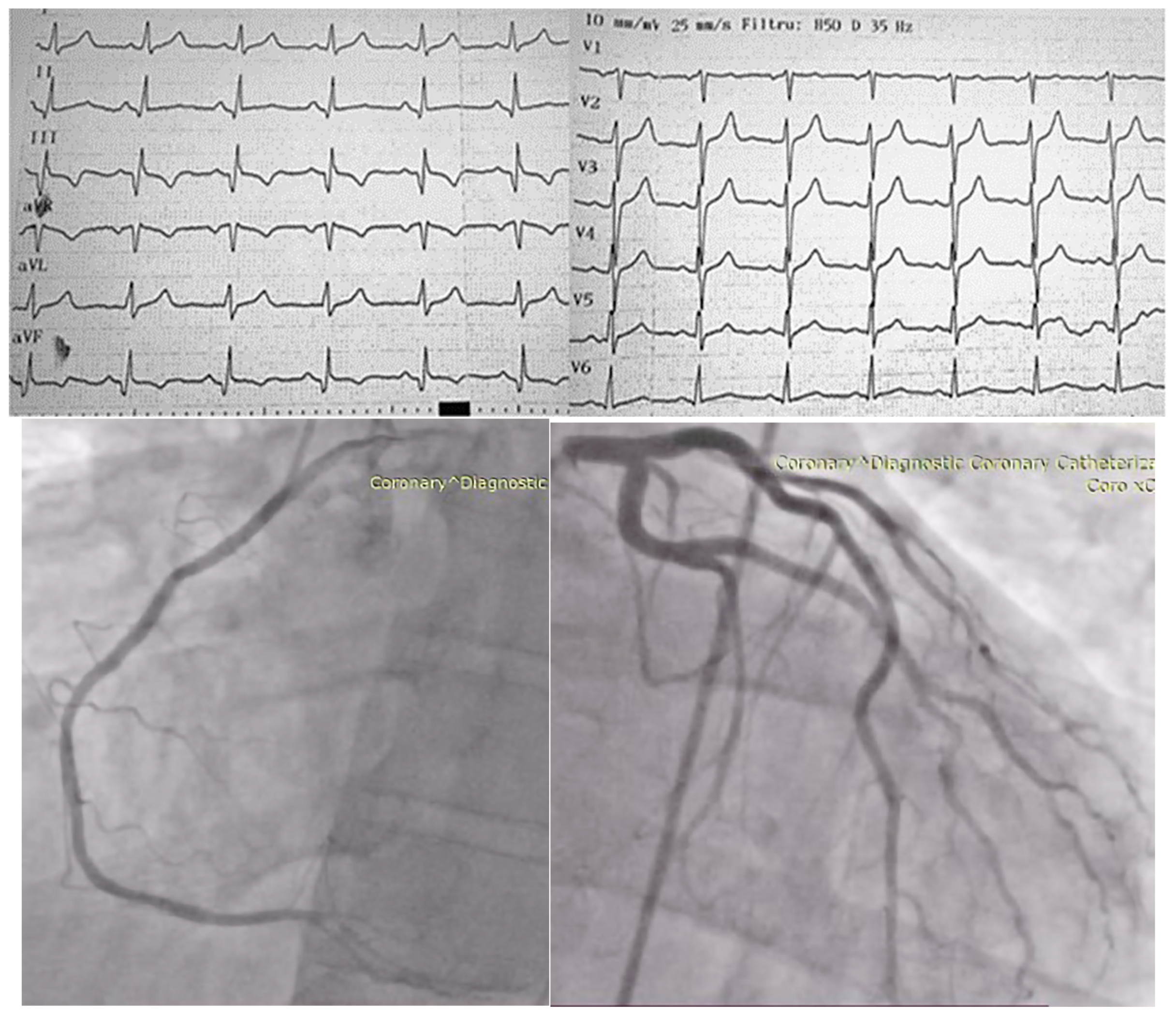
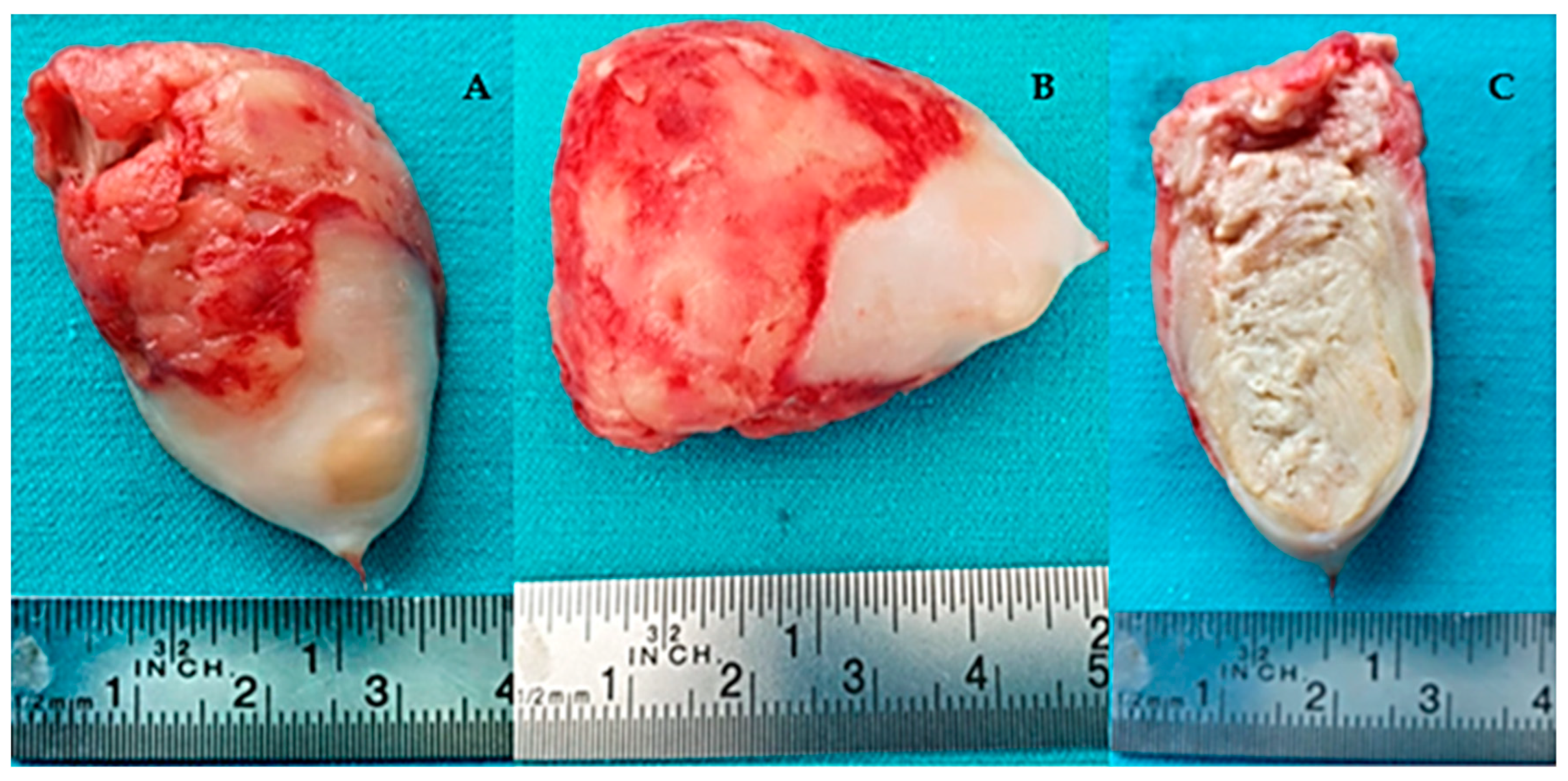

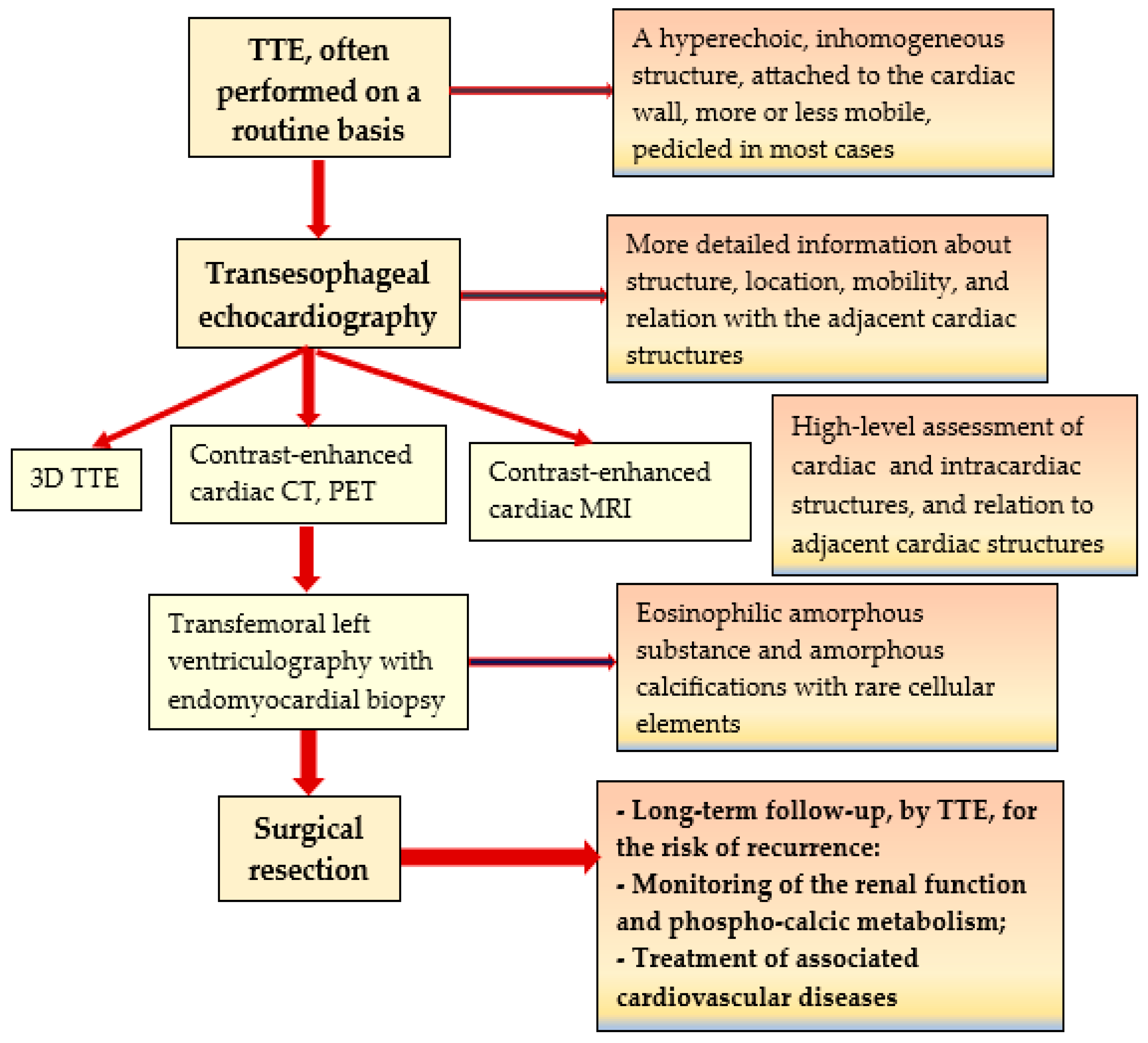
| No. | Authors/Year | No. of Patients/Gender/Age | Associated Conditions | Location in LV | Symptoms | Renal Status | CAT Evolution |
|---|---|---|---|---|---|---|---|
| 1. | Tsushima et al./2024 [7] | 1/M/58 | Hemodialysis | A mass attached to the posterior mitral leaflet and dense mitral annular calcification | Acute stroke | Heamodyalisis for 2 years | Unknown |
| 2. | Hatori et al./2024 [13] | 1/F/77 | Systemic hypertension, lung cancer, coronary artery disease | A mobile mass in the LV outflow tract attached to the non-coronary and the right coronary aortic cusp | Dyspnea, chest pain | Creatinine = 0.85 mg/dL | Unknown |
| 3. | Eizawa et al./2023 [8] | 1/F/80 | Calcified mitral annulus | Mobile mass originating from the mitral annulus of the posterior leaflet | Left eye vision loss due to an embolus at the bifurcation of the retinal artery | NR | Unknown |
| 4. | Ufuc et al./2023 [3] | 1/M/58 | Not significant | Calcified, irregular tubular, and infiltrative mass in the inferior-basal segment of LV, extending in the mid-inferior wall, mid-inferior septum, and inferior papillary muscle | Chest pain, palpitations | NR | 3 years |
| 5. | Ghaballi et al./2023 [14] | 1/M/37 | Not significant | An irregular mass with a wide base attached to the atrial wall | Chest pain | NR | 5 years |
