Post-Capillary Pulmonary Hypertension: Clinical Review
Abstract
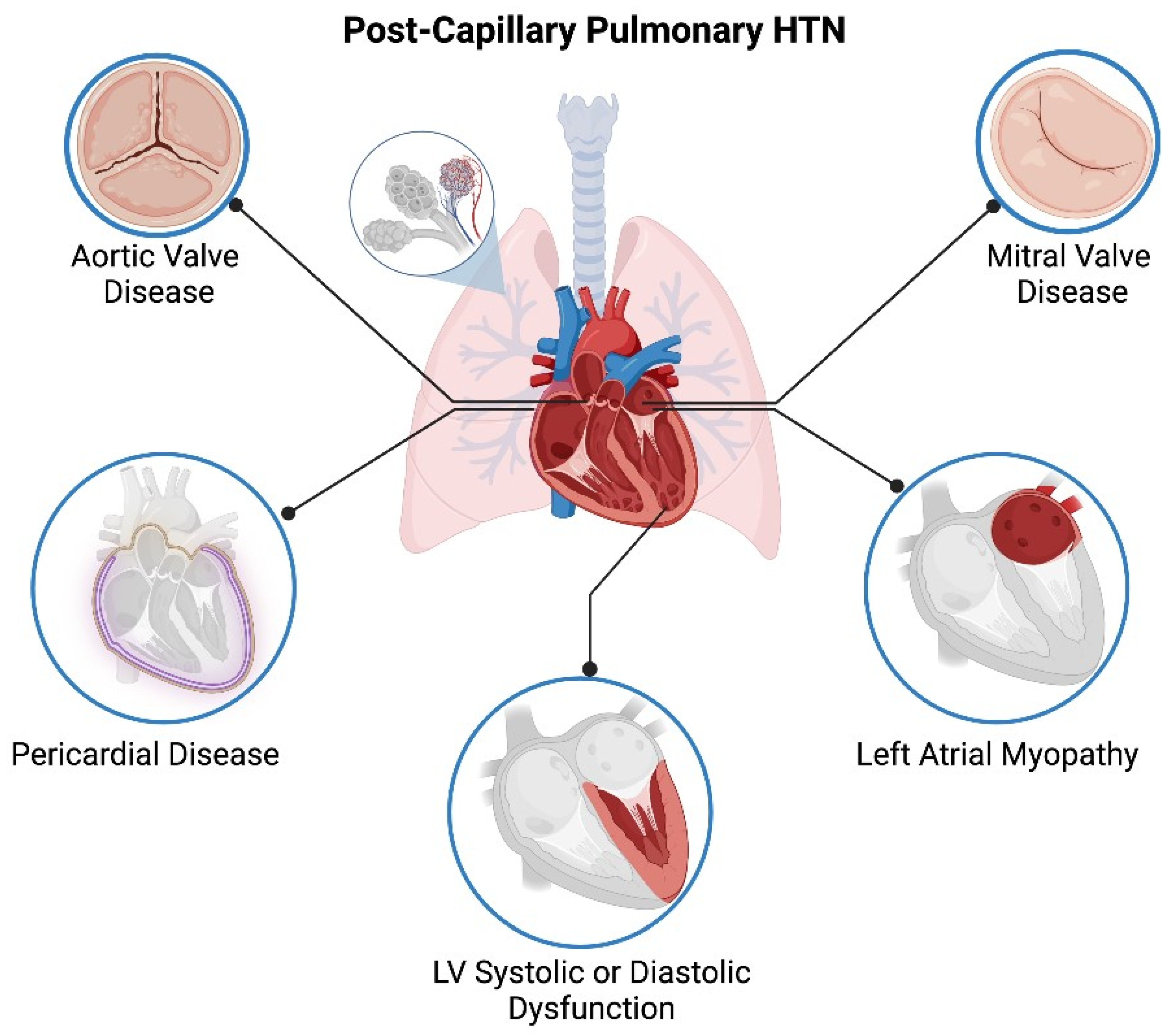
1. Introduction
2. Epidemiology
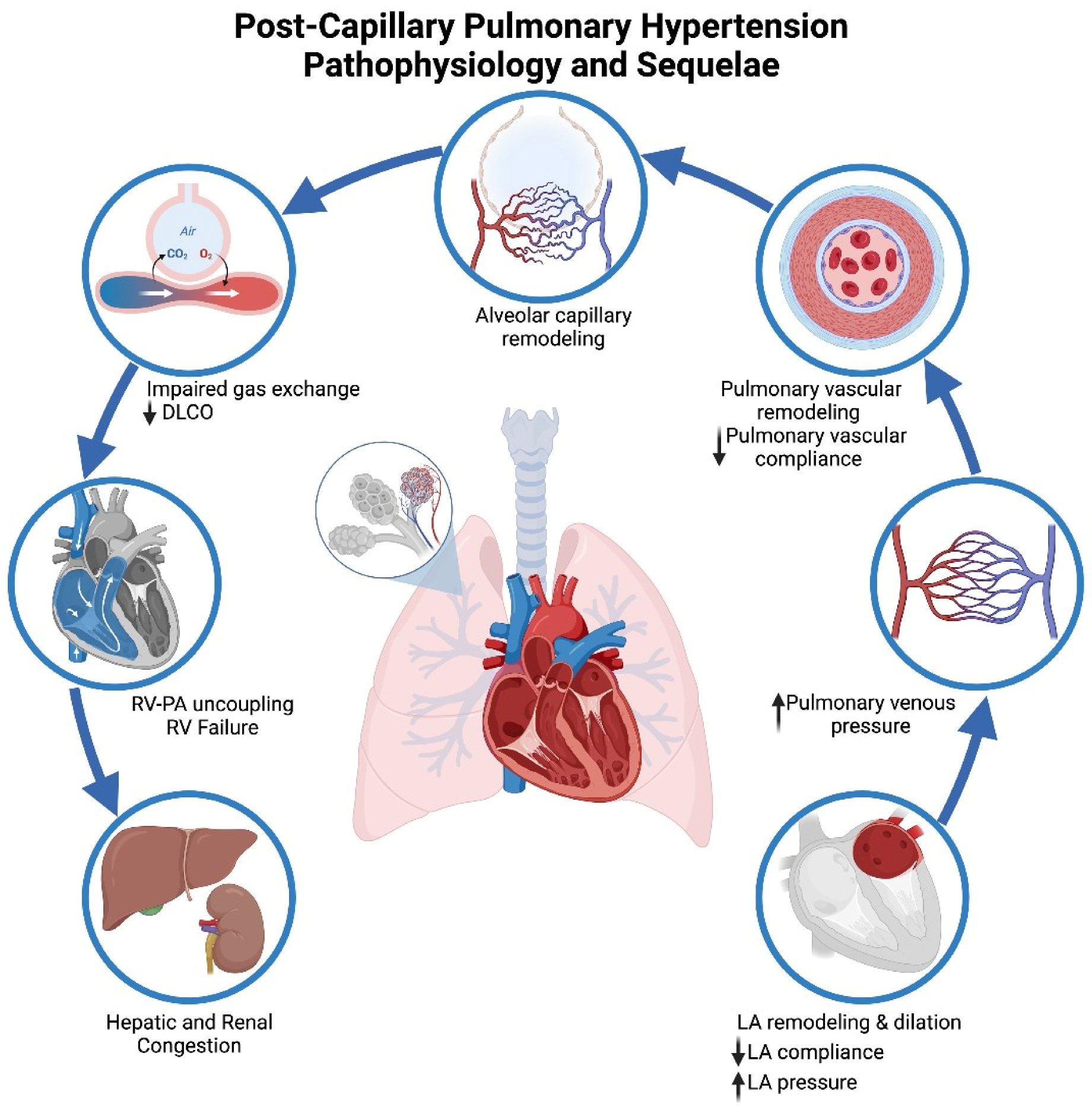
3. Pathophysiology
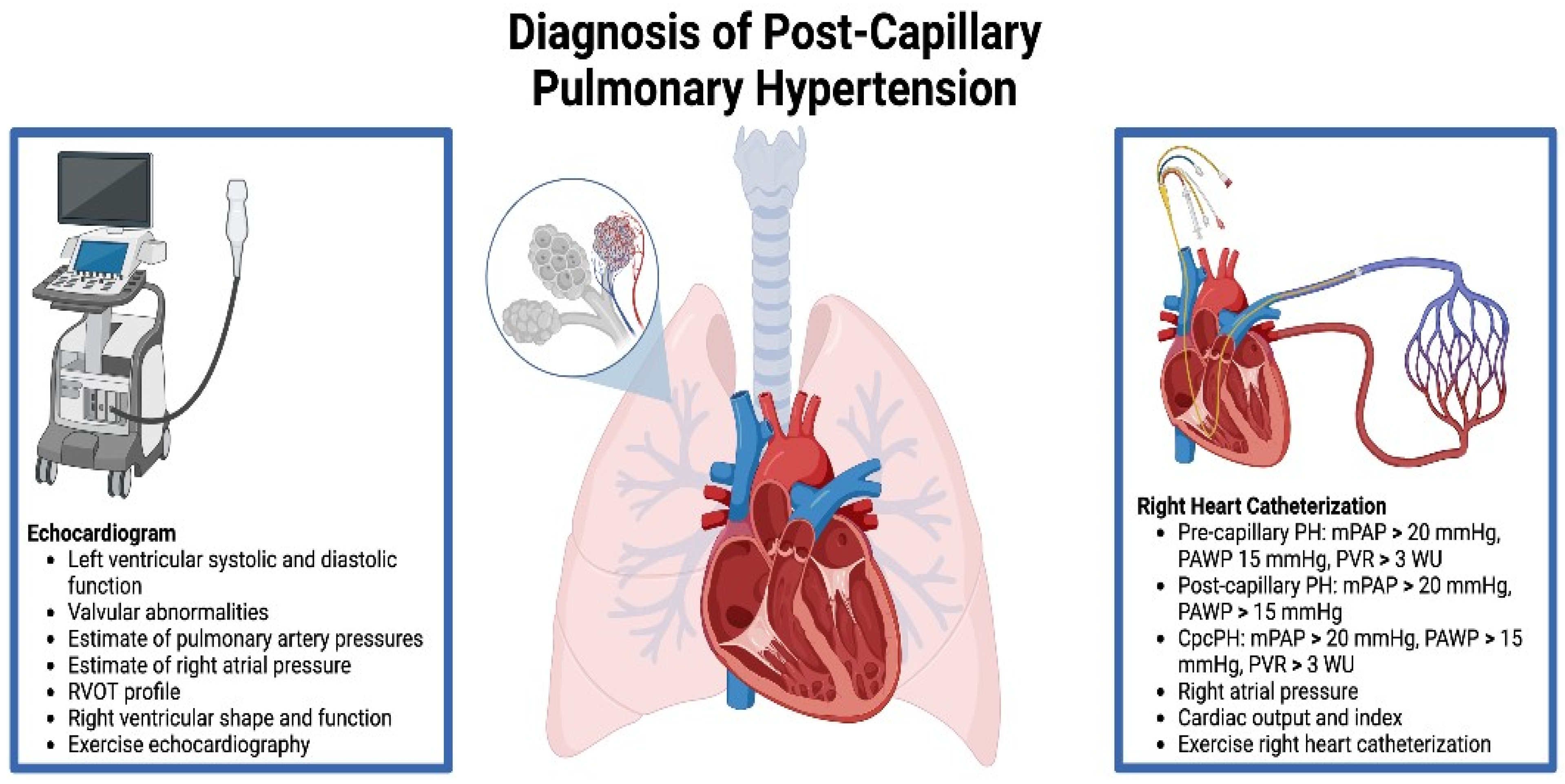
4. Diagnosis
5. Treatment
5.1. Heart Failure with Reduced Ejection Fraction
5.2. Heart Failure with Preserved Ejection Fraction
5.3. Advanced Heart Failure
5.4. Preventative Care in Heart Failure
5.5. Valvular Dysfunction
5.5.1. Mitral Stenosis
5.5.2. Mitral Regurgitation
5.5.3. Aortic Stenosis
6. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenkranz, S.; Gibbs, J.S.R.; Wachter, R.; De Marco, T.; Vonk-Noordegraaf, A.; Vachiéry, J.-L. Left ventricular heart failure and pulmonary hypertension. Eur. Heart J. 2016, 37, 942–954. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Guazzi, M.; Colombo, P.C.; Yuzefpolskaya, M. A right ventricular state of mind in the progression of heart failure with reduced ejection fraction: Implications for left ventricular assist device therapy. Heart Fail. Rev. 2021, 26, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Vachiéry, J.L.; Adir, Y.; Barberà, J.A.; Champion, H.; Coghlan, J.G.; Cottin, V.; De Marco, T.; Galiè, N.; Ghio, S.; Gibbs, J.S.R.; et al. Pulmonary hypertension due to left heart diseases. J. Am. Coll. Cardiol. 2013, 62, D100–D108. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, D.T.; Lajkosz, K.; Brogly, S.B.; Lougheed, M.D.; Jiang, L.; Housin, A.; Barber, D.; Johnson, A.; Doliszny, K.M.; Archer, S.L. Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e003973. [Google Scholar] [CrossRef]
- Lam, C.S.; Roger, V.L.; Rodeheffer, R.J.; Borlaug, B.A.; Enders, F.T.; Redfield, M.M. Pulmonary hypertension in heart failure with preserved ejection fraction: A community-based study. J. Am. Coll. Cardiol. 2009, 31, 1119–1126. [Google Scholar] [CrossRef]
- Gerges, M.; Gerges, C.; Pistritto, A.M.; Lang, M.B.; Trip, P.; Jakowitsch, J.; Binder, T.; Lang, I.M. Pulmonary Hypertension in Heart Failure. Epidemiology, Right Ventricular Function, and Survival. Am. J. Respir. Crit. Care Med. 2015, 192, 1234–1246. [Google Scholar] [CrossRef]
- Miller, W.L.; Grill, D.E.; Borlaug, B.A. Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: Pulmonary hypertension and heart failure. JACC Heart Fail. 2013, 1, 290–299. [Google Scholar] [CrossRef]
