Risk of Hepatitis B Virus Reactivation in COVID-19 Patients Receiving Immunosuppressive Treatment: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Definitions
2.4. Statistical Analysis
3. Results
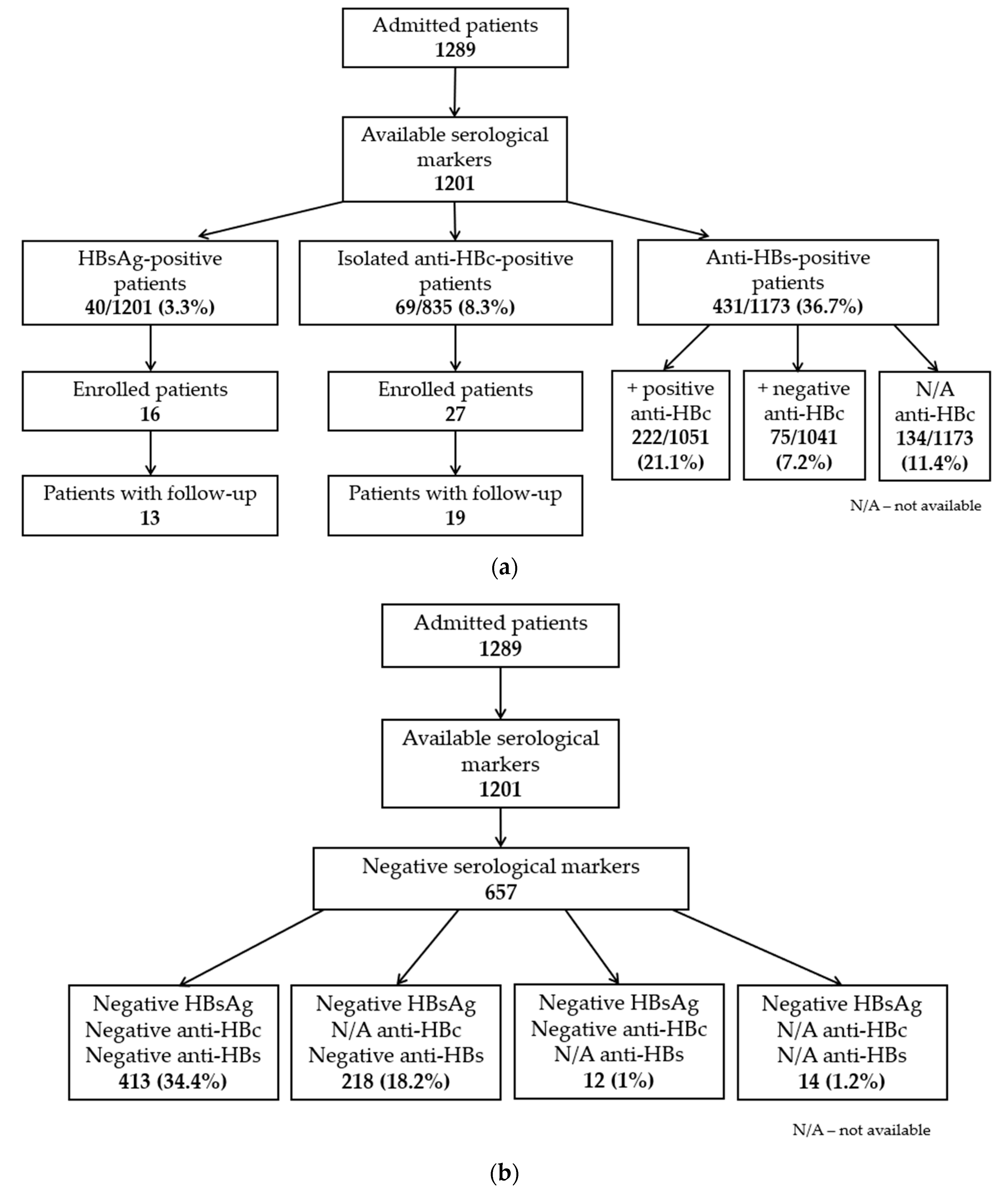
3.1. Seroprevalence of HBV Infection in COVID-19 Patients
3.2. General Characteristics of Study Participants
3.3. Hepatitis B Virus Status of the Study Participants
3.3.1. HBsAg-Positive Patients
3.3.2. HBsAg-Negative/Anti-HBc-Positive Patients
3.4. Biochemical and Hematological Parameters of Study Participants
3.5. Immunosuppressive COVID-19 Treatment Administered during Hospitalization
3.6. Patients with HBVr
- Patient no. 8, aged 56, with positive HBsAg and a baseline HBV DNA level of 179 (2.25 log) IU/mL, was treated with IV dexamethasone (8 mg/day for 5 days and then 4 mg/day for 2 days). The patient was initially re-evaluated 1 month after discharge, and at that time, HBV DNA was 1660 (3.22 log) IU/mL. At 3 months, a viral load of 17,378 (4.24 log) IU/mL was detected. FibroMax was also performed, which revealed F1/A0-A1/S3/N2/H0. According to the decision of her attending physician, treatment with entecavir 0.5 mg/day was then initiated.
