Evaluation of Red Blood Cell Biochemical Markers and Coagulation Profiles Following Cell Salvage in Cardiac Surgery: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
2.2. Data Extraction
2.3. Quality and Bias Risk Assessment
2.4. Data Synthesis and Statistical Analysis
3. Results
3.1. Results Obtained in the Selection of Articles
3.2. Descriptive Analysis of the Results Found
3.3. Bias Risk Assessment of the Selected Studies and Publication Bias
3.4. Results of the Meta-Analysis
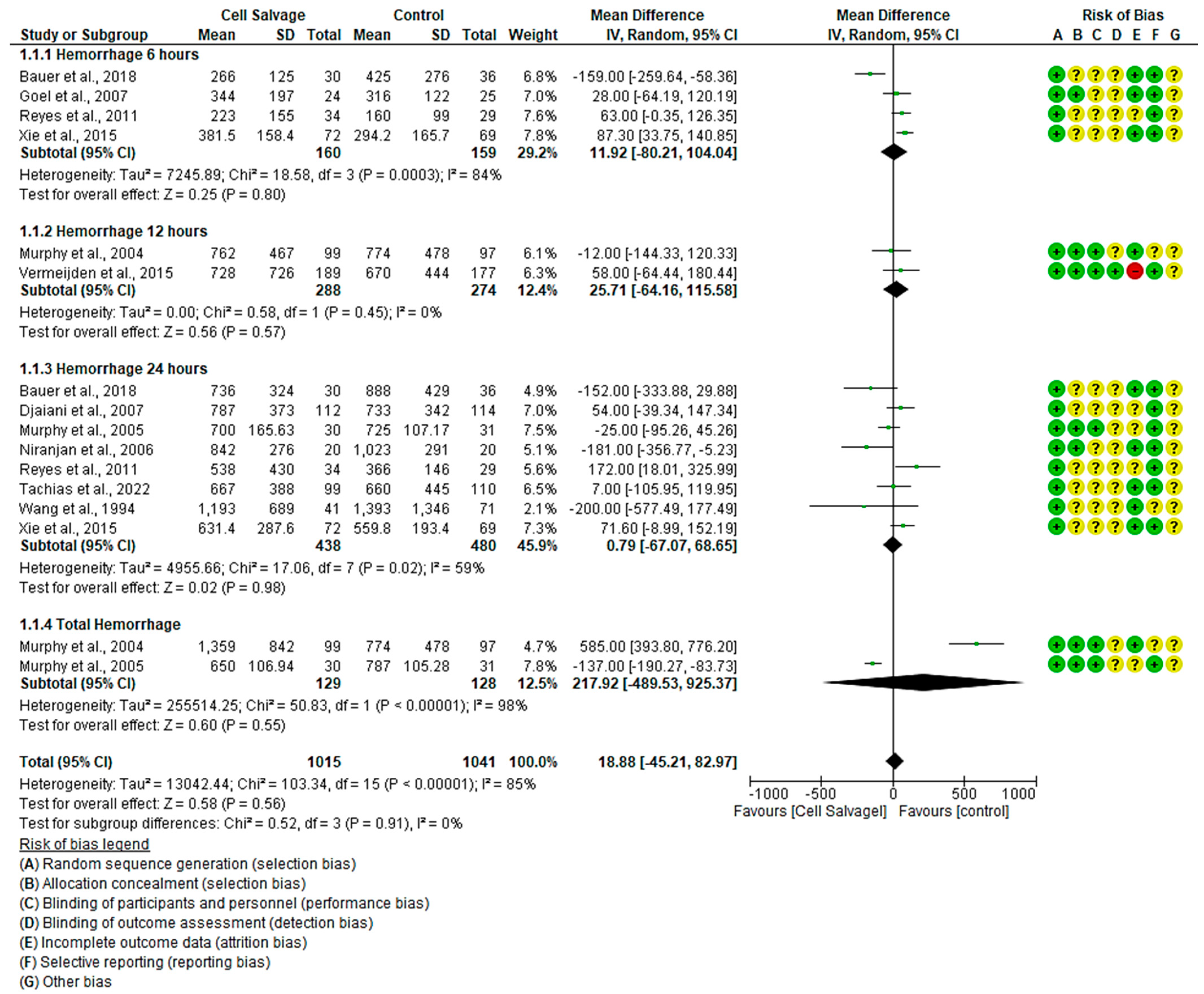
3.4.1. Efficacy of Cell Salvage in Hemorrhage
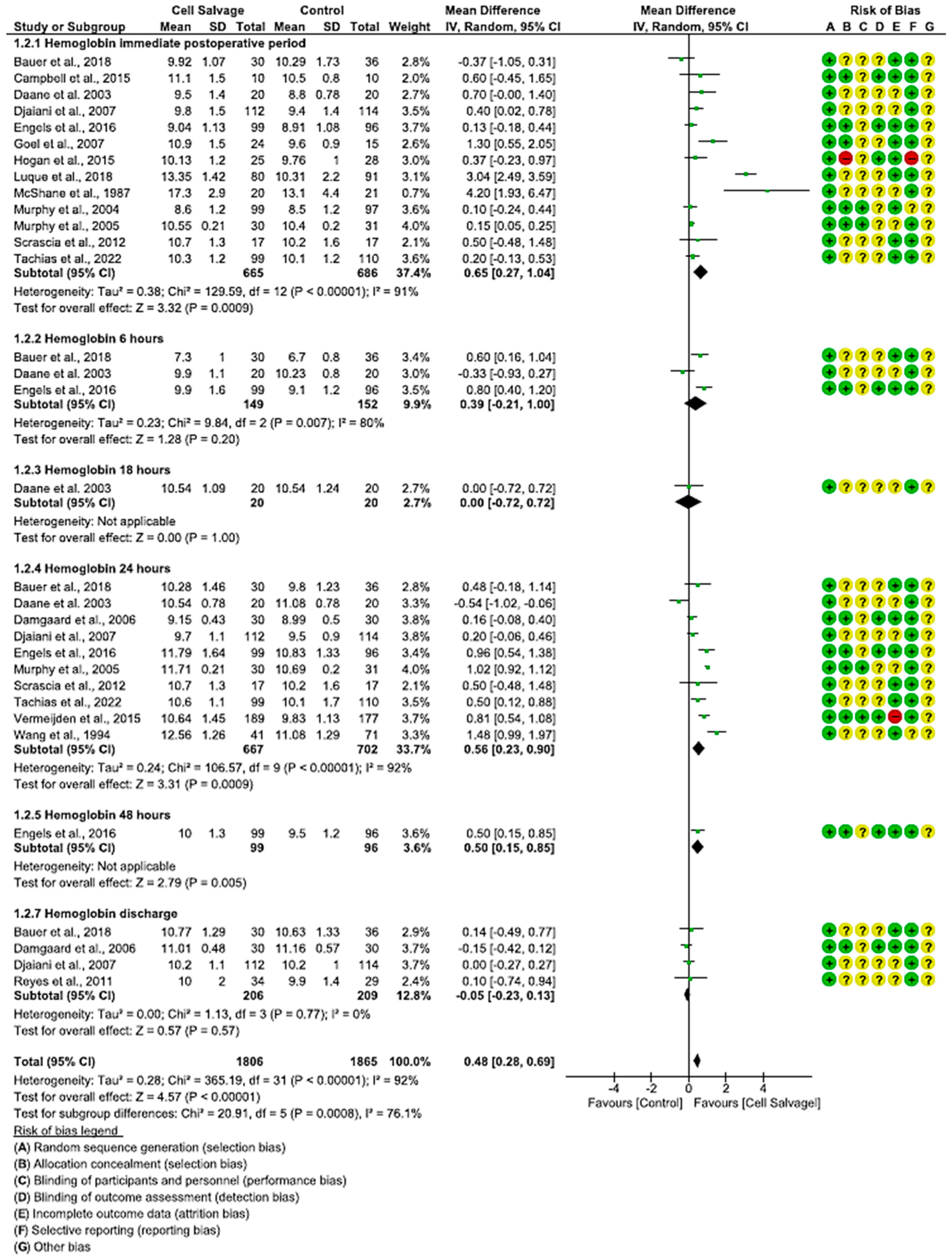
3.4.2. Efficacy of Cell Salvage on Hemoglobin Levels
3.4.3. Efficacy of Cell Salvage on Hematocrit
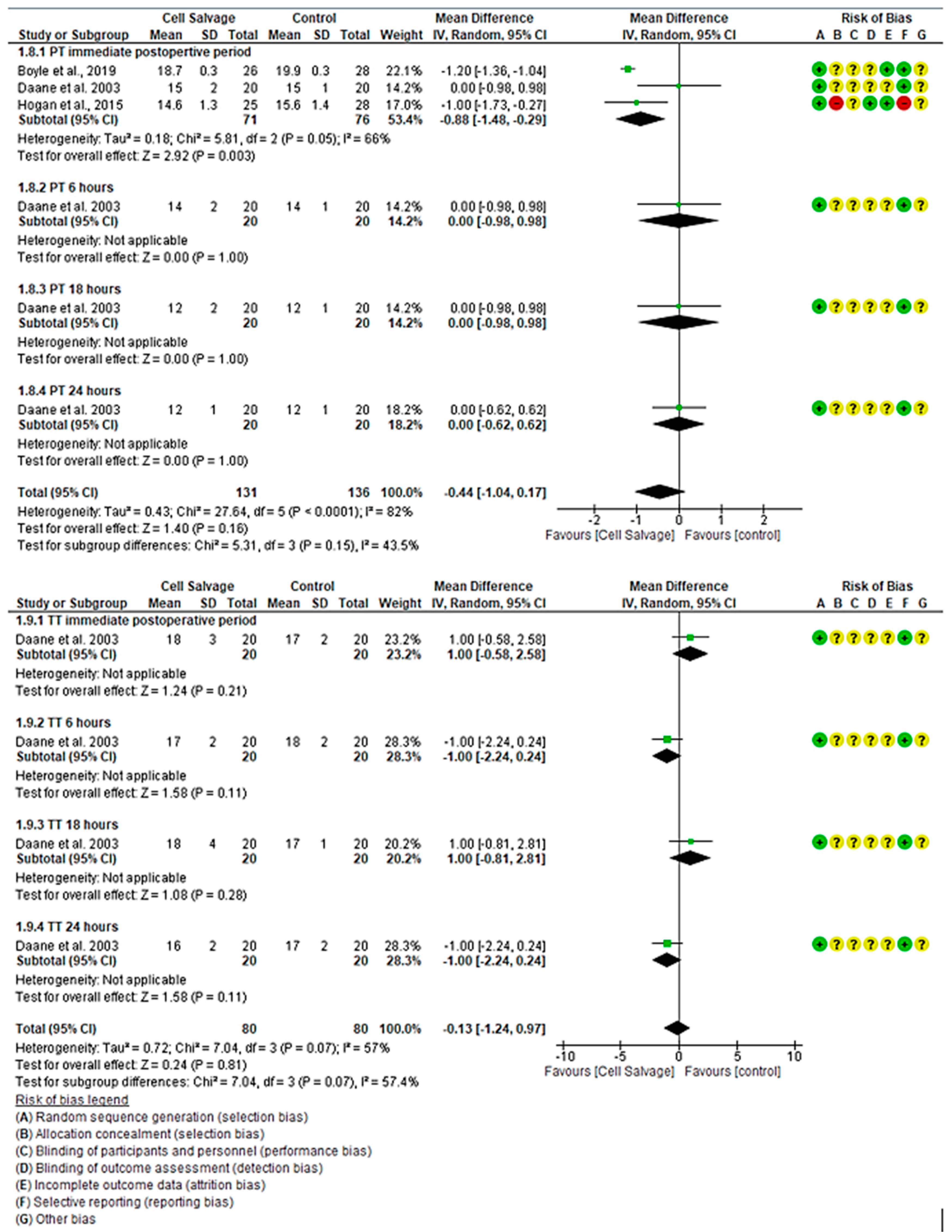
3.4.4. Efficacy of Cell Salvage on Coagulation Parameters
3.4.5. The Efficacy of Cell Salvage in Fresh Frozen Plasma Transfusion
3.4.6. The Efficacy of Cell Salvage on Other Coagulation Factors
4. Discussion
4.1. Cell Salvage-Associated Hemorrhage and Red Series Effectiveness
4.2. Hemoglobin and Hematocrit Levels after Use of Cell Salvage
4.3. Effectiveness of Cell Salvage on Clotting Times
4.4. Gender Differences in Cell Salvage Effectiveness
4.5. Limitations and Strengths
4.6. Clinical Prospective
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NICE. NICE Recommends New Tests for Bleeding Problems during and after Cardiac Surgery. 2014. Available online: https://www.nice.org.uk/guidance/dg13/chapter/1-recommendations (accessed on 26 July 2024).
- Geissler, R.G.; Rotering, H.; Buddendick, H.; Franz, D.; Bunzemeier, H.; Roeder, N.; Kwiecien, R.; Sibrowski, W.; Scheld, H.H.; Martens, S.; et al. Utilisation of blood components in cardiac surgery: A single-centre retrospective analysis with regard to diagnosis-related procedures. Transfus. Med. Hemother. 2015, 42, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Stoicea, N.; Bergese, S.D.; Ackermann, W.; Moran, K.R.; Hamilton, C.; Joseph, N.; Steiner, N.; Barnett, C.J.; Smith, S.; Ellis, T.J. Current status of blood transfusion and antifibrinolytic therapy in orthopedic surgeries. Front. Surg. 2015, 12, 3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McKay, C. Perfusion approaches to blood conservation. Semin. Cardiothorac. Vasc. Anesth. 2007, 11, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Bainbridge, D.; Martin, J.; Cheng, D. The efficacy of an intraoperative cell saver during cardiac surgery: A meta-analysis of randomized trials. Anesth. Analg. 2009, 109, 320–330. [Google Scholar] [CrossRef]
- Stover, P.E.; Siegel, L.C.; Parks, R.; Levin, J.; Body, S.C.; Maddi, R.; D’Ambra, M.N.; Mangano, D.T.; Spiess, B.D. Variability in transfusion practice for coronary artery bypass surgery persists despite national consensus guidelines: A 24-institution study. Institutions of the Multicenter Study of Perioperative Ischemia Research Group. Anesthesiology 1998, 88, 327–333. [Google Scholar] [CrossRef]