- Kjaergaard, J.; Akkan, D.; Iversen, K.K.; Kjoller, E.; Køber, L.; Torp-Pedersen, C.; Hassager, C. Prognostic importance of pulmonary hypertension in patients with heart failure. Am. J. Cardiol. 2007, 99, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Hess, E.; Maddox, T.M.; Opotowsky, A.R.; Tedford, R.J.; Lahm, T.; Joynt, K.E.; Kass, D.J.; Stephens, T.; Stanislawski, M.A.; et al. Association of Borderline Pulmonary Hypertension with Mortality and Hospitalization in a Large Patient Cohort: Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 2016, 133, 1240–1248. [Google Scholar] [CrossRef]
- Gall, H.; Felix, J.F.; Schneck, F.K.; Milger, K.; Sommer, N.; Voswinckel, R.; Franco, O.H.; Hofman, A.; Schermuly, R.T.; Weissmann, N.; et al. The Giessen Pulmonary Hypertension Registry: Survival in pulmonary hypertension subgroups. J. Heart Lung Transplant. 2017, 36, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Gavazzi, A.; Campana, C.; Inserra, C.; Klersy, C.; Sebastiani, R.; Arbustini, E.; Recusani, F.; Tavazzi, L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J. Am. Coll. Cardiol. 2001, 37, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Gheorghiade, M.; Triposkiadis, F.; Solomon, S.D.; Pieske, B.; Butler, J. Left atrium in heart failure with preserved ejection fraction: Structure, function, and significance. Circ. Heart Fail. 2014, 7, 1042–1049. [Google Scholar] [CrossRef]
- Guazzi, M.; Ghio, S.; Adir, Y. Pulmonary Hypertension in HFpEF and HFrEF: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Hwang, S.J.; Redfield, M.M.; Zakeri, R.; Lin, G.; Borlaug, B.A. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ. Heart Fail. 2015, 8, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, J.J.; Arora, R.; Green, D.; Greenland, P.; Lee, D.C.; Lloyd-Jones, D.M.; Markl, M.; Ng, J.; Shah, S.J. Evaluating the Atrial Myopathy Underlying Atrial Fibrillation: Identifying the Arrhythmogenic and Thrombogenic Substrate. Circulation 2015, 132, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef]
- Tedford, R.J.; Hassoun, P.M.; Mathai, S.C.; Girgis, R.E.; Russell, S.D.; Thiemann, D.R.; Cingolani, O.H.; Mudd, J.O.; Borlaug, B.A.; Redfield, M.M.; et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 2012, 125, 289–297. [Google Scholar] [CrossRef]