- Patient no. 14, aged 75, was known to have chronic HBV infection. However, we found isolated anti-HBc and undetectable HBV DNA at the time of admission. He received a single dose (100 mg) of subcutaneous anakinra, a single dose (400 mg) of IV tocilizumab, and IV dexamethasone (8 mg/day for 3 days; then, 6 mg/day for 5 days, followed by 4 mg/day for 7 days). At follow-up, a detectable viral load was found (HBV DNA < 10 IU/mL).
- Patient no. 20, aged 63, with isolated anti-HBc and initially undetectable HBV DNA, developed a critical form of COVID-19 requiring admission to the Intensive Care Unit and non-invasive ventilation. During hospitalization, she received a single dose (800 mg) of IV tocilizumab, oral baricitinib (4 mg/day for 3 days), and IV dexamethasone in gradually decreasing doses (initially, 8 mg every 12 h for 1 day; then, 12 mg/day for 3 days; then, 8 mg/day for 7 days, 6 mg/day for 4 days, 4 mg/day for 1 day, and 2 mg/day for 2 days). At follow-up, a detectable viral load was found (HBV DNA < 10 IU/mL).
- Patient no. 28, aged 80, with isolated anti-HBc and initially undetectable HBV DNA, was treated with IV dexamethasone (6 mg/day for 7 days; then, 4 mg/day for 2 days). At follow-up, detectable HBV viral load (10 IU/mL) was found.
4. Discussion
4.1. Seroprevalence of HBV Infection in COVID-19 Patients
4.2. Definitions of HBVr
4.3. Risk of HBVr in Patients Receiving Immunosuppressive Treatment for COVID-19
4.4. Risk of HBVr in Patients Receiving Immunosuppressive Treatment for Non-COVID-19 Diseases
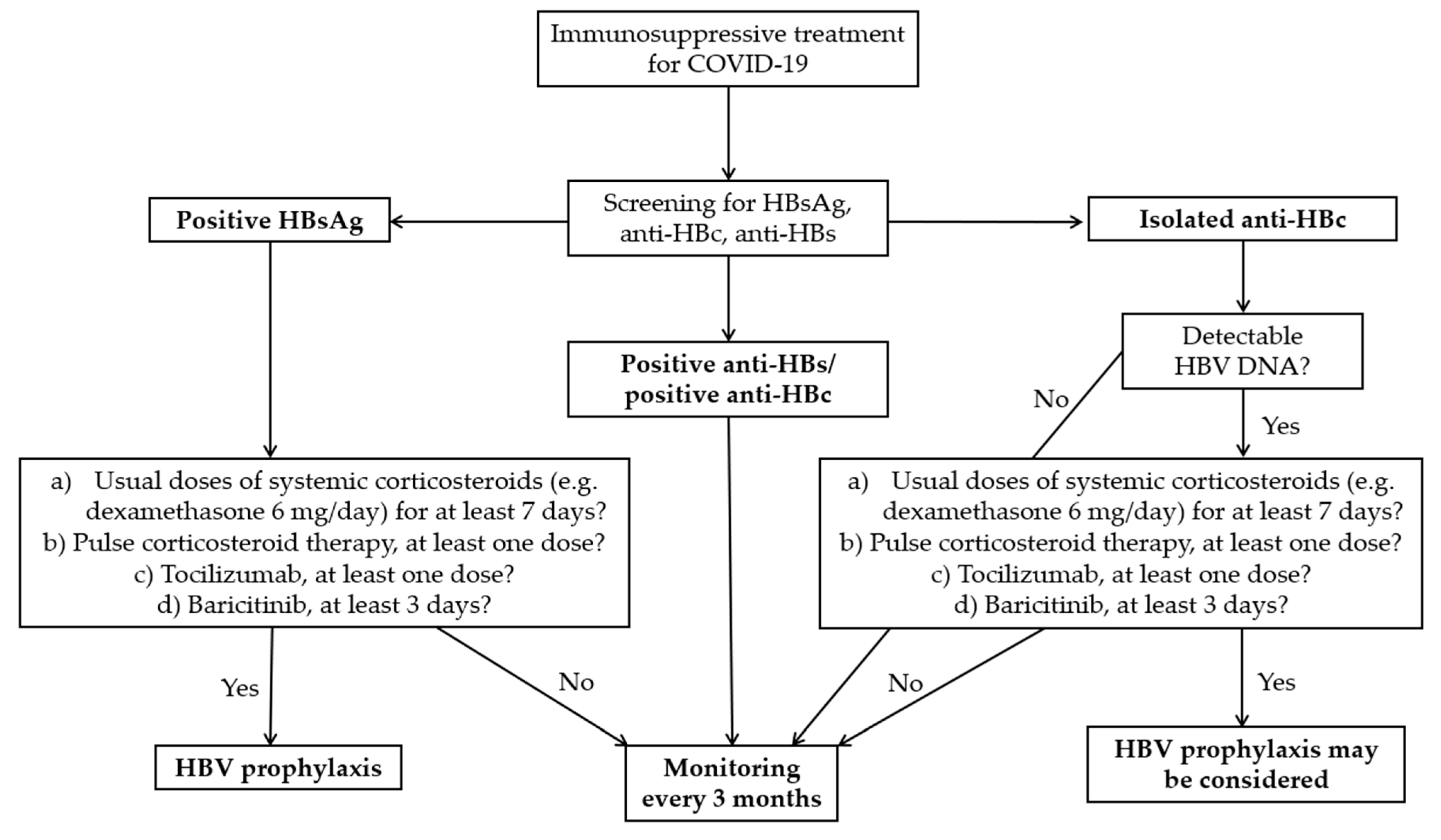
4.5. Management of HBV–SARS-CoV-2 Co-Infected Patients Receiving Immunosuppressive Treatment
4.6. Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO COVID-19 Dashboard. Available online: https://covid19.who.int/ (accessed on 10 September 2024).
- Montazersaheb, S.; Khatibi, S.M.H.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Sorbeni, F.G.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef]
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://www.ncbi.nlm.nih.gov/books/NBK570371/pdf/Bookshelf_NBK570371.pdf (accessed on 8 August 2024).
