First Worldwide Report of a Total Colectomy with the Hugo RAS Platform
Abstract
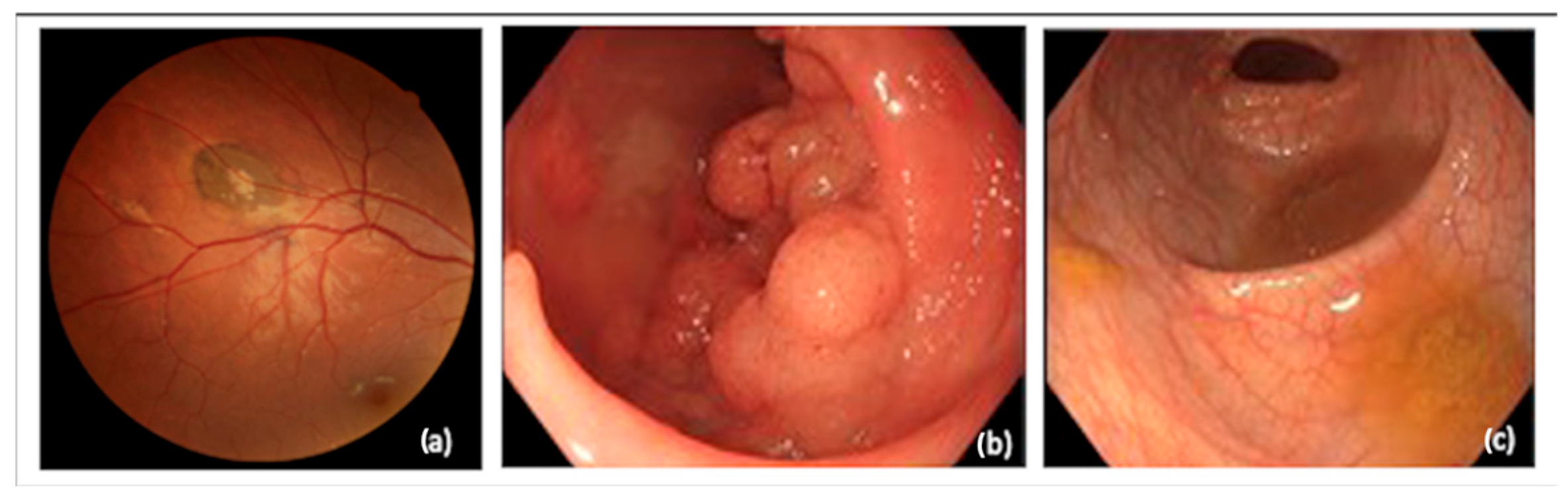
1. Introduction
2. Materials and Methods
2.1. Patient
2.2. Platform Hugo RAS
2.3. Surgical Procedure
2.3.1. Summary
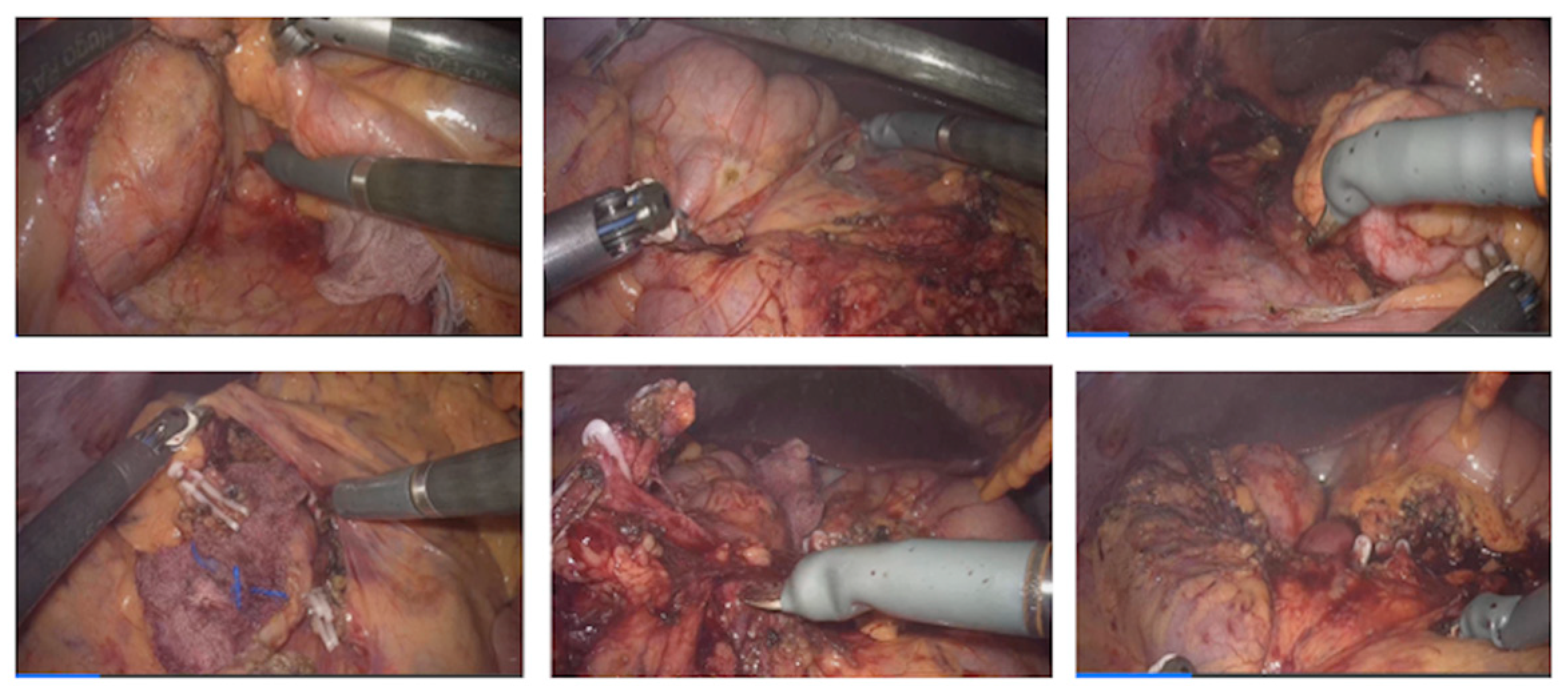
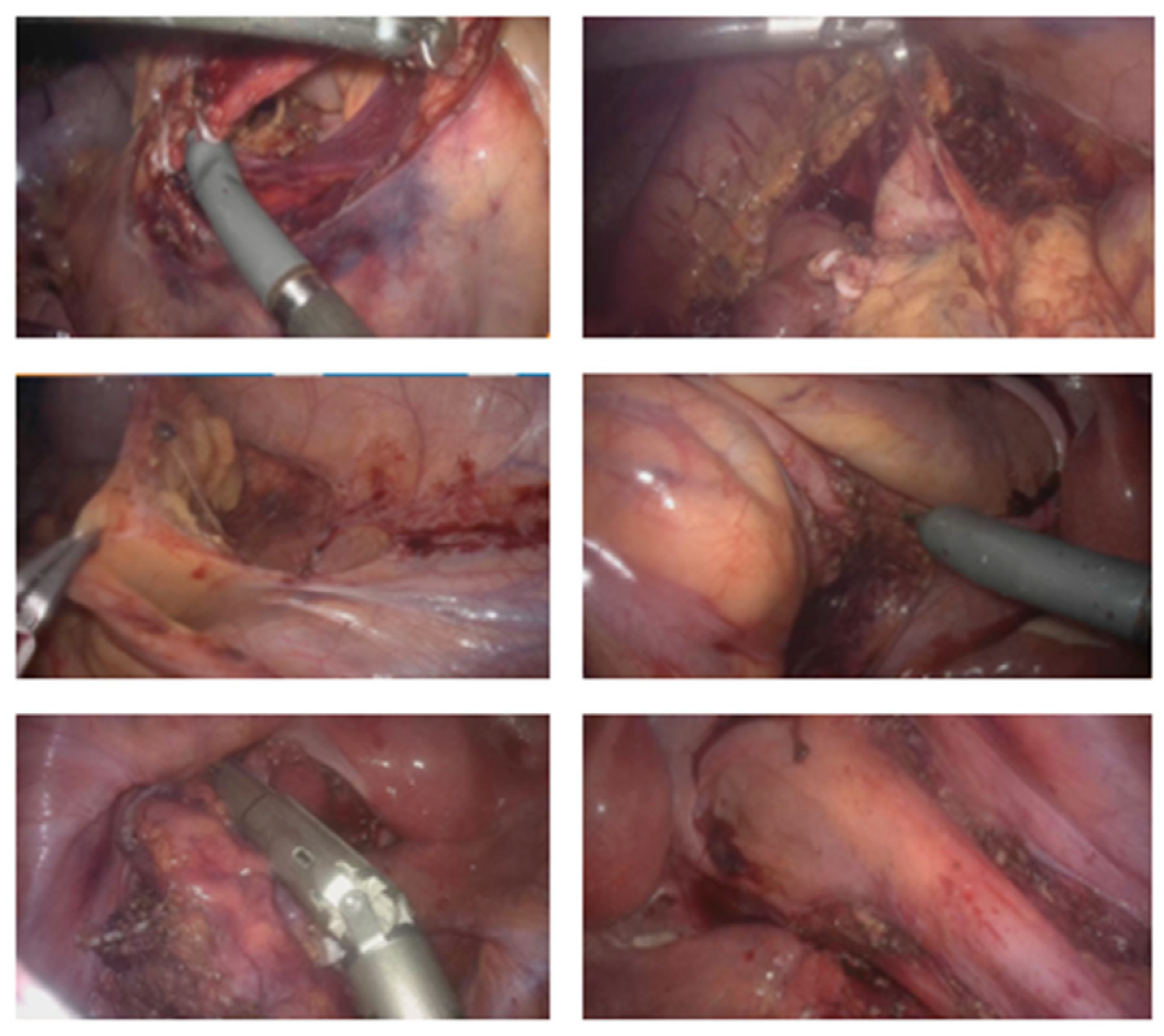
2.3.2. Description of the Procedure Steps
- 8 mm (arm in lateral-superior left position): Tilt (+30°)—dock—45°—reserve;
- 11 mm (arm in the lateral-inferior right position): Tilt (+30°)—dock—145°—endoscope/assistant port;
- 8 mm (arm in the lateral-inferior right position): Tilt (−15°)—dock—225°—surgeon’s left hand (bipolar);
- 11 mm (arm in lateral-superior left position): Tilt (−15°)—dock—315°—surgeon’s right hand (monopolar curved shears); also used for laparoscopic Hem-o-lok application.
- Assistant port and insufflation/aspiration system
- 8 mm (arm in the lateral-superior left position): Tilt (−15°)—dock—45°—surgeon’s left hand with Cadiere grasper;
- 8 mm (arm in the lateral-inferior right position): Tilt (−15°)—dock—145°—surgeon’s right hand (monopolar curved shears);
- 11 mm (arm in the lateral-inferior right position): Tilt (+30°)—dock—225°—endoscope;
- 11 mm (arm in the lateral-superior right position): Tilt (+30°)—dock—315°—reserve (Cadiere bipolar forceps).
- Assistant port and insufflation/aspiration system
- A.
- Suprapubic incision;
- B.
- Extraction of the surgical specimen;
- C.
- Ileorectal anastomosis.