- Al-Omary, M.S.; Sugito, S.; Boyle, A.J.; Sverdlov, A.L.; Collins, N.J. Pulmonary Hypertension Due to Left Heart Disease: Diagnosis, Pathophysiology, and Therapy. Hypertension 2020, 75, 1397–1408. [Google Scholar] [CrossRef]
- Guazzi, M.; Naeije, R. Pulmonary Hypertension in Heart Failure: Pathophysiology, Pathobiology, and Emerging Clinical Perspectives. J. Am. Coll. Cardiol. 2017, 69, 1718–1734. [Google Scholar] [CrossRef]
- Omote, K.; Sorimachi, H.; Obokata, M.; Reddy, Y.N.V.; Verbrugge, F.H.; Omar, M.; DuBrock, H.M.; Redfield, M.M.; Borlaug, B.A. Pulmonary vascular disease in pulmonary hypertension due to left heart disease: Pathophysiologic implications. Eur. Heart J. 2022, 43, 3417–3431. [Google Scholar] [CrossRef]
- Kovacs, G.; Berghold, A.; Scheidl, S.; Olschewski, H. Pulmonary arterial pressure during rest and exercise in healthy subjects: A systematic review. Eur. Respir. J. 2009, 34, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Chemla, D.; Lau, E.M.; Papelier, Y.; Attal, P.; Herve, P. Pulmonary vascular resistance and compliance relationship in pulmonary hypertension. Eur. Respir. J. 2015, 46, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Brittain, E.L.; Hess, E.; Waldo, S.W.; Baron, A.E.; Huang, S.; Goldstein, R.H.; Assad, T.; Wertheim, B.M.; Alba, G.A.; et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Kovacs, G.; Vaidya, A.; Bhatt, D.L.; Nishimura, R.A.; Mak, S.; Guazzi, M.; Tedford, R.J. Cardiopulmonary Hemodynamics in Pulmonary Hypertension and Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 76, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.R.; Forfia, P.R.; Chamera, E.; Housten-Harris, T.; Champion, H.C.; Girgis, R.E.; Corretti, M.C.; Hassoun, P.M. Accuracy of Doppler Echocardiography in the Hemodynamic Assessment of Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2009, 179, 615–621. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.M.; Assad, T.R.; Xu, M.; Farber-Eger, E.; Wells, Q.S.; Hemnes, A.R.; Brittain, E.L. Lack of a Tricuspid Regurgitation Doppler Signal and Pulmonary Hypertension by Invasive Measurement. J. Am. Heart Assoc. 2018, 7, e009362. [Google Scholar] [CrossRef]
- Arkles, J.S.; Opotowsky, A.R.; Ojeda, J.; Rogers, F.; Liu, T.; Prassana, V.; Marzec, L.; Palevsky, H.I.; Ferrari, V.A.; Forfia, P.R. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2011, 183, 268–276. [Google Scholar] [CrossRef]