| 6. | Odujoko et al./2023 [15] | 1/F/34 | Type 1 diabetes mellitus, hypertension, Hashimoto thyroiditis, mesenteric ischemia, recent myocardial infarction of the posterolateral LV, moderate/severe coronary atherosclerosis, end-stage renal disease under hemodialysis | Masses on the mitral valve, the endocardium, and subendocardial portions of the left and right ventricles | Abdominal pain, gluteal pain, and diarrhea | Creatinine = 3.52 mg/dL, CKD | Autopsy finding |
| 7. | Ushioda et al./2022 [9] | 1/F/86 | 90% stenosis of the right coronary artery requiring coronary artery bypass, preserved ejection fraction, severe mitral annular calcifications | A mobile mass within the anterior annulus of the mitral valve | Episodes of syncope | NR | Unknown |
| 8. | Endo et al./2022 [10] | 1/F/71 | Diabetes mellitus, multiple myeloma | A mobile, nonobstructive mass attached to the anterior leaflet of the mitral valve | Asymptomatic | NR | Unknown |
| 9. | Kimura et al./2022 [16] | 1/F/82 | Mitral annular calcification, repeated strokes, dental infection | A calcified mass on the posterior leaflet | Acute stroke | NR | 7 months |
| 10. | Nishiguchi et al./2021 [12] | 1/F/67 | Stroke, systolic hypertension, hyperlipidemia | A pedunculated mass attached to the posterior leaflet of the mitral valve | Visual impairment | Creatinine = 1.45 mg/dL | 5 months |