- Liu, J.; Wang, T.; Cai, Q.; Sun, L.; Huang, D.; Zhou, G.; He, Q.; Wang, F.; Liu, L.; Chen, J. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol. Res. 2020, 50, 1211–1221. [Google Scholar] [CrossRef]
- Rodríguez-Tajes, S.; Miralpeix, A.; Costa, J.; López-Suñé, E.; Laguno, M.; Pocurull, A.; Lens, S.; Mariño, Z.; Forns, X. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J. Viral Hepat. 2021, 28, 89–94. [Google Scholar] [CrossRef]
- Camarero, J.G.; Aranda, E.B.; Castro, R.Q.; Chumillas, R.M.S.; Vega, L.A.; Ruiz, S.D.; Manero, N.G.; Campo, R.V.; Plaza, F.J. Hepatitis B and C screening in hospitalized patients with SARS-CoV-2 infection. Gastroenterol. Hepatol. 2022, 45, 256–264. [Google Scholar] [CrossRef]
- Foo, H.; Phan, F.; Bagatella, M.; Petrovski, I.; Nagendra, V.; Acharya, P.; Levy, M.; Prakoso, E. Risk of hepatitis B reactivation following baricitinib or tocilizumab for treatment of COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 799–801. [Google Scholar] [CrossRef]
- MacLachlan, J.H.; Cowie, B.C. Hepatitis B virus epidemiology. CSH Perspect. Med. 2015, 5, a021410. [Google Scholar] [CrossRef]
- Trickey, A.; Bivegete, S.; Duffell, E.; McNaughton, A.L.; Nerlander, L.; Walker, J.G.; Fraser, H.; Hickman, M.; Vickerman, P.; Brooks-Pollock, E.; et al. Estimating hepatitis B virus prevalence among key population groups for European Union and European Economic Area countries and the United Kingdom: A modelling study. BMC Infect. Dis. 2023, 23, 457. [Google Scholar] [CrossRef]
- Conners, E.E.; Panagiotakopoulos, L.; Hofmeister, M.G.; Spradling, P.R.; Hagan, L.M.; Harris, A.M.; Rogers-Brown, J.S.; Wester, C.; Nelson, N.P. Screening and testing for hepatitis B virus infection: CDC recommendations—United States, 2023. MMWR Recomm. Rep. 2023, 72, 1–25. [Google Scholar] [CrossRef]
- Perrillo, R.P.; Gish, R.; Falck-Ytter, Y.T. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015, 148, 221–244.e3. [Google Scholar] [CrossRef]
- Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of chronic hepatitis B. Clin. Mol. Hepatol. 2019, 25, 93–159. [Google Scholar] [CrossRef]
- Lau, G.; Yu, M.-L.; Wong, G.; Thompson, A.; Ghazinian, H.; Hou, J.-L.; Piratvisuth, T.; Jia, J.-D.; Mizokami, M.; Cheng, G.; et al. APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatol. Int. 2021, 15, 1031–1048. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.-M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Jiménez, M.L.; Garrido, M.L.; Cano, M.C.F. Letter to the editor: Reactivation of HBV triggered by SARS-CoV-2 in a patient with cirrhosis. Hepatology 2022, 75, 765–766. [Google Scholar] [CrossRef]
- Giugliano, L.; Pinon, M.; Calvo, P.L. COVID-19 as a trigger of acute-on-chronic hepatitis B presenting with undetectable INR due to hypercoagulability in a 16-year-old girl. Pediatr. Infect. Dis. J. 2023, 42, 143–145. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Du, M.; Liu, M.; Liu, Y.; He, Y. Patients with COVID-19 and HBV coinfection are at risk of poor prognosis. Infect. Dis. Ther. 2022, 11, 1229–1242. [Google Scholar] [CrossRef]
- Mastroianni, A.; Greco, S.; Chidichimo, L.; Mauro, M.V.; Urso, F.; Vangeli, V. Antiviral prophylaxis for hepatitis B virus in COVID-19 patients treated with immunosuppressive drug therapy. Antivir. Ther. 2022, 27, 13596535211067602. [Google Scholar] [CrossRef]
- Wu, Y.-F.; Yu, W.-J.; Jiang, Y.-H.; Chen, Y.; Zhang, B.; Zhen, R.-B.; Zhang, J.-T.; Wang, Y.-P.; Li, Q.; Xu, F.; et al. COVID-19 or treatment associated immunosuppression may trigger hepatitis B virus reactivation: A case report. World J. Clin. Cases 2021, 9, 5266–5269. [Google Scholar] [CrossRef]