- Raza, F.; Dillane, C.; Mirza, A.; Brailovsky, Y.; Weaver, S.; Keane, M.G.; Forfia, P. Differences in right ventricular morphology, not function, indicate the nature of increased afterload in pulmonary hypertensive subjects with normal left ventricular function. Echocardiography 2017, 34, 1584–1592. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- Opotowsky, A.R.; Hess, E.; Maron, B.A.; Brittain, E.L.; Barón, A.E.; Maddox, T.M.; Alshawabkeh, L.I.; Wertheim, B.M.; Xu, M.; Assad, T.R.; et al. Thermodilution vs Estimated Fick Cardiac Output Measurement in Clinical Practice: An Analysis of Mortality From the Veterans Affairs Clinical Assessment, Reporting, and Tracking (VA CART) Program and Vanderbilt University. JAMA Cardiol. 2017, 2, 1090–1099. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Preston, I.R. Right heart catheterisation: Best practice and pitfalls in pulmonary hypertension. Eur. Respir. Rev. 2015, 24, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Mathier, M.A. The Nuts and Bolts of Interpreting Hemodynamics in Pulmonary Hypertension Associated With Diastolic Heart Failure. Adv. Pulm. Hypertens. 2011, 10, 33–40. [Google Scholar] [CrossRef]
- Tonelli, A.R.; Mubarak, K.K.; Li, N.; Carrie, R.; Alnuaimat, H. Effect of Balloon Inflation Volume on Pulmonary Artery Occlusion Pressure in Patients With and Without Pulmonary Hypertension. Chest 2011, 139, 115–121. [Google Scholar] [CrossRef]
- Zhang, F.; Liang, Y.; Chen, X.; Xu, L.; Zhou, C.; Fan, T.; Yan, J. Echocardiographic evaluation of left ventricular end diastolic pressure in patients with diastolic heart failure. Medicine 2020, 99, e22683. [Google Scholar] [CrossRef] [PubMed]
- Halpern, S.D.; Taichman, D.B. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest 2009, 136, 37–43. [Google Scholar] [CrossRef]
- Mascherbauer, J.; Zotter-Tufaro, C.; Duca, F.; Binder, C.; Koschutnik, M.; Kammerlander, A.A.; Aschauer, S.; Bonderman, D. Wedge Pressure Rather Than Left Ventricular End-Diastolic Pressure Predicts Outcome in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2017, 5, 795–801. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Shah, A.N.; Vaduganathan, M.; Butler, J.; Bonow, R.O.; Rosano, G.M.; Taylor, S.; Kupfer, S.; Misselwitz, F.; Sharma, A.; et al. Recognizing hospitalized heart failure as an entity and developing new therapies to improve outcomes: Academics’, clinicians’, industry’s, regulators’, and payers’ perspectives. Heart Fail. Clin. 2013, 9, 285. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.; Sato, N.; Shah, A.N.; et al. The Global Health and Economic Burden of Hospitalizations for Heart Failure: Lessons Learned From Hospitalized Heart Failure Registries. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef]
- Anker, S.D.; Schroeder, S.; Atar, D.; Bax, J.J.; Ceconi, C.; Cowie, M.R.; Crisp, A.; Dominjon, F.; Ford, I.; Ghofrani, H.A.; et al. Traditional and new composite endpoints in heart failure clinical trials: Facilitating comprehensive efficacy assessments and improving trial efficiency. Eur. J. Heart Fail. 2016, 18, 482–489. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.; Metra, M.; Adamo, M.; Gardner, R.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Kaluski, E.; Cotter, G.; Leitman, M.; Milo-Cotter, O.; Krakover, R.; Kobrin, I.; Moriconi, T.; Rainisio, M.; Caspi, A.; Reizin, L.; et al. Clinical and hemodynamic effects of bosentan dose optimization in symptomatic heart failure patients with severe systolic dysfunction, associated with secondary pulmonary hypertension—A multi-center randomized study. Cardiology 2008, 109, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Shah, R.; Shahzad, K.; Camuso, J.M.; Pappagianopoulos, P.P.; Hung, J.; Tawakol, A.; Gerszten, R.E.; Systrom, D.M.; Bloch, K.D.; et al. Sildenafil Improves Exercise Capacity and Quality of Life in Patients With Systolic Heart Failure and Secondary Pulmonary Hypertension. Circulation 2007, 116, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, T.; Zhou, Q.; Li, S.; Huang, L. Additional use of a phosphodiesterase 5 inhibitor in patients with pulmonary hypertension secondary to chronic systolic heart failure: A meta-analysis. Eur. J. Heart Fail. 2014, 16, 444–453. [Google Scholar] [CrossRef]