2.3.3. General Description of the Procedure
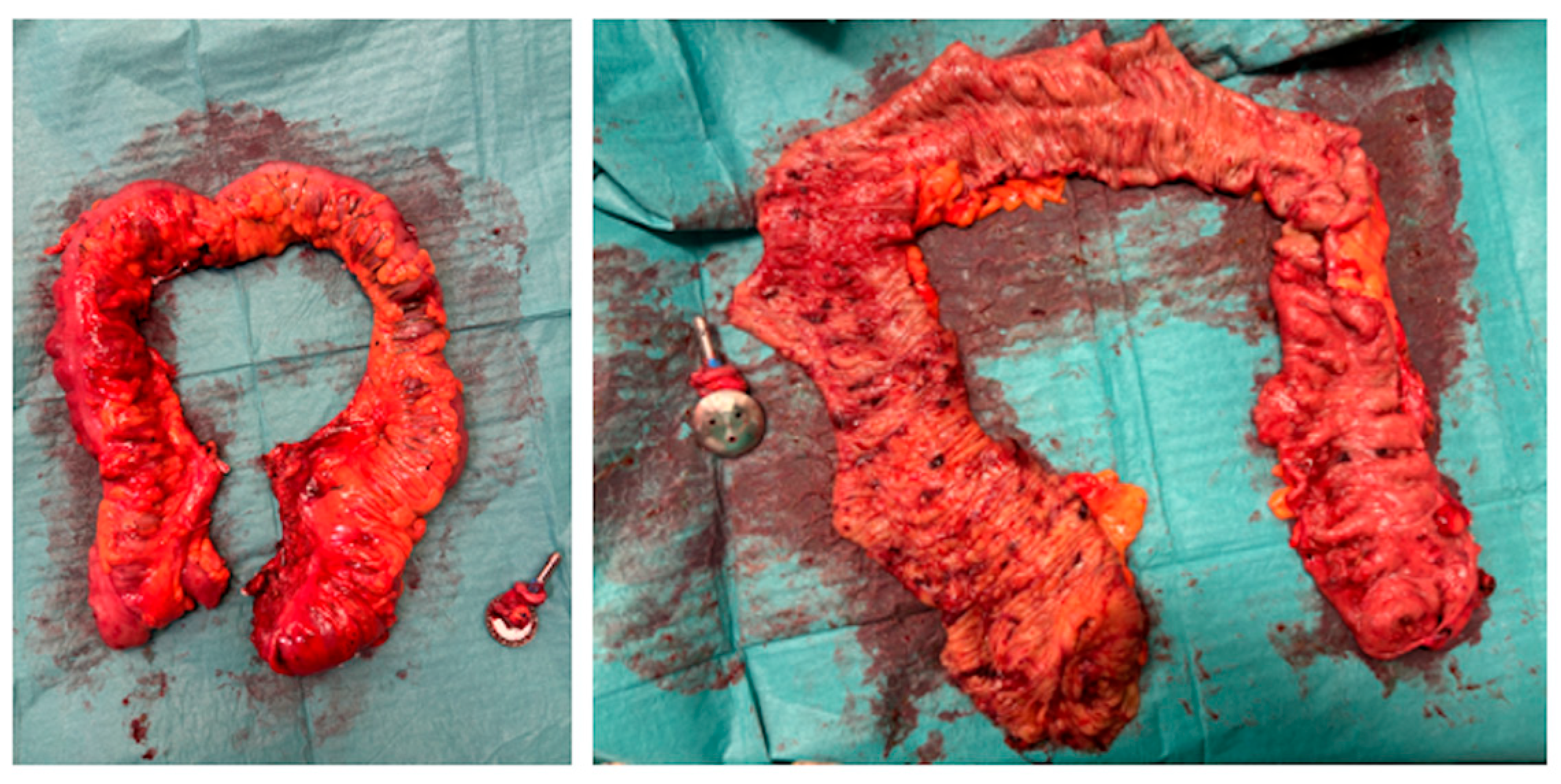
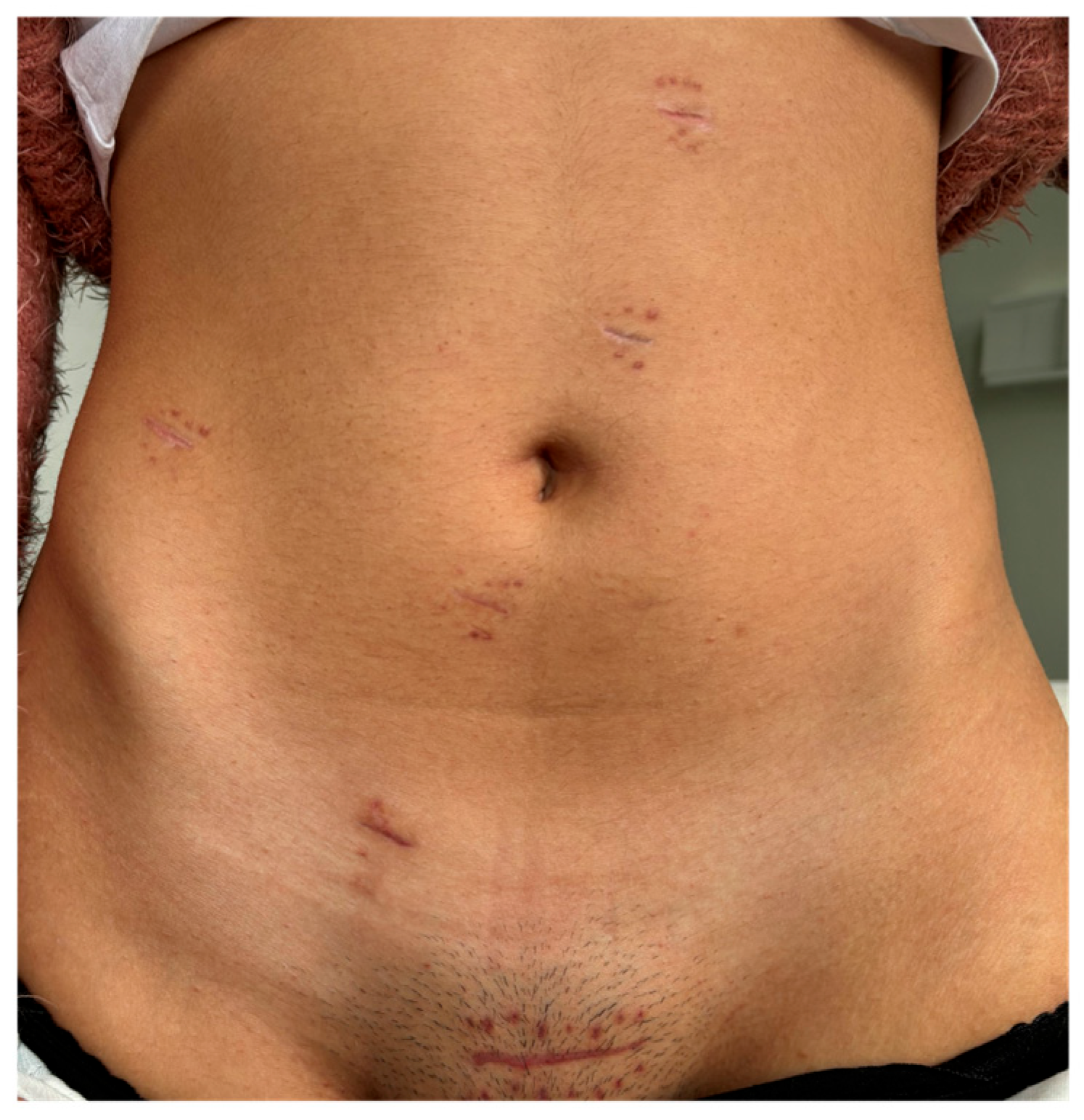
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moghadamyeghaneh, Z.; Hanna, M.H.; Carmichael, J.C.; Pigazzi, A.; Stamos, M.J.; Mills, S. Comparison of open, laparoscopic, and robotic approaches for total abdominal colectomy. Surg. Endosc. 2016, 30, 2792–2798. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Oh, B.Y.; Chung, S.S.; Lee, R.A.; Noh, G.T. Short-term outcomes of single-incision robotic colectomy versus conventional multiport laparoscopic colectomy for colon cancer. J. Robot. Surg. 2023, 17, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S.S.; D’Adamo, C.; Mavanur, A.; Wolf, J.H. A retrospective comparison of 90-day outcomes, length of stay, and readmissions between robotic-assisted and laparoscopic colectomy. J. Robot. Surg. 2023, 17, 2205–2209. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Rodriguez, R.M.; Quezada-Diaz, F.; Tchack, M.; Pappou, E.; Wei, I.H.; Smith, J.J.; Nash, G.M.; Guillem, J.G.; Paty, P.B.; Weiser, M.R.; et al. Use of the Xi robotic platform for total abdominal colectomy: A step forward in minimally invasive colorectal surgery. Surg. Endosc. 2019, 33, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Hollandsworth, H.M.; Stringfield, S.; Klepper, K.; Zhao, B.; Abbadessa, B.; Lopez, N.E.; Parry, L.; Ramamoorthy, S.; Eisenstein, S. Multiquadrant surgery in the robotic era: A technical description and outcomes for da Vinci Xi robotic subtotal colectomy and total proctocolectomy. Surg. Endosc. 2020, 34, 5153–5159. [Google Scholar] [CrossRef] [PubMed]