- Sagnelli, C.; Montella, L.; Grimaldi, P.; Pisaturo, M.; Alessio, L.; De Pascalis, S.; Sagnelli, E.; Coppola, N. COVID-19 as another trigger for HBV reactivation: Clinical case and review of literature. Pathogens 2022, 11, 816. [Google Scholar] [CrossRef]
- Braimakis, I.; Vasileiadi, S.; Trifylli, E.-M.; Papadopoulos, N.; Deutsch, M. Can hepatitis B virus (HBV) reactivation result from a mild COVID-19 infection? Livers 2023, 3, 347–353. [Google Scholar] [CrossRef]
- Figueredo, C.J.; Haider, T.; Guddati, H.; Massoumi, H. A case of hepatitis B reactivation associated hepatitis after tocilizumab therapy in a patient with SARS-CoV-2 infection. Am. J. Gastroenterol. 2021, 116, S1205–S1206. [Google Scholar] [CrossRef]
- Wong, G.L.; Yuen, B.W.; Chan, H.L.; Tse, Y.; Yip, T.C.; Lam, K.L.; Lui, G.C.; Wong, V.W. Impact of dose and duration of corticosteroid on the risk of hepatitis flare in patients with chronic hepatitis B. Liver Int. 2019, 39, 271–279. [Google Scholar] [CrossRef]
- Wong, G.L.-H.; Wong, V.W.-S.; Yuen, B.W.-Y.; Tse, Y.-K.; Yip, T.C.-F.; Luk, H.W.-S.; Lui, G.C.-Y.; Chan, H.L.-Y. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J. Hepatol. 2020, 72, 57–66. [Google Scholar] [CrossRef]
- Chen, L.-F.; Mo, Y.-Q.; Jing, J.; Ma, J.-D.; Zheng, D.-H.; Dai, L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: A prospective clinical observation. Int. J. Rheum. Dis. 2017, 20, 859–869. [Google Scholar] [CrossRef]
- Kuo, M.H.; Tseng, C.-W.; Lu, M.-C.; Tung, C.-H.; Tseng, K.-C.; Huang, K.-Y.; Lee, C.-H.; Lai, N.-S. Risk of hepatitis B virus reactivation in rheumatoid arthritis patients undergoing tocilizumab-containing treatment. Dig. Dis. Sci. 2021, 66, 4026–4234. [Google Scholar] [CrossRef]
- Chen, M.-H.; Liu, C.-Y.; Tsai, C.-Y.; Huang, D.-F.; Lin, H.-Y.; Lee, M.-H.; Huang, Y.-H. Hepatitis B virus reactivation in rheumatoid arthritis patients undergoing biologics treatment. J. Infect. Dis. 2016, 215, 566–573. [Google Scholar] [CrossRef]
- Sonneveld, M.J.; Murad, S.D.; van der Eijk, A.; de Man, R. Fulminant liver failure due to hepatitis B reactivation during treatment with tocilizumab. ACG Case Rep. J. 2019, 6, e00243. [Google Scholar] [CrossRef]
- Biehl, A.; Harinstein, L.; Brinker, A.; Glaser, R.; Muñoz, M.; Avigan, M. A case series analysis of serious exacerbations of viral hepatitis and non-viral hepatic injuries in tocilizumab-treated patients. Liver Int. 2021, 41, 515–528. [Google Scholar] [CrossRef]
- Campbell, C.; Andersson, M.I.; Ansari, M.A.; Moswela, O.; Misbah, S.A.; Klenerman, P.; Matthews, P.C. Risk of reactivation of hepatitis B virus (HBV) and tuberculosis (TB) and complications of hepatitis C virus (HCV) following tocilizumab therapy: A systematic review to inform risk assessment in the COVID-19 era. Front. Med. 2021, 8, 706482. [Google Scholar] [CrossRef]
- Yip, T.C.-F.; Gill, M.; Wong, G.L.-H.; Liu, K. Management of hepatitis B virus reactivation due to treatment of COVID-19. Hepatol. Int. 2022, 16, 257–268. [Google Scholar] [CrossRef]
- Harigai, M.; Winthrop, K.; Takeuchi, T.; Hsieh, T.-Y.; Chen, Y.-M.; Smolen, J.S.; Burmester, G.; Walls, C.; Wu, W.-S.; Dickson, C.; et al. Evaluation of hepatitis B virus in clinical trials of baricitinib in rheumatoid arthritis. RMD Open 2020, 6, e001095. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury—Anakinra. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548615/ (accessed on 8 August 2024).