- Wells, A.W.; Mounter, P.J.; Chapman, C.E.; Stainsby, D.; Wallis, J.P. Where does blood go? Prospective observational study of red cell transfusion in north England. BMJ 2002, 325, 803. [Google Scholar] [CrossRef]
- Pati, I.; Velati, C.; Mengoli, C.; Franchini, M.; Masiello, F.; Marano, G.; Veropalumbo, E.; Vaglio, S.; Piccinini, V.; Pupella, S.; et al. A forecasting model to estimate the drop in blood supplies during the SARS-CoV-2 pandemic in Italy. Transfus. Med. 2021, 31, 200–205. [Google Scholar] [CrossRef]
- Robich, M.P.; Koch, C.G.; Johnston, D.R.; Schiltz, N.; Pillai, A.C.; Hussain, S.T.; Soltesz, E.G. Trends in blood utilization in United States cardiac surgical patients. Transfusion 2015, 55, 805–814. [Google Scholar] [CrossRef]
- Goodnough, L.T.; Shafron, D.; Marcus, R.E. The impact of preoperative autologous blood donation on orthopaedic surgical practice. Vox Sang. 1990, 59, 65–69. [Google Scholar] [CrossRef]
- Toy, P.T.; Kaplan, E.B.; McVay, P.A.; Lee, S.J.; Strauss, R.G.; Stehling, L.C. Blood loss and replacement in total hip arthroplasty: A multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion 1992, 32, 63–67. [Google Scholar] [CrossRef]
- Stehling, L.; Zauder, H.L. Acute normovolemic hemodilution. Transfusion 1991, 31, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.J.; Kessler, R.; Wernly, J.A. Blood conservation in cardiac surgery. Ann. Thorac. Surg. 1990, 50, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Rubens, F.; Wells, P. Tranexamic acid use during coronary artery bypass grafting. Ann. Thorac. Surg. 1996, 61, 774–775. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, L.T. Clinical application of recombinant erythropoietin in the perioperative period. Hematol. Oncol. Clin. N. Am. 1994, 8, 1011–1020. [Google Scholar] [CrossRef]
- Brainard, D.M.D. Amputation of the thigh for disease of the knee joint: Transfusion of blood. Chic. Med. J. 1860, 18, 116–117. [Google Scholar]
- Klein, A.; Agarwal, S.; Cholley, B.; Fassl, J.; Griffin, M.; Kaakinen, T.; Mzallassi, Z.; Paulus, P.; Rex, S.; Siegemund, M.; et al. A survey of patient blood management for patients undergoing cardiac surgery in nine European countries. J. Clin. Anesth. 2021, 72, 110311. [Google Scholar] [CrossRef]
- Schulz, K.F.; Chalmers, I.; Hayes, R.J.; Altman, D.G. Empirical evidence of bias: Dimensions of methodological quality associated with estimates of treatment effect in controlled trials. JAMA 1995, 273, 408–412. [Google Scholar] [CrossRef]
- Van Klarenbosch, J.; Van den Heuvel, E.R.; Van Oeveren, W.; De Vries, A.J. Does Intraoperative Cell Salvage Reduce Postoperative Infection Rates in Cardiac Surgery? J. Cardiothorac. Vasc. Anesth. 2020, 34, 1457–1463. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, W.; Fang, W.; Meng, G.; Zhang, L.; Zhou, Y.; Gu, E.; Liu, X. Safety, efficacy, and cost-effectiveness of intraoperative blood salvage in OPCABG with different amount of bleeding: A single-center, retrospective study. J. Cardiothorac. Surg. 2018, 13, 109. [Google Scholar] [CrossRef]