- Bonderman, D.; Ghio, S.; Felix, S.B.; Ghofrani, H.-A.; Michelakis, E.; Mitrovic, V.; Oudiz, R.J.; Boateng, F.; Scalise, A.-V.; Roessig, L.; et al. Riociguat for Patients With Pulmonary Hypertension Caused by Systolic Left Ventricular Dysfunction. Circulation 2013, 128, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Yusuf, S.; Pfeffer, M.A.; Swedberg, K.; Granger, C.B.; Held, P.; McMurray, J.J.; Michelson, E.L.; Olofsson, B.; Östergren, J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-Preserved Trial. Lancet 2003, 362, 777–781. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Massie, B.M.; Carson, P.E.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Zile, M.R.; Anderson, S.; Donovan, M.; Iverson, E.; Staiger, C.; et al. Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction. N. Engl. J. Med. 2008, 359, 2456–2467. [Google Scholar] [CrossRef]
- Ahmed, A.; Rich, M.W.; Fleg, J.L.; Zile, M.R.; Young, J.B.; Kitzman, D.W.; Love, T.E.; Aronow, W.S.; Adams, K.F.; Gheorghiade, M. Effects of Digoxin on Morbidity and Mortality in Diastolic Heart Failure. Circulation 2006, 114, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Vachiéry, J.-L.; Delcroix, M.; Al-Hiti, H.; Efficace, M.; Hutyra, M.; Lack, G.; Papadakis, K.; Rubin, L.J. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur. Respir. J. 2018, 51, 1701886. [Google Scholar] [CrossRef] [PubMed]
- Koller, B.; Steringer-Mascherbauer, R.; Ebner, C.H.; Weber, T.; Ammer, M.; Eichinger, J.; Pretsch, I.; Herold, M.; Schwaiger, J.; Ulmer, H.; et al. Pilot Study of Endothelin Receptor Blockade in Heart Failure with Diastolic Dysfunction and Pulmonary Hypertension (BADDHY-Trial). Heart Lung Circ. 2017, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Vicenzi, M.; Arena, R.; Guazzi, M.D. Pulmonary Hypertension in Heart Failure With Preserved Ejection Fraction. Circulation 2011, 124, 164–174. [Google Scholar] [CrossRef]
- Hoendermis, E.S.; Liu, L.C.Y.; Hummel, Y.M.; Van Der Meer, P.; De Boer, R.A.; Berger, R.M.F.; Van Veldhuisen, D.J.; Voors, A.A. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: A randomized controlled trial. Eur. Heart J. 2015, 36, 2565–2573. [Google Scholar] [CrossRef]
- Crawford, T.C.; Leary, P.J.; Fraser, C.D., 3rd; Suarez-Pierre, A.; Magruder, J.T.; Baumgartner, W.A.; Zehr, K.J.; Whitman, G.J.; Masri, S.C.; Sheikh, F.; et al. Impact of the New Pulmonary Hypertension Definition on Heart Transplant Outcomes: Expanding the Hemodynamic Risk Profile. Chest 2020, 157, 151–161. [Google Scholar] [CrossRef]