- Barone, M.; Notarnicola, A.; Lopalco, G.; Viggiani, M.T.; Sebastiani, F.; Covelli, M.; Iannone, F.; Avolio, A.W.; Di Leo, A.; Cantarini, L.; et al. Safety of long-term biologic therapy in rheumatologic patients with a previously resolved hepatitis B viral infection. Hepatology 2015, 62, 40–46. [Google Scholar] [CrossRef]
- Pérez, J.V.; Chinesta, J.R. Risk of hepatitis B reactivation associated with treatment against SARS-CoV-2 (COVID-19) with corticosteroids. Rev. Clin. Esp. 2020, 220, 535–536. [Google Scholar] [CrossRef]
- Spera, A.M. Hepatitis B virus infection reactivation in patients under immunosuppressive therapies: Pathogenesis, screening, prevention and treatment. World J. Virol. 2022, 11, 275–282. [Google Scholar] [CrossRef]
- Satsangi, S.; Gupta, N.; Kodan, P. Current and new drugs for COVID-19 treatment and its effects on the liver. J. Clin. Transl. Hepatol. 2021, 9, 436–446. [Google Scholar] [CrossRef]
- Wong, G.L.; Chan, H.L.; Yuen, B.W.; Tse, Y.; Luk, H.W.; Yip, T.C.; Hui, V.W.; Liang, L.Y.; Lee, H.; Lui, G.C.; et al. The safety of stopping nucleos(t)ide analogue treatment in patients with HBeAg-negative chronic hepatitis B. Liver Int. 2020, 40, 549–557. [Google Scholar] [CrossRef]
| Variables | All Patients (N = 32) |
|---|---|
| Age (years), median (IQR) | 67 (58.5–71.5) |
| Male, n (%) | 19 (59.4%) |
| BMI (kg/m2), median (IQR) | 28.5 (26.3–32.5) |
| Comorbidity, n (%) | |
| Hypertension | 20 (62.5%) |
| Obesity | 13 (40.6%) |
| Chronic pulmonary diseases | 8 (25%) |
| Diabetes mellitus | 7 (21.9%) |
| Alcohol use disorder | 3 (9.4%) |
| Other comorbidities | 4 (12.5%) |
| No comorbidities, n (%) | 4 (12.5%) |
| COVID-19 severity, n (%) | |
| Mild | 3 (9.4%) |
| Moderate | 18 (56.3%) |
| Severe | 9 (28.1%) |
| Critical | 2 (6.3%) |
| COVID-19 diagnosis during the Omicron variant circulation, n (%) | 18 (56.3%) |
| Immunosuppressive COVID-19 treatment administered during hospitalization, n (%) | |
| Systemic corticosteroids | 23 (71.9%) |
| Anakinra | 9 (28.1%) |
| Tocilizumab | 4 (12.5%) |
| Baricitinib | 4 (12.5%) |
| No immunosuppressive COVID-19 treatment, n (%) | 9 (28.1%) |
| Hepatitis B virus status, n (%) | |
| HBsAg-positive | 13 (40.6%) |
| HBsAg-negative/anti-HBc-positive | 19 (59.4%) |
| (a) HBsAg-Positive Patients | ||||||
|---|---|---|---|---|---|---|
| No. | Sex | Age (Years) | Treatment—Duration, Cumulative Dose | Viral Load at Baseline (IU/mL) | Viral Load at Follow-Up (IU/mL) | HBVr |