- Yao, Y.; Yuan, X.; He, L.; Yu, Y.; Du, Y.; Liu, G.; Tian, L.; Ma, Z.; Zhang, Y.; Ma, J. Patient Blood Management: Single Center Evidence and Practice at Fuwai Hospital. Chin. Med. Sci. J. 2022, 37, 246–260. [Google Scholar] [CrossRef]
- Koçyiğit, M.; Koçyiğit, Ö.I.; Güllü, A.Ü.; Şenay, Ş.; Alhan, C. Postoperative Atrial Fibrillation Reduced by Intraoperative and Postoperative Cell Saver System in Coronary Artery Bypass Graft Surgery. Turk. J. Anaesthesiol. Reanim. 2022, 50, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Spence, R.K.; Erhard, J. History of patient blood management. Best. Pract. Res. Clin. Anaesthesiol. 2013, 27, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Faught, C.; Wells, P.; Fergusson, D.; Laupacis, A. Adverse effects of methods for minimizing perioperative allogeneic transfusion: A critical review of the literature. Transfus. Med. Rev. 1998, 12, 206–225. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.A.; Silva, J.P.; Silva Lda, F.; Sousa, A.G.; Piotto, R.F.; Baumgratz, J.F. Therapeutic options to minimize allogeneic blood transfusions and their adverse effects in cardiac surgery: A systematic review. Rev. Bras. Cir. Cardiovasc. 2014, 29, 606–621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, S.; McEwen, C.; Basha, A.; Panchal, P.; Eqbal, A.; Wu, N.; Belley-Cote, E.P.; Whitlock, R. Retrograde autologous priming in cardiac surgery: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2021, 60, 1245–1256. [Google Scholar] [CrossRef]
- Garg, P.; Malhotra, A.; Desai, M.; Sharma, P.; Bishnoi, A.K.; Tripathi, P.; Rodricks, D.; Pandya, H. Pretransfusion Comparison of Dialyser-Based Hemoconcentrator With Cell Saver System for Perioperative Cell Salvage. Innovations 2015, 10, 334–341. [Google Scholar] [CrossRef]
- Stoneham, M.D.; Barbosa, A.; Maher, K.; Douglass, P.; Desborough, M.J.R.; Von Kier, S. Intraoperative cell salvage using swab wash and serial thromboelastography in elective abdominal aortic aneurysm surgery involving massive blood loss. Br. J. Haematol. 2023, 200, 652–659. [Google Scholar] [CrossRef]
- Al Khabori, M.; Al Riyami, A.; Siddiqi, M.S.; Sarfaraz, Z.K.; Ziadinov, E.; Al Sabti, H. Impact of cell saver during cardiac surgery on blood transfusion requirements: A systematic review and meta-analysis. Vox Sang. 2019, 114, 553–565. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Estarli, M.; Martínez-Rodríguez, R.; Baladia, E.; Camacho, S.; Buhring, K.; Herrero-López, A. Reference items for publishing protocols of systematic reviews and meta-analyses: PRISMA-P 2015 statement. Rev. Esp. Nutr. Hum. Diet. 2016, 20, 148. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration: London, UK, 2011; Available online: https://training.cochrane.org/handbook/current (accessed on 25 July 2024).
- GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime. 2022. Available online: https://Gradepro.org (accessed on 1 July 2024).