- Velleca, A.; Shullo, M.A.; Dhital, K.; Azeka, E.; Colvin, M.; DePasquale, E.; Farrero, M.; Garcia-Guereta, L.; Jamero, G.; Khush, K.; et al. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J. Heart. Lung Transplant. 2023, 42, e1–e141. [Google Scholar] [CrossRef]
- Selim, A.M.; Wadhwani, L.; Burdorf, A.; Raichlin, E.; Lowes, B.; Zolty, R. Left Ventricular Assist Devices in Pulmonary Hypertension Group 2 With Significantly Elevated Pulmonary Vascular Resistance: A Bridge to Cure. Heart Lung Circ. 2019, 28, 946–952. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Farhoud, M.; Zacharias, M.; Ginwalla, M.B.; ElAmm, C.A.; Benatti, R.D.; Oliveira, G.H. Left Ventricular Assist Devices or Inotropes for Decreasing Pulmonary Vascular Resistance in Patients with Pulmonary Hypertension Listed for Heart Transplantation. J. Card. Fail. 2017, 23, 209–215. [Google Scholar] [CrossRef]
- Imamura, T.; Chung, B.; Nguyen, A.; Rodgers, D.; Sayer, G.; Adatya, S.; Sarswat, N.; Kim, G.; Raikhelkar, J.; Ota, T.; et al. Decoupling Between Diastolic Pulmonary Artery Pressure and Pulmonary Capillary Wedge Pressure as a Prognostic Factor After Continuous Flow Ventricular Assist Device Implantation. Circ. Heart Fail. 2017, 10, e003882. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Garg, S.; Khunger, M.; Darden, D.; Ayers, C.; Kumbhani, D.J.; Mayo, H.G.; De Lemos, J.A.; Berry, J.D. Dose–Response Relationship Between Physical Activity and Risk of Heart Failure. Circulation 2015, 132, 1786–1794. [Google Scholar] [CrossRef]
- Suskin, N.; Sheth, T.; Negassa, A.; Yusuf, S. Relationship of current and past smoking to mortality and morbidity in patients with left ventricular dysfunction. J. Am. Coll. Cardiol. 2001, 37, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2015, 373, 2103–2116. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Holland, R. Systematic review of multidisciplinary interventions in heart failure. Heart 2005, 91, 899–906. [Google Scholar] [CrossRef]
- O’Sullivan, C.J.; Wenaweser, P.; Ceylan, O.; Rat-Wirtzler, J.; Stortecky, S.; Heg, D.; Spitzer, E.; Zanchin, T.; Praz, F.; Tüller, D.; et al. Effect of Pulmonary Hypertension Hemodynamic Presentation on Clinical Outcomes in Patients With Severe Symptomatic Aortic Valve Stenosis Undergoing Transcatheter Aortic Valve Implantation. Circ. Cardiovasc. Interv. 2015, 8, e002358. [Google Scholar] [CrossRef]