| Patients who received tocilizumab, anakinra, and/or baricitinib | ||||||
| 1 | F | 58 | ANK—7 days, 1000 mg DEX—12 days, 82 mg | 164 | 362 | No |
| 2 | M | 43 | TCZ—1 dose, 400 mg ANK—5 days, 1400 mg DEX—10 days, 128 mg | 34 | 18 | No |
| 3 | F | 70 | ANK—7 days, 1000 mg DEX—7 days, 72 mg | <10 | <10 | No |
| 4 | F | 53 | ANK—7 days, 1000 mg DEX—9 days, 68 mg | 342 | 723 | No |
| 5 o | M | 61 | ANK—4 days, 700 mg DEX—9 days, 76 mg | 18 | 66 | No |
| Patients who received systemic corticosteroids | ||||||
| 6 | M | 67 | DEX—16 days, 146 mg | 18 | <10 | No |
| 7 o | F | 55 | DEX—2 days, 16 mg | U | 17 | No |
| 8 o | F | 56 | DEX—7 days, 48 mg | 179 | 17,378 | Yes |
| Patients who did not receive any immunosuppressive treatment | ||||||
| 9 | M | 61 | - | 126 | 225 | No |
| 10 o | M | 49 | - | 646 | 1190 | No |
| 11 o | F | 34 | - | 77 | <10 | No |
| 12 o | F | 77 | - | 11 | 118 | No |
| 13 o | F | 67 | - | U | 16 | No |
| (b) HBsAg-Negative/Anti-HBc-Positive Patients | ||||||
| No. | Sex | Age (Years) | Treatment—Duration, Cumulative Dose | Viral Load at Baseline a (IU/mL) | Viral Load at Follow-Up (IU/mL) | HBVr |
| Patients who received tocilizumab, anakinra, and/or baricitinib | ||||||
| 14 | M | 75 | TCZ—1 dose, 400 mg ANK—1 day, 100 mg DEX—15 days, 82 mg | U | <10 | Yes |
| 15 | F | 73 | ANK—7 days, 1000 mg DEX—14 days, 152 mg | U | Positive anti-HBs b | No |
| 16 | M | 66 | TCZ—1 dose, 400 mg ANK—6 days, 1200 mg DEX—33 days, 252 mg | U | U | No |
| 17 | M | 56 | ANK—1 day, 200 mg BARI—9 days, 36 mg DEX—9 days, 96 mg | - | U | No |
| 18 o | M | 67 | BARI—14 days, 56 mg DEX—9 days, 98 mg | - | U | No |
| 19 o | M | 75 | BARI—7 days, 28 mg DEX—7 days, 48 mg | - | U | No |
| 20 o | F | 63 | TCZ—1 dose, 800 mg BARI—3 days, 12 mg DEX—18 days, 140 mg | U | <10 | Yes |
| Patients who received systemic corticosteroids | ||||||
| 21 | F | 59 | DEX—5 days, 30 mg | - | U | No |
| 22 | M | 68 | DEX—5 days, 30 mg | - | U | No |
| 23 | M | 62 | DEX—19 days, 60 mg | - | U | No |
| 24 | M | 83 | DEX—12 days, 104 mg | - | U | No |
| 25 o | M | 67 | DEX—7 days, 38 mg | - | U | No |
| 26 o | M | 62 | DEX—5 days, 36 mg | - | U | No |
| 27 o | F | 68 | DEX—5 days, 30 mg | - | U | No |
| 28 o | M | 80 | DEX—9 days, 50 mg | U | 10 | Yes |
| Patients who did not receive any immunosuppressive treatment | ||||||
| 29 o | M | 75 | - | - | U | No |
| 30 o | F | 69 | - | - | U | No |
| 31 o | M | 74 | - | - | U | No |
| 32 o | M | 70 | - | - | U | No |