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Hausmann, H.; Schaarschmidt, J.; Scharpenberg, M.; Troitzsch, D.; Johansen, P.; Nygaard, H.; Eberle, T.; Hasenkam, J.M. Shed-blood-separation and cell-saver: An integral Part of MiECC? Shed-blood-separation and its influence on the perioperative inflammatory response during coronary revascularization with minimal invasive extracorporeal circulation systems - a randomized controlled trial. Perfusion 2018, 33, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Boyle, G.; Kuffel, A.; Parmar, K.; Gibson, K.; Smith, M.; Grehan, A.; Hunt, B.J.; Chambers, D.J. A comparison of haemostatic biomarkers during low-risk patients undergoing cardiopulmonary bypass using either conventional centrifugal cell salvage or the HemoSep device. Perfusion 2019, 34, 76–83. [Google Scholar] [CrossRef]
- Campbell, J.; Holland, C.; Richens, D.; Skinner, H. Impact of cell salvage during cardiac surgery on the thrombelastomeric coagulation profile: A pilot study. Perfusion 2012, 27, 221–224. [Google Scholar] [CrossRef]
- Daane, C.R.; Golab, H.D.; Meeder, J.H.; Wijers, M.J.; Bogers, A.J. Processing and transfusion of residual cardiopulmonary bypass volume: Effects on haemostasis, complement activation, postoperative blood loss and transfusion volume. Perfusion 2003, 18, 115–121. [Google Scholar] [CrossRef]
- Damgaard, S.; Steinbrüchel, D.A. Autotransfusion with cell saver for off-pump coronary artery bypass surgery: A randomized trial. Scand. Cardiovasc. J. 2006, 40, 194–198. [Google Scholar] [CrossRef]
- Djaiani, G.; Fedorko, L.; Borger, M.A.; Green, R.; Carroll, J.; Marcon, M.; Karski, J. Continuous-flow cell saver reduces cognitive decline in elderly patients after coronary bypass surgery. Circulation 2007, 116, 1888–1895. [Google Scholar] [CrossRef]
- Engels, G.E.; Van Klarenbosch, J.; Gu, Y.J.; Van Oeveren, W.; de Vries, A.J. Intraoperative cell salvage during cardiac surgery is associated with reduced postoperative lung injury. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Goel, P.; Pannu, H.; Mohan, D.; Arora, R. Efficacy of cell saver in reducing homologous blood transfusions during OPCAB surgery: A prospective randomized trial. Transfus. Med. 2007, 17, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.; Needham, A.; Ortmann, E.; Bottrill, F.; Collier, T.J.; Besser, M.W.; Klein, A.A. Haemoconcentration of residual cardiopulmonary bypass blood using Hemosep®: A randomised controlled trial. Anaesthesia 2015, 70, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.A.; Nashef, S.A.M.; Sharples, L.; Bottrill, F.; Dyer, M.; Armstrong, J.; Vuylsteke, A. A randomized controlled trial of cell salvage in routine cardiac surgery. Anesth. Analg. 2008, 107, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Laub, G.W.; Dharan, M.; Riebman, J.B.; Chen, C.; Moore, R.; Bailey, B.M.; Fernandez, J.; Adkins, M.S.; Anderson, W.; McGrath, L.B. The impact of intraoperative autotransfusion on cardiac surgery. A prospective randomized double-blind study. Chest 1993, 104, 686–689. [Google Scholar] [CrossRef]
- Luque Oliveros, M.; Domínguez Baños, M.A.; Gutiérrez Plata, M. Autologous blood for reinfusion using a cell saver in cardiac patients in response to blood transfusions. Cardiocore 2018, 53, 122–127. [Google Scholar] [CrossRef]
- Marcheix, B.; Carrier, M.; Martel, C.; Cossette, M.; Pellerin, M.; Bouchard, D.; Perrault, L.P. Effect of pericardial blood processing on postoperative inflammation and the complement pathways. Ann. Thorac. Surg. 2008, 85, 530–535. [Google Scholar] [CrossRef]
- McGill, N.; O’Shaughnessy, D.; Pickering, R.; Herbertson, M.; Gill, R. Mechanical methods of reducing blood transfusion in cardiac surgery: Randomised controlled trial. BMJ 2002, 324, 1299. [Google Scholar] [CrossRef]
- Mcshane, A.J.; Power, C.; Jackson, J.F.; Murphy, D.F.; Macdonald, A.; Moriarty, D.C.; Otridge, B.W. Autotransfusion: Quality of blood prepared with a red cell processing device. Br. J. Anaesth. 1987, 59, 1035–1039. [Google Scholar] [CrossRef]
- Merville, C.; Charlet, P.; Zerr, C.; Bricard, H. Efficacité respective du Cell Saver et de la récupération du circuit de CEC ultrafiltré en chirurgie cardiaque. Ann. Fr. Anesth. Reanim. 1991, 10, 548–553. [Google Scholar] [CrossRef]
- Murphy, G.J.; Allen, S.M.; Unsworth-White, J.; Lewis, C.T.; Dalrymple-Hay, M.J. Safety and efficacy of perioperative cell salvage and autotransfusion after coronary artery bypass grafting: A randomized trial. Ann. Thorac. Surg. 2004, 77, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Rogers, C.; Lansdowne, W.; Channon, I.; Alwair, H.; Cohen, A.; Caputo, M.; Angelini, G. Safety, efficacy, and cost of intraoperative cell salvage and autotransfusion after off-pump coronary artery bypass surgery: A randomized trial. J. Thorac. Cardiovasc. Surg. 2005, 130, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, G.; Asimakopoulos, G.; Karagounis, A.; Cockerill, G.; Thompson, M.; Chandrasekaran, V. Effects of cell saver autologous blood transfusion on blood loss and homologous blood transfusion requirements in patients undergoing cardiac surgery on- versus off-cardiopulmonary bypass: A randomised trial. Eur. J. Cardiothorac. Surg. 2006, 30, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Reyes, G.; Prieto, M.; Alvarez, P.; Orts, M.; Bustamante, J.; Santos, G.; Sarraj, A.; Planas, A. Cell saving systems do not reduce the need of transfusion in low-risk patients undergoing cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Scrascia, G.; Rotunno, C.; Nanna, D.; Rociola, R.; Guida, P.; Rubino, G.; Schinosa, L.d.L.T.; Paparella, D. Pump blood processing, salvage and re-transfusion improves hemoglobin levels after coronary artery bypass grafting, but affects coagulative and fibrinolytic systems. Perfusion 2012, 27, 270–277. [Google Scholar] [CrossRef]
- Tachias, F.; Samara, E.; Petrou, A.; Karakosta, A.; Siminelakis, S.; Apostolakis, E.; Tzimas, P. The Effect of Cell Salvage on Bleeding and Transfusion Needs in Cardiac Surgery. Anesth. Res. Pract. 2022, 2022, 3993452. [Google Scholar] [CrossRef]
- Vermeijden, W.J.; van Klarenbosch, J.; Gu, Y.J.; Mariani, M.A.; Buhre, W.F.; Scheeren, T.W.; Hagenaars, J.A.; Tan, M.E.S.; Haenen, J.S.; Bras, L.; et al. Effects of cell-saving devices and filters on transfusion in cardiac surgery: A multicenter randomized study. Ann. Thorac. Surg. 2015, 99, 26–32. [Google Scholar] [CrossRef]
- Wang, M.J.; Chen, T.L.; Huang, C.H.; Chao, A.; Chu, S.H. Difference in efficacy of the Cell Saver in coronary bypass grafting surgery and cardiac valvular reoperations. J. Formos. Med. Assoc. 1994, 93, 117–121. [Google Scholar]
- Wang, X.; Ji, B.; Zhang, Y.; Zhu, X.; Liu, J.; Long, C.; Zheng, Z. Comparison of the effects of three cell saver devices on erythrocyte function during cardiopulmonary bypass procedure--a pilot study. Artif. Organs 2012, 36, 931–935. [Google Scholar] [CrossRef]
- Xie, Y.; Shen, S.; Zhang, J.; Wang, W.; Zheng, J. The efficacy, safety and cost-effectiveness of intra-operative cell salvage in high-bleeding-risk cardiac surgery with cardiopulmonary bypass: A prospective randomized and controlled trial. Int. J. Med. Sci. 2015, 12, 322–328. [Google Scholar] [CrossRef]
- Vonk, A.B.; Meesters, M.I.; Garnier, R.P.; Romijn, J.W.; van Barneveld, L.J.; Heymans, M.W.; Jansen, E.K.; Boer, C. Intraoperative cell salvage is associated with reduced postoperative blood loss and transfusion requirements in cardiac surgery: A cohort study. Transfusion 2013, 53, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Huët, C.; Salmi, L.R.; Fergusson, D.; Koopman-van Gemert, A.W.; Rubens, F.; Laupacis, A. A meta-analysis of the effectiveness of cell salvage to minimize perioperative allogeneic blood transfusion in cardiac and orthopedic surgery. International Study of Perioperative Transfusion (ISPOT) Investigators. Anesth. Analg. 1999, 89, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.-P.; Fullerton, H.J.; et al. Heart Disease and Strole Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, 38–360. [Google Scholar] [CrossRef]
- Drouet, N. European transfusion practices:the SANGUIS survey. Cah. Anesthesiol. 1994, 42, 425–428. [Google Scholar] [PubMed]
- Murphy, G.J.; Pike, K.; Rogers, C.A.; Wordsworth, S.; Stokes, E.A.; Angelini, G.D.; Reeves, B.C.; TITRe2 Investigators. Liberal or restrictive transfusion after cardiac surgery. N. Engl. J. Med. 2015, 372, 997–1008, Erratum in N. Engl. J. Med. 2015, 372, 2274. [Google Scholar] [CrossRef]
- Working with the Haemonetics® Cell Saver® 5. Available online: https://manualzz.com/doc/27212870/working-with-the-haemonetics®-cell-saver®-52022 (accessed on 1 July 2024).