- Ngu, J.C.; Lin, C.C.; Sia, C.J.; Teo, N.Z. A narrative review of the Medtronic Hugo RAS and technical comparison with the Intuitive da Vinci robotic surgical system. J. Robot. Surg. 2024, 18, 99. [Google Scholar] [CrossRef] [PubMed]
- Prata, F.; Ragusa, A.; Tempesta, C.; Iannuzzi, A.; Tedesco, F.; Cacciatore, L.; Raso, G.; Civitella, A.; Tuzzolo, P.; Calle, P.; et al. State of the Art in Robotic Surgery with Hugo RAS System: Feasibility, Safety and Clinical Applications. J. Pers. Med. 2023, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Brime Menendez, R.; Garcia Rojo, E.; Hevia Palacios, V.; Feltes Ochoa, J.A.; Justo Quintas, J.; Lista Mateos, F.; Fraile, A.; Manfredi, C.; Belli, S.; Bozzini, G.; et al. Da Vinci vs. Hugo RAS for robot-assisted radical prostatectomy: A prospective comparative single-center study. World J. Urol. 2024, 42, 336. [Google Scholar] [CrossRef] [PubMed]
- Garcia Rojo, E.; Hevia Palacios, V.; Brime Menendez, R.; Feltes Ochoa, J.A.; Justo Quintas, J.; Lista Mateos, F.; Touijer, K.; Romero Otero, J. Da Vinci and Hugo RAS Platforms for robot-assisted partial nephrectomy: A preliminary prospective comparative analysis of the outcomes. Minerva Urol. Nephrol. 2024, 76, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.C.; Marian, L.; Li, C.C.; Juan, Y.S.; Tung, M.C.; Shih, H.J.; Chang, C.P.; Chen, J.T.; Yang, C.H.; Ou, Y.C. Robot-Assisted Radical Prostatectomy by the Hugo Robotic-Assisted Surgery (RAS) System and the da Vinci System: A Comparison between the Two Platforms. Cancers 2024, 16, 1207. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, P.P.; Salaj, A.; Rocco, B.; Formisano, G. First worldwide report on Hugo RAS surgical platform in right and left colectomy. Updates Surg. 2023, 75, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Romero-Marcos, J.M.; Sampson-Davila, J.G.; Cuenca-Gomez, C.; Altet-Torne, J.; Gonzalez-Abos, S.; Ojeda-Jimenez, I.; Galaviz-Sosa, M.L.; Delgado-Rivilla, S. Colorectal procedures with the novel Hugo RAS system: Training process and case series report from a non-robotic surgical team. Surg. Endosc. 2024, 38, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Belyaev, O.; Fahlbusch, T.; Slobodkin, I.; Uhl, W. Use of Hugo(TM) RAS in General Surgery: The First 70 Cases at a German Centre and a Systematic Review of the Literature. J. Clin. Med. 2024, 13, 3678. [Google Scholar] [CrossRef] [PubMed]
- Caputo, D.; Cammarata, R.; Farolfi, T.; Coppola, R.; La Vaccara, V. First worldwide report on rectal resections with Hugo surgical system: Description of docking angles and tips for an effective setup. ANZ J. Surg. 2024, 94, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Vicente, E.; Quijano, Y.; Ferri, V. New era of robotic surgery: First case in Spain of right hemicolectomy on Hugo RAS surgical platform. BMJ Case Rep. 2023, 16, e256035. [Google Scholar] [PubMed]
- Bourla, C.; Taoum, C.; Kante, S.; Mourregot, A.; Rouanet, P.; Colombo, P.E. Port placement strategies and management of the robotic system during total colectomy or total coloproctectomy for cancer—A video vignette. Color. Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2023, 25, 1733–1735. [Google Scholar] [CrossRef] [PubMed]
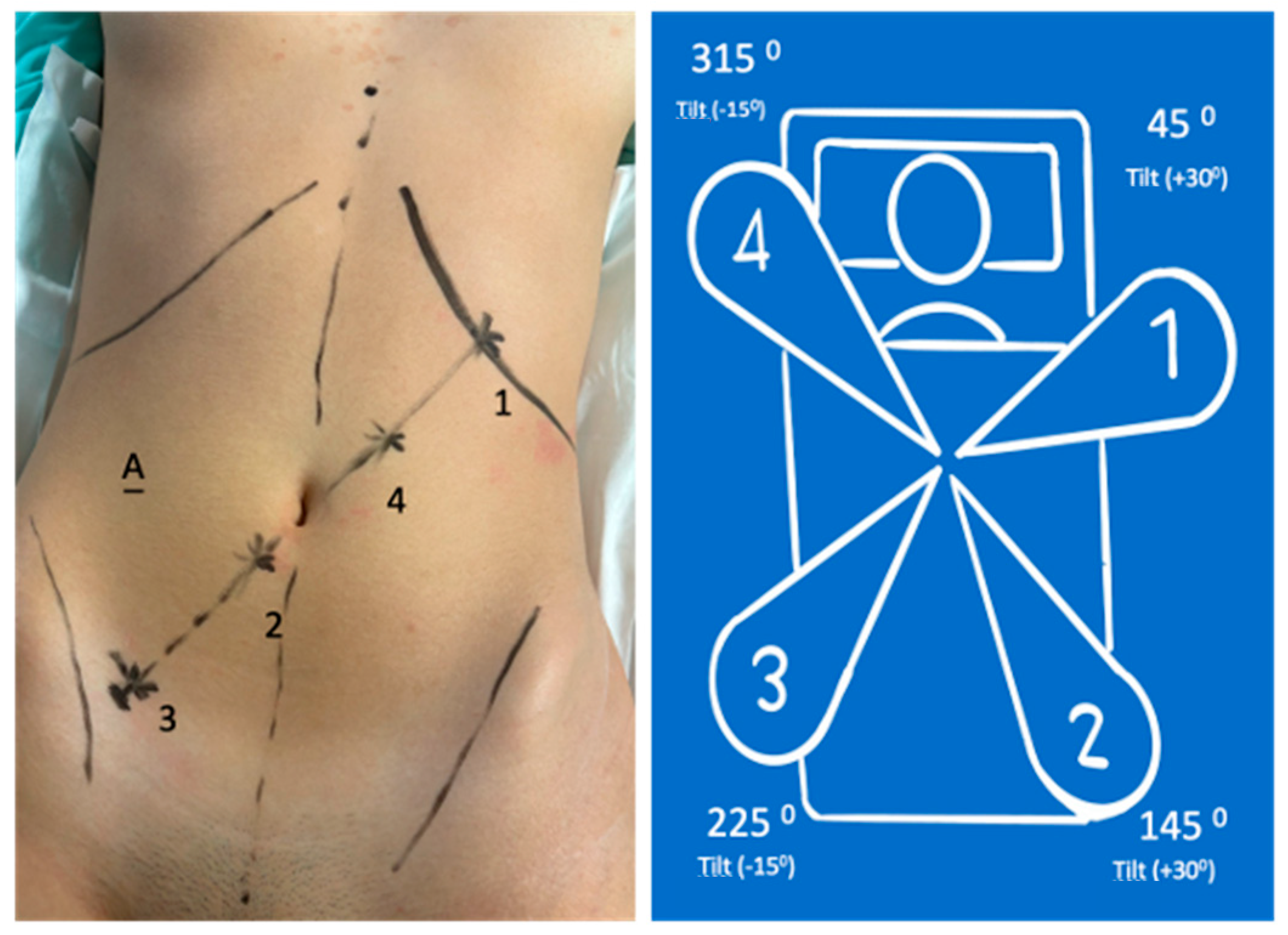

| Phase | Docking Time | Patient Position | Port Placement and System Configuration | Main Steps |
|---|---|---|---|---|