| 11. | Kumar et al./2021 [2] | 1/F/46 | Chronic kidney disease stage 4, right thromboembolic frontoparietal infarct (2 months ago), coronary artery disease requiring coronary artery by-pass, heart failure with reduced ejection fraction | A pedunculated mobile mass attached to the intraventricular septum | Signs and symptoms of decompensated heart failure and decreased urine output | NR | Unknown |
| 12. | Suzue et al./2021 [11] | 1/F/83 | Coronary artery bypass graft surgery, mitral annular calcification | Mobile, pediculated mass adherend to the posterior commissure of the mitral valve | Asymptomatic | Creatinine—0.43 mg/dL | 4 months |
| 13. | Koyama et al./2021 [17] | 1/F/83 | Aortic valve stenosis | Encapsulated mass between the noncoronary and the left coronary aortic cusp | Symptoms attributed to aortic valve stenosis | NR | Unknown |
| 14. | Yamanaka et al./2020 [18] | 1/F/82 | Systemic hypertension, diabetes mellitus type 2, multiple myeloma, chemotherapy, MAC | An immobile, calcified mass on the mitral annulus | Asymptomatic | Creatinine = 0.61 mg/dL | 18 months |
| 15. | Okazaki et al./2020 [19] | 1/M/67 | Chronic hemodialysis, heart failure, systemic hypertension, gastric cancer, infectious endocarditis with Enterococcus faecalis | A mobile, cystic lesion with a mass inside the cyst attached to the apex of the LV wall | Fatigue, stomatitis, and diarrhea | ESRD | 5 months |