- Liao, X.-Y.; Zuo, S.-S.; Meng, W.-T.; Zhang, J.; Huang, Q.; Gou, D.-M. Intraoperative blood salvage may shorten the lifespan of red blood cells within 3 days postoperatively. Medicine 2017, 96, e8143. [Google Scholar] [CrossRef]
- Álvarez Gallesio, J.M.; Bertolino, T.; Méndez, M.M.; David, M.; Tenorio Núñez, O.M.; Borracci, R.A. Recuperación rutinaria de sangre con cell saver durante la cirugía cardíaca electiva. Rev. Argent. Cardiol. 2020, 88, 276–278. [Google Scholar] [CrossRef]
- Al-Riyami, A.Z.; Al-Khabori, M.; Baskaran, B.; Siddiqi, M.; Al-Sabti, H. Intra-operative cell salvage in cardiac surgery may increase platelet transfusion requirements: A cohort study. Vox Sang. 2015, 109, 280–286. [Google Scholar] [CrossRef]
- Gunaydin, S.; Robertson, C.; Budak, A.B.; Gourlay, T. Comparative evaluation of blood salvage techniques in patients undergoing cardiac surgery with cardiopulmonary bypass. Perfusion 2018, 33, 105–109. [Google Scholar] [CrossRef]
- Rubens, F.D.; Boodhwani, M.; Mesana, T.; Wozny, D.; Wells, G.; Nathan, H.J. Cardiotomy Investigators The cardiotomy trial: A randomized, double-blind study to assess the effect of processing of shed blood during cardiopulmonary bypass on transfusion and neurocognitive function. Circulation 2007, 116, I89–I97. [Google Scholar]
- Adam, E.H.; Funke, M.; Zacharowski, K.; Meybohm, P.; Keller, H.; Weber, C.F. Impact of intraoperative cell salvage on blood coagulation factor concentrations in patients undergoing cardiac surgery. Anesth. Analg. 2020, 130, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Terwindt, L.; Karlas, A.; Eberl, S.; Wijnberge, M.; Driessen, A.; Veelo, D.; Geerts, B.; Hollmann, M.; Vlaar, A. Patient blood management in the cardiac surgical setting: An updated overview. Transfus. Apher. Sci. 2019, 58, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Boer, C.; Meesters, M.I.; Milojevic, M.; Benedetto, U.; Bolliger, D.; von Heymann, C.; Jeppsson, A.; Koster, A.; Osnabrugge, R.L.; Ranucci, M.; et al. 2017 EACTS/EACTA guidelines on patient blood management for adult cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2018, 32, 88–120. [Google Scholar] [CrossRef] [PubMed]
- Raphael, J.; Mazer, C.D.; Subramani, S.; Schroeder, A.; Abdalla, M.; Ferreira, R.; Roman, P.E.; Patel, N.; Welsby, I.; Greilich, P.E.; et al. Society of cardiovascular anesthesiologists clinical practice improvement advisory for management of perioperative bleeding and hemostasis in cardiac surgery patients. Anesth. Analg. 2019, 129, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, D.; Buser, A.; Erb, J.M. Patient blood management in cardiac surgery. Curr. Anesthesiol. Rep. 2019, 9, 215–222. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, J.; Wang, W.; Zheng, J.; Xie, Y. Impact of intra-operative cell salvage on blood coagulation in high-bleeding-risk patients undergoing cardiac surgery with cardiopulmonary bypass: A prospective randomized and controlled trial. J. Transl. Med. 2016, 14, 228. [Google Scholar] [CrossRef]
- Jobes, D.R.; Aitken, G.L.; Shaffer, G.W. Increased accuracy and precision of heparin and protamine dosing reduces blood loss and transfusion in patients undergoing primary cardiac operations. J. Thorac. Cardiovasc. Surg. 1995, 110, 36–45. [Google Scholar] [CrossRef][Green Version]
- Khuri, S.F.; Valeri, C.R.; Loscalzo, J.; Weinstein, M.J.; Birjiniuk, V.; Healey, N.A.; MacGregor, H.; Doursounian, M.; Zolkewitz, M.A. Heparin causes platelet dysfunction and induces fibrinolysis before cardiopulmonary bypass. Ann. Thorac. Surg. 1995, 60, 1008–1014. [Google Scholar] [CrossRef]
- Koch, C.G.; Weng, Y.-S.; Zhou, S.X.; Savino, J.S.; Mathew, J.P.; Hsu, P.H.; Saidman, L.J.; Mangano, D.T. Prevalence of risk factors, and not gender per se, determines short- and long-term survival after coronary artery bypass surgery. J. Cardiothorac. Vasc. Anesth. 2003, 17, 585–593. [Google Scholar] [CrossRef]
- Ried, M.; Lunz, D.; Kobuch, R.; Rupprecht, L.; Keyser, A.; Hilker, M.; Schmid, C.; Diez, C. Gender’s impact on outcome in coronary surgery with minimized extracorporeal circulation. Clin. Res. Cardiol. 2012, 101, 437–444. [Google Scholar] [CrossRef]
- Othman, H.; Khambatta, S.; Seth, M.; Lalonde, T.A.; Rosman, H.S.; Gurm, H.S.; Mehta, R.H. Differences in sex-related bleeding and outcomes after percutaneous coronary intervention: Insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) registry. Am. Heart J. 2014, 168, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mehran, R.; Grinfeld, L.; Xu, K.; Nikolsky, E.; Brodie, B.R.; Witzenbichler, B.; Kornowski, R.; Dangas, G.D.; Lansky, A.J.; et al. Sex-based differences in bleeding and long term adverse events after percutaneous coronary intervention for acute myocardial infarction: Three year results from the HORIZONS-AMI trial. Catheter. Cardiovasc. Interv. 2015, 85, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Egger, M. Misleading meta-analysis: Lessons from “an effective, safe, simple” intervention that wasn’t. BMJ 1995, 310, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Cappelleri, J.C.; Ioannidis, J.P.; Schmid, C.H.; de Ferranti, S.D.; Aubert, M.; Chalmers, T.C.; Lau, J. Large trials vs meta-analysis of smaller trials: How do their results compare? JAMA 1996, 276, 1332–1338. [Google Scholar] [CrossRef]
- Grégoire, G.; Derderian, F.; Le Lorier, J. Selecting the language of the publications included in a meta-analysis: Is there a tower of Babel bias? J. Clin. Epidemiol. 1995, 48, 159–163. [Google Scholar] [CrossRef]
- Egger, M.; Zellweger-Zähner, T.; Schneider, M.; Junker, C.; Lengeler, C.; Antes, G. Language bias in randomised controlled trials published in English and German. Lancet 1997, 350, 326–329. [Google Scholar] [CrossRef]




| Study (Author/Year) Evidence Level | Country | Design | Intervention | Sample (M/F) Average Age | Objectives | Implementation Details | Outcomes | Parameters Assessed |
|---|---|---|---|---|---|---|---|---|
| Bauer et al. (2017) (1+) [38] | Germany | Prospective, randomized, controlled clinical trial | CS (MiECCB System©) No CS (control) | 30 (23/7) 36 (29/7) CS: 67.5 ± 9.9 No CS: 66.7 ± 8.8 | To investigate the impact of cell washing shed blood from the operating field versus direct return to the ECC on the biomarkers for systemic inflammation. | CS: Suction blood was separated, and CS was performed before the blood was re-transfused as an autologous RBC concentrate. Control: The suction blood was separated and directly re-transfused without any treatment. | CS has positive effects on hemorrhage. | Hemorrhage HB |
| Boyle et al., (2019) (1+) [39] | United Kingdom | Randomized clinical trial | CS (HemoSep©) No CS (control) | 26 (NR) 28 (NR) Age (NR) | To assess homeostatic markers in patients who underwent surgeries where they received conventional CS. | CS: Patients allocated into CS group, blood from the bypass reservoir was drained into two treatment bags with half the volume in each. Control: Received autologous blood. | CS device demonstrated a slight increase in hemostatic markers in low-risk cardiac surgery. | aPTT PT Fibrinogen D-dimer |