| Phase I Initial Steps and System Configuration | Docking Time: 3 min 25 s | - Operating table at 60 cm height; - Supine position in modified lithotomy; - 10° Trendelenburg; - 10° left lateral tilt. | Ports: - Four ports positioned diagonally from the left hypochondrium to the right iliac fossa; - Two robotic trocars of 8 mm; - Two robotic trocars of 11 mm (one for the camera, one for traditional laparoscopic instruments); - A 12 mm trocar for insufflation and assistance. System Configuration: - Arm 1 (8 mm): Tilt (+30°), dock, 45°—reserve; - Arm 2 (11 mm): Tilt (+30°), dock, 145°—endoscope/assistant port; - Arm 3 (8 mm): Tilt (−15°), dock, 225°—surgeon’s left hand (bipolar); - Arm 4 (11 mm): Tilt (−15°), dock, 315°—surgeon’s right hand (monopolar curved shears). | - Port placement; - System configuration and robotic arm docking. |
| Phase II Assisted Robotic Right Colectomy | N/A | Same as Phase I | As configured in Phase I | - Access to retroperitoneum between the superior mesenteric vein and ileocolic vessels; - Dissection and division of ileocolic vessels; - Identification and control of right colic and middle colic vessels; - Medial-to-lateral mobilization of the right colon; - Opening of the greater omentum to access the lesser sac and complete mobilization of the right colon. |
| Phase III System modification | Docking Time: 2 min 55 s | - 10–15° Trendelenburg; - 10–15° right lateral tilt. | System Configuration: - Arm 1 (8 mm): Tilt (−15°), dock, 45°—surgeon’s left hand (Cadiere grasper); - Arm 2 (8 mm): Tilt (−15°), dock, 145°—surgeon’s right hand (monopolar curved shears); - Arm 3 (11 mm): Tilt (+30°), dock, 225°—endoscope; - Arm 4 (11 mm): Tilt (+30°), dock, 315°—reserve (Cadiere bipolar forceps). | - Detachment of robotic arms; - Patient repositioning; - Redocking of robotic arms with a new configuration for left colectomy. |