| HBVr in HBsAg-Positive Patients | HBVr in HBsAg-Negative/Anti-HBc-Positive Patients | |
|---|---|---|
| KASL 2022 [12] |
|
|
| APASL 2021 [13] |
|
|
| AASLD 2018 [14] |
|
|
| AGA 2015 [11] |
|
|
| Medical Association | HBsAg-Positive Patients Who Experienced HBVr/ All Patients Followed, n/n | Study | HBsAg-Negative/ Anti-HBc-Positive Patients Who Experienced HBVr/ All Patients Followed, n/n | Study |
|---|---|---|---|---|
| KASL 2022 [12] | 2/5 | Liu et al. [4] | 3/15 | This work |
| 0/3 | Camarero et al. [6] | |||
| 1/8 | This work | |||
| Pooled rate of HBVr, % | 18.75% (3/16) | 20% (3/15) | ||
| APASL 2021 [13] | 2/5 | Liu et al. [4] | 0/15 | This work |
| 0/3 | Camarero et al. [6] | |||
| 1/8 | This work | |||
| Pooled rate of HBVr, % | 18.75% (3/16) | 0% | ||
| AASLD 2018 [14] | 2/5 | Liu et al. [4] | 2/6 | Tajez et al. [5] b |
| 0/3 | Camarero et al. [6] | 3/15 | This work | |
| 1/8 | This work | |||
| Pooled rate of HBVr, % | 18.75% (3/16) | 23.8% (5/21) | ||
| AGA 2015 [11] | 2/5 | Liu et al. [4] | 3/15 | This work |
| 1/3 | Camarero et al. [6] | |||
| 2/8 | This work | |||
| Pooled rate of HBVr, % | 31.25% (5/16) | 20% (3/15) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, N.; Olariu, M.C.; Ganea, O.-A.; Adamescu, A.-I.; Molagic, V.; Aramă, Ș.S.; Tilișcan, C.; Aramă, V. Risk of Hepatitis B Virus Reactivation in COVID-19 Patients Receiving Immunosuppressive Treatment: A Prospective Study. J. Clin. Med. 2024, 13, 6032. https://doi.org/10.3390/jcm13206032
Mihai N, Olariu MC, Ganea O-A, Adamescu A-I, Molagic V, Aramă ȘS, Tilișcan C, Aramă V. Risk of Hepatitis B Virus Reactivation in COVID-19 Patients Receiving Immunosuppressive Treatment: A Prospective Study. Journal of Clinical Medicine. 2024; 13(20):6032. https://doi.org/10.3390/jcm13206032
Chicago/Turabian StyleMihai, Nicoleta, Mihaela Cristina Olariu, Oana-Alexandra Ganea, Aida-Isabela Adamescu, Violeta Molagic, Ștefan Sorin Aramă, Cătălin Tilișcan, and Victoria Aramă. 2024. "Risk of Hepatitis B Virus Reactivation in COVID-19 Patients Receiving Immunosuppressive Treatment: A Prospective Study" Journal of Clinical Medicine 13, no. 20: 6032. https://doi.org/10.3390/jcm13206032
APA StyleMihai, N., Olariu, M. C., Ganea, O.-A., Adamescu, A.-I., Molagic, V., Aramă, Ș. S., Tilișcan, C., & Aramă, V. (2024). Risk of Hepatitis B Virus Reactivation in COVID-19 Patients Receiving Immunosuppressive Treatment: A Prospective Study. Journal of Clinical Medicine, 13(20), 6032. https://doi.org/10.3390/jcm13206032