| Campbell et al., (2011) (1++) [40] | United Kingdom | Randomized clinical trial | CS (C.A.T.S., Fresenius Hemocare GmbH©, Bad Homberg, Germany) No CS (control) | 10 (9/1) 10 (9/1) CS: 62.0 ± 10 No CS: 64.0 ± 10 | To examine the relationship between processing the residual CPB volume and the viscoelastic properties of clot formation in patients undergoing cardiac surgery. | CS: In this group, a continuous autotransfusion system was used prior to the administration of heparin and after reversal with protamine, and the residual CPB volume was processed before transfusion after bypass. Control: CS was not used in this group, and the residual CPB volume was transfused unprocessed after protamine administration. | There was a strong association between clot formation time after surgery and blood loss. The increase in blood loss was 4.1 mL for every one-second increase in clot formation time. CS of the residual cardiopulmonary bypass volume reduced platelet numbers and prolonged clot formation time and maximum clot firmness was less in this group. | HB Platelet count |
| Daane et al., (2003) (1−) [41] | The Netherlands | Prospective randomized clinical trial | CS (Haemolite 2plus, Haemonetics Corp., Braintree, MA, USA©) No CS (control) | 20 (11/9) 20 (13/7) CS: 65.0 ± 10 No CS: 65.0 ± 14 | To compare the effects of the transfusion of unprocessed and CS volume on hemostasis, complement activation, postoperative blood loss, and transfusion requirements after elective cardiac surgery. | CS: Patients in this group were transfused with processed blood using a CS device and the residual CPB volume. Control: Patients were transfused with unprocessed residual volume obtained from the extracorporeal circuit. | Processing CPB volume in combination with processing preoperative blood loss may result in reducing the volume of transfusion needed of allogeneic blood product. | HB HCT aPTT PT TT Platelet count Fibrinogen D-dimer |
| Damgaard et al. (2006) (1++) [42] | Denmark | Randomized clinical trial | CS (Autolog Medtronic, Minneapolis, MN, USA©) No CS (control) | 30 (19/11) 30 (16/14) CS: 77.0 ± 3.70 No CS: 76.0 ± 6.70 | To clarify the effect of using a CS intraoperatively. | CS: Patients in this group were transfused with blood processed with a blood CS, device residual CPB volume. Control: Patients were transfused with unprocessed residual volume obtained from the ECC. | Use of CS reduced intraoperative RBC loss and seemed to reduce transfusions by 1 unit per patient. | HB HCT |
| Djaiani et al., (2007) (1−) [43] | Canada | Randomized clinical trial | CS (Fresenius corporation, Concord, CA, USA©) No CS (control) | 112 (100/12) 114 (103/9) CS: 67.5 ± 6.0 No CS: 67.0 ± 6.1 | To determine whether the replacement of cardiotomy suction with a continuous-flow CS device would improve neuroprotection by minimizing cerebral microembolization and reduce cognitive decline in elderly patients after coronary artery bypass graft surgery. | CS: The continuous-flow CS was used to process shed blood before returning it back to the patient. Control: Cardiotomy suction was used in a standard closed venous reservoir where cardiotomy blood was collected and reinfused through the arterial circuit back to the patient. | Processing of shed blood with CS results in a clinically significant reduction in postoperative cognitive dysfunction after cardiac surgery. | Hemorrhage HB aPTT FFP Platelet count INR |
| Engels et al. (2016) (1++) [44] | The Netherlands | Randomized, prospective, multicenter clinical trial | CS (Continuous AutoTransfusion System Fresenius©) No CS (control) | 99 (69/30) 96 (62/34) CS: 66.0 ± 10.0 No CS: 68.0 ± 9.0 | To assess whether intraoperative CS may reduce lung injury following cardiac surgery by removing cytokines, neutrophilic proteases, and lipids that are present in cardiotomy suction blood. | CS: Blood was collected from skin incision until closure of the sternum including cardiotomy suction blood and residual heart–lung machine blood processed with a CS device. Control: Conventional cardiotomy suction device was used, and the residual blood from the heart–lung machine was re-transfused to the patient through a standard blood transfusion set. | First, patients in the cell saver group had higher HB levels during the first 24 h after surgery; however, the perioperative packed RBC transfusion rates were similar between the 2 groups. Second, patients in the CS group had a higher INR ratio and lower platelet count at the time of ICU admission. | HB Platelet count |
| Goel et al., (2007) (1+) [45] | India | Prospective randomized trial | CS (Dideco, Mirandola, Italy) No CS (control) | 24 (21/3) 24 (21/3) CS: 58.2 ± 8.7 No CS: 61.9 ± 10.0 | To evaluate the safety and efficacy of this modality in patients undergoing off-pump coronary artery bypass grafting. | CS: CS was used to salvage and autotransfuse shed blood from the time of incision. Control: This group was administered banked homologous packed RBCs as the only blood replacement therapy and served as the control. | The use of CS reduced the requirement for HB transfusion. Its use is not associated with any clinically significant bleeding diathesis. | HB FFP |
| Hogan et al. (2015) (1−) [46] | United Kingdom | Randomized controlled trial | CS (HemoSep©) No CS (control) | 25 (19/6) 28 (24/4) CS: 70.5 ± 10.2 No CS: 67.7 ± 10.2 | To compare the autotransfusion of residual CPB blood with residual blood concentrated using the novel HemoSep© device. | CS: In patients allocated to the CS group, blood from the bypass reservoir was drained into two treatment bags with half the volume in each. Control: The blood was re-transfused to the patient at a rate determined by the anesthetist. | There was no difference in the HB concentration in both groups. HemoSep© reduced the weight of the blood in comparison to the control group. | HB aPTT PT Platelet count |
| Klein et al., (2008) (1++) [47] | United Kingdom | Randomized clinical trial | CS (C.A.T.S—Fresenius Hemocare, France) No CS (control) | 111 (84/27) 102 (78/24) CS: 67.4 ± 10.2 No CS: 68.6 ± 9.6 | To examine the hypothesis that the use of CS during and after routine cardiac surgery would reduce the proportion of patients exposed to allogeneic blood transfusion. | CS: The CS was prepared before the start of surgery, and it was used exclusively for surgical suction before and after CPB in the CS group. Control: After CPB, any remaining blood in the bypass machine tubing and reservoir was collected in a bag and transfused directly to the patient. | There was no difference between the two groups in the proportion of patients exposed to allogeneic blood (32% in both groups, relative risk 1.0 p = 0.89). | FFP |
| Laub et al. (1993) (1−) [48] | USA | Randomized clinical trial | CS (Cell Salver 4, Haemonetics Corp.) No CS (control) | 19 (15/4) 19 (15/4) CS: 65.0 ± 2.4 No CS: 64.4 ± 2.1 | Assessing intraoperative autotransfusion | CS: The shed intraoperative and pump blood remaining at the conclusion of bypass was washed with CS and autotransfused. Control: Shed blood was discarded, and pump blood was re-transfused without washing. | Intraoperative use of CS decreased the volume of blood products required. | FFP |
| Luque et al. (2018) (1−) [49] | Spain | Randomized clinical trial | CS (Continuous Autologous Autotransfusion System, C.A.T.S, Fresenius) No CS (control) | 80 (63/17) 91 (78/13) CS: 65.0 ± 6.2 No CS: 67.0 ± 5.4 | To analyze the evidence on the effectiveness of CS to reduce blood transfusions. | CS: Lost blood was recovered from the operative field with CS, and after processing, the obtained RBCs were reinfused into the patient continuously during surgery. Control: Traditional transfusion system. | Both HB and HCT were lower in the control group in comparison to the CS group, both during and after surgery. Blood transfusions were higher in the control group both during and after surgery. | HB HCT Platelet count |