| Phase IV Assisted Robotic Left Colectomy | N/A | Same as Phase III | As configured in Phase III | - Division of inferior mesenteric artery and vein; - Medial-to-lateral dissection of the left mesocolon; - Opening of the left paracolic gutter up to the splenic flexure; - Mobilization of the splenic flexure; - Disconnection of the greater omentum from the left transverse colon; - Complete mobilization of the transverse colon; - Partial mesorectal dissection and division of the rectum below the rectosigmoid junction. |
| Phase V Laparoscopic Assisted Phase | N/A | - Suprapubic (Pfannenstiel) incision | - Removal of robotic trocars; - Use of traditional laparoscopic instruments. | - Incision for extraction of surgical specimen; - Division of the ileum and introduction of a 28 mm EEA circular stapler anvil; - Transanal ileorectal anastomosis; - Integrity test of the anastomosis with methylene blue; - Closure of the ports. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, M.D.d.; Brandão, P. First Worldwide Report of a Total Colectomy with the Hugo RAS Platform. J. Clin. Med. 2024, 13, 6071. https://doi.org/10.3390/jcm13206071
Santos MDd, Brandão P. First Worldwide Report of a Total Colectomy with the Hugo RAS Platform. Journal of Clinical Medicine. 2024; 13(20):6071. https://doi.org/10.3390/jcm13206071
Chicago/Turabian StyleSantos, Marisa Domingues dos, and Pedro Brandão. 2024. "First Worldwide Report of a Total Colectomy with the Hugo RAS Platform" Journal of Clinical Medicine 13, no. 20: 6071. https://doi.org/10.3390/jcm13206071
APA StyleSantos, M. D. d., & Brandão, P. (2024). First Worldwide Report of a Total Colectomy with the Hugo RAS Platform. Journal of Clinical Medicine, 13(20), 6071. https://doi.org/10.3390/jcm13206071