| 16 | Formelli et al./2020 [20] | 1/F/79 | Systemic hypertension, polyglobulia | An echodense, not mobile left atrial mass | Ischemic stroke | NR | 3 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streian, C.G.; Tudoran, C.; Staicu, R.E.; Negru, A.G.; Mederle, A.L.; Borza, C.; Lascu, A. Particularities of a Cardiac Amorphous Left Ventricular Tumor in a Patient with Coronary Artery Disease—Diagnostic and Therapeutic Challenges: A Case Report and Literature Review. J. Clin. Med. 2024, 13, 6092. https://doi.org/10.3390/jcm13206092
Streian CG, Tudoran C, Staicu RE, Negru AG, Mederle AL, Borza C, Lascu A. Particularities of a Cardiac Amorphous Left Ventricular Tumor in a Patient with Coronary Artery Disease—Diagnostic and Therapeutic Challenges: A Case Report and Literature Review. Journal of Clinical Medicine. 2024; 13(20):6092. https://doi.org/10.3390/jcm13206092
Chicago/Turabian StyleStreian, Caius Glad, Cristina Tudoran, Raluca Elisabeta Staicu, Alina Gabriela Negru, Alexandra Laura Mederle, Claudia Borza, and Ana Lascu. 2024. "Particularities of a Cardiac Amorphous Left Ventricular Tumor in a Patient with Coronary Artery Disease—Diagnostic and Therapeutic Challenges: A Case Report and Literature Review" Journal of Clinical Medicine 13, no. 20: 6092. https://doi.org/10.3390/jcm13206092
APA StyleStreian, C. G., Tudoran, C., Staicu, R. E., Negru, A. G., Mederle, A. L., Borza, C., & Lascu, A. (2024). Particularities of a Cardiac Amorphous Left Ventricular Tumor in a Patient with Coronary Artery Disease—Diagnostic and Therapeutic Challenges: A Case Report and Literature Review. Journal of Clinical Medicine, 13(20), 6092. https://doi.org/10.3390/jcm13206092