| Marchiex et al. (2008) (1+) [50] | Canada | Randomized clinical trial | Groups A and B: Non-CS. Groups C and D: CS. | A: 25 (22/3) B: 25 (24/1) C: 25 (21/4) D: 25 (22/3) A: 60 ± 6.1 B: 61.2 ± 7.1 C: 59.7 ± 8.5 D: 59.2 ± 9.7 | To determine the effect of processing of pericardial blood with a CS and vacuum-assisted cardiopulmonary bypass (VACPB) on the reduction of postoperative inflammation. | Group A: No CS and no VACPB. Group B: VACPB alone. Group C: CS alone. Group D: CS and VACPB. The processed blood in groups C and D was re-transfused immediately after weaning from CPB. Patients in groups A and B received re-transfusion of suctioned blood during CPB. | The use of CS decreases the activation of the complement alternative pathway and reduces neurologic injury and exposure to allogeneic blood transfusion. | FFP |
| McGill et al. (2022) (1−) [51] | USA | Randomized clinical trial | 1–Intraoperative CS (Dideco Compact, Dideco, Mirandola, Italy), intraoperative 2–CS with acute perioperative normovolemic hemodilution, 3–No CS (control) | 84 (75/9) 84 (74/10) CS: 63.8 ± 7.8 No CS: 63.4 ± 9.1 | To assess the effectiveness of two mechanical methods of blood conservation in reducing the need for allogeneic RBC or coagulation products during cardiac surgery. | CS: Intraoperative blood salvage with CS. This blood was re-transfused at the termination of bypass. Control: Shed blood was discarded. | The need for allogeneic RBC transfusion in elective coronary artery bypass grafting can be reduced by using intraoperative CS. | FFP |
| McShane et al., (1987) (1−) [52] | Ireland | Clinical trial | CS (Continuous Autologous Autotransfusion System, C.A.T.S, Fresenius) No CS (control) | 20 (12/8) 21 (16/5) Age (NR) | To measure HB, HCT, pH, and the concentrations of lysozyme, potassium, and red cell 2,3-DPG, and to compare these values with those in donor blood. | CS: An intra-surgical CS was used. Control: A conventional pressure suction drain was used. | The autotransfusor is a useful aid for blood conservation, producing good quality RBCs with relatively normal pH and potassium values. However, modification of the centrifugation and washing is required to lessen the high white cell count and heparin concentrations found in the saved blood. | HB HCT |
| Merville et al., (1991) (1−) [53] | France | Prospective randomized clinical trial | CS (Haemonetics®) Control: filter | 60 (44/16) 60 (41/19) CS: 61.0 ± 11.0 No CS: 63.4 ± 10.0 | To determine, in adult cardiac surgery, the effectiveness of two intraoperative blood collection techniques. | CS: The residual blood of the circuit of the circulatory system is also treated by CS. This treated blood can be returned to the patient in the operating room or during their transfer to the ICU. Control: The residual blood of the extracorporeal circuit is ultrafiltered. The blood reconcentrated by gentle hemofiltration is collected in a transfer bag. This blood is returned to the patient in the ICU. | The use of CS associated with the use of normovolemic hemodilution was more effective. It makes it possible to carry out 65% of cardiac surgery interventions under extracorporeal circulation without homologous blood supply. | FFP |
| Murphy et al., (2004) (1+) [54] | United Kingdom | Randomized controlled trial | CS (Autolog Medtronic, Watford, UK) No CS (control) | 97 (74/23) 99 (86/13) CS: 62.3 ± 18.7 No CS: 64.3 ± 9.2 | To compare the effects of autotransfusion of washed salvaged RBCs on coagulation pathway function and blood loss after cardiac surgery in a randomized controlled trial. | CS: All blood loss from skin incision to commencement of CPB and then after administration of protamine to skin closure was salvaged at high pressure suction. All blood remaining in the CPB circuit after discontinuation of bypass was re-transfused. Control: All blood spilled before commencement of CPB and after administration of protamine was aspirated using a high-pressure sucker and discarded. | Autotransfusion is a safe and effective method of reducing the use of homologous bank blood after routine first-time coronary artery bypass grafting. | Hemorrhage HB aPTT ratio Platelet count D-dimer |
| Murphy et al., (2005) (1+) [55] | United Kingdom | Randomized controlled trial | CS (Dideco, Gloucester, UK) No CS (control) | 30 (25/5) 31 (23/8) CS: 66.4 ± 7.6 No CS: 62.3 ± 9.3 | To evaluate the safety and effectiveness of intraoperative CS and autotransfusion of washed salvaged RBCs after first-time coronary artery bypass grafting performed on the beating heart. | CS: Patients underwent intraoperative CS, with autotransfusion of washed, salvaged RBCs at the completion of the operative procedure. All blood lost, from skin incision to skin closure, was salvaged at high-pressure suction, washed, and autotransfused. Control: All blood spilled, from skin incision to skin closure, was aspirated with a high-pressure sucker and discarded. | The postoperative HB concentration was significantly higher in the autotransfusion patients. HB levels were similar in the 2 groups after protamine administration and at 1 h (p = 0.71 and p = 0.60, respectively), but at 24 h, the mean level was, on average, 1.02 g/dL lower in the control group (p = 0.0007). A similar difference was noted with HCT. At 24 h, the mean HCT level was significantly lower (0.03 L/L) in the control group (p = 0.0008). | Hemorrhage HB HCT aPTT ratio FFP Platelet count Fibrinogen |
| Niranjan et al., (2006) (1+) [56] | United Kingdom | Randomized clinical trial | CS (Dideco, Gloucester, UK) | 20 (16/4) 20 (16/4) CS: 66.3 ± 7.3 No CS: 66.1 ± 10.8 | To investigate the potential additive effects of autologous CS blood transfusion and CPB on blood loss, homologous blood transfusion requirements and clotting parameters in patients undergoing CABG for the first time. | CS: The device was used to collect blood lost from skin incision to skin closure in the off-CPB group and from skin incision to commencement of CPB and returned to the venous reservoir. Any remaining blood in the CPB circuit after discontinuation from bypass was re-transfused via the aortic cannula before decannulation. Control: In the off-pump group without CS, all lost blood from skin incision to closure was suctioned with a high-pressure sucker into a waste container. All blood lost from skin incision to commencement of CPB and protamine reversal to skin closure was aspirated into a waste sucker. | Off-pump CABG is associated with a significant reduction in intraoperative mediastinal blood loss and homologous transfusion requirements. Autologous transfusion of salvaged washed mediastinal blood reduced homologous transfusion significantly in the on-CPB group. CS caused no significant adverse impact on coagulation parameters in on- or off-CPB CABG. Postoperative morbidity and blood loss were not affected using CPB or autologous blood transfusion. | Hemorrhage |
| Reyes et al., (2010) (1−) [57] | Spain | Randomized clinical trial | CS (CATS, Fresenius Hemocare, France) No CS (control) | 34 (24/10) 29 (18/11) CS: 65.5 ± 12.1 No CS: 63.7 ± 12.7 | To analyze if the use of CS systems reduces the need for blood products in low-risk patients undergoing cardiac surgery. | CS: Device was used in the CS group. CS was used all through the procedure. At the end of surgery, all remaining blood inside the circuits was recovered and concentrated by the CS. All recovered blood was transfused to the patients, and cardiotomy suction was used and the blood transfused to the patient. Control: All blood in the surgical field was aspirated only using the cardiotomy suction. | In low-risk patients, the CS system did not reduce the need for blood transfusion. Clinical outcomes were similar regardless of the use of a CS saver system. A low preoperative HB level and a low BSA were related to the use of blood products. | Hemorrhage HB FFP Platelet count |
| Scrascia et al. (2012) (1−) [58] | Italy | Prospective, randomized, controlled trial | CS (Hemonetics©) No CS (control) | 17 (8/9) 17 (13/4) CS: 71.0 ± 8.0 No CS: 66.0 ± 10 | To evaluate the influence of residual pump blood salvage on inflammatory, coagulative, and fibrinolytic system activation and on postoperative HB levels and transfusion rates in patients undergoing coronary artery bypass grafting. | CS: The CS system was used to collect residual blood remaining inside the bypass CPB at the end of the surgery. This blood was transferred into a sterile collecting bag and transfused to the patient via a standard blood-giving set at the time of skin closure. Control: Blood samples were collected from a peripheral arterial line after the induction of anesthesia and 24 h later. Samples were also taken from the collecting bag after the washing and concentration procedure and prior to infusion into the patient. | The recovery of blood with the use of the CS improves postoperative HB levels but induces the generation of thrombin and activation of fibrinolysis, which increase the potential for coagulopathies. | HB FFP |
| Tachias et al., (2022) (1−) [59] | Greece | Prospective randomized clinical trial | CS (Haemonetics Cell Saver®) No CS (control) | 99 (75/24) 110 (87/23) CS: 66.3 ± 10.0 No CS: 67.1 ± 10.0 | To investigate the potential effects of the centrifuged end-product on bleeding, transfusion rates, and other transfusion-related variables in adult cardiac surgery patients submitted to ECC. | CS: The device was used for all patients and collected lost blood from the moment of pericardiotomy to the ECC, and after ECC weaning to the end of the surgery. The CS concentrate was transfused to the patients. Control: Patients underwent surgery without CS use. | Within the study’s constraints, the perioperative use of the CS concentrate does not seem to affect bleeding or transfusion variables, although it could probably ameliorate postoperative oxygenation in adult cardiac surgery patients. A tendency to promote coagulation disturbances was detected. | Hemorrhage HB aPTT Platelet count Fibrinogen INR |
| Vermeijden et al. (2015) (1−) [60] | The Netherlands | Multicenter, factorial randomized, partially blinded clinical trial | CS (Continuous Autologous Autotransfusion System, C.A.T.S, Fresenius; Haemonetics, Braintree; Sorin, Milan, Italy). No CS (control) | 175 (140/35) 177 (66/111) CS: 66.0 ± 9.5 No CS: 65.0 ± 9.7 | To investigate the effect of CS, LD filters, and their combination on transfusion requirements in cardiac surgical patients. | CS group: Cardiotomy suction blood, blood from the surgical field, and residual heart–lung machine blood was collected. This blood was washed in the CS. Control: Neither CS nor a filter was used. A conventional cardiotomy suction device was used, and blood from the surgical field was discarded after the reversal of heparin. | There was no significant effect of CS or the filter on the total number of blood products. Using a CS reduced RBC transfusions within 24 h but not during the hospital stay. The use of a CS was also significantly associated with increased transfusions of FFP and the percentage of patients who received any transfusion but not with platelets, whereas filters were not associated significantly. | Hemorrhage HB |
| Wang et al., (1994) (1−) [61] | Taiwan | Prospective clinical trial | CS (Haemonetics, Braintree, MA, USA) No CS (control) | 41 (35/6) 70 (54/16) CS: 57.8 ± 9.1 No CS: 57.7 ± 7.6 | To assess the efficacy of this newly introduced blood conservation technique in terms of reducing postoperative transfusion requirements in two different categories of patients who underwent corrective cardiac surgical procedures. | CS: CS was used as the blood conservation method during surgery. Control: Patients underwent surgery without CS use. | The use of CS did not increase the postoperative chest tube drainage in either the CABG or the redo patients. CS is useful in CABG patients, as far as the reduction in transfusion requirements is concerned. | Hemorrhage HB HCT Platelet count |
| Wang et al., (2012) (1−) [62] | China | Randomized clinical trial | CS: (Cell Saver 5+; Haemonetics) CS: (autolog; Medtronic) CS: CATS; Fresenius HemoCare) | 10 (NR) 10 (NR) 10 (NR) Age (NR) | To evaluate three commercially available CS devices in terms of erythrocyte function and the quality of washed RBCs during CPB. | The salvaged blood was processed by different CS devices, which were set up and operated according to the manufacturers’ recommended instructions. Filling and emptying of the centrifugal chambers were performed in automatic mode. | CS devices use the same theory of centrifugation; however, based on different designs, the function of the washed RBCs and the undesirable content removal efficiency differ widely from one device to another. | HB HCT |
| Xie et al. (2015) (1−) [63] | China | Prospective, randomized, controlled trial | CS (Haemonetics, USA) No CS (Control) | 72 (35/37) 69 (29/40) CS: 51.7 ± 15.6 No CS: 53.1 ± 15.1 | To evaluate the efficacy, safety, and cost-effectiveness of intraoperative CS in CPB surgery. | CS: Shed blood from the wound and mediastina were sucked into the CS reservoir. At the end of the surgery, residual blood in the CPB circuit was sucked into the reservoir directly. After being filtrated, centrifugated, washed, and concentrated, the recovered blood became autologous blood and was then transfused back to the patients. Control: Shed blood from the wound and mediastina during the period of non-heparinization and residual blood were sucked into the suction apparatus and were discarded. | The proportion and quantity of perioperative allogeneic RBC transfusion were significantly lower in the CS group. The incidence of residual heparin and total impairment of blood coagulative function in the 24 h after surgery and the incidence of postoperative excessive bleeding were significantly higher in the CS group. The costs of allogeneic RBC transfusion and total allogeneic blood transfusion were also significantly lower in the CS group, but the cost of total blood transfusion was significantly higher in this group. | Hemorrhage FFP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres-Matos, R.; Luque-Oliveros, M.; Pabón-Carrasco, M. Evaluation of Red Blood Cell Biochemical Markers and Coagulation Profiles Following Cell Salvage in Cardiac Surgery: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 6073. https://doi.org/10.3390/jcm13206073
Cáceres-Matos R, Luque-Oliveros M, Pabón-Carrasco M. Evaluation of Red Blood Cell Biochemical Markers and Coagulation Profiles Following Cell Salvage in Cardiac Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024; 13(20):6073. https://doi.org/10.3390/jcm13206073
Chicago/Turabian StyleCáceres-Matos, Rocío, Manuel Luque-Oliveros, and Manuel Pabón-Carrasco. 2024. "Evaluation of Red Blood Cell Biochemical Markers and Coagulation Profiles Following Cell Salvage in Cardiac Surgery: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 13, no. 20: 6073. https://doi.org/10.3390/jcm13206073
APA StyleCáceres-Matos, R., Luque-Oliveros, M., & Pabón-Carrasco, M. (2024). Evaluation of Red Blood Cell Biochemical Markers and Coagulation Profiles Following Cell Salvage in Cardiac Surgery: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 13(20), 6073. https://doi.org/10.3390/jcm13206073