- Tigges, E.; Blankenberg, S.; Von Bardeleben, R.S.; Zürn, C.; Bekeredjian, R.; Ouarrak, T.; Sievert, H.; Nickenig, G.; Boekstegers, P.; Senges, J.; et al. Implication of pulmonary hypertension in patients undergoing MitraClip therapy: Results from the German transcatheter mitral valve interventions (TRAMI) registry. Eur. J. Heart Fail. 2018, 20, 585–594. [Google Scholar] [CrossRef]
- Weitsman, T.; Weisz, G.; Farkash, R.; Klutstein, M.; Butnaru, A.; Rosenmann, D.; Hasin, T. Pulmonary Hypertension with Left Heart Disease: Prevalence, Temporal Shifts in Etiologies and Outcome. Am. J. Med. 2017, 130, 1272–1279. [Google Scholar] [CrossRef]
- Magne, J.; Pibarot, P.; Sengupta, P.P.; Donal, E.; Rosenhek, R.; Lancellotti, P. Pulmonary Hypertension in Valvular Disease. JACC Cardiovasc. Imaging 2015, 8, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Debenedictus, C.; Watt, T.; Farley, S.; Salita, A.; Hornsby, W.; Wu, X.; Herbert, M.; Likosky, D.; Bolling, S.F. The impact of concomitant pulmonary hypertension on early and late outcomes following surgery for mitral stenosis. J. Thorac. Cardiovasc. Surg. 2016, 152, 394–400.e391. [Google Scholar] [CrossRef] [PubMed]
- Ross, J., Jr.; Braunwald, E.; Morrow, A.G. Clinical and hemodynamic observations in pure mitral insufficiency. Am. J. Cardiol. 1958, 2, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Kainuma, S.; Taniguchi, K.; Toda, K.; Funatsu, T.; Kondoh, H.; Nishino, M.; Daimon, T.; Sawa, Y. Pulmonary hypertension predicts adverse cardiac events after restrictive mitral annuloplasty for severe functional mitral regurgitation. J. Thorac. Cardiovasc. Surg. 2011, 142, 783–792. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Davidson Jr, W.R.; Chambers, C.E.; Pae, W.E.; Sun, B.; Campbell, D.B.; Pu, M. Preoperative pulmonary hypertension is associated with postoperative left ventricular dysfunction in chronic organic mitral regurgitation: An echocardiographic and hemodynamic study. J. Am. Soc. Echocardiogr. 2006, 19, 1051–1055. [Google Scholar] [CrossRef]
- Le Tourneau, T.; Richardson, M.; Juthier, F.; Modine, T.; Fayad, G.; Polge, A.S.; Ennezat, P.V.; Bauters, C.; Vincentelli, A.; Deklunder, G. Echocardiography predictors and prognostic value of pulmonary artery systolic pressure in chronic organic mitral regurgitation. Heart 2010, 96, 1311–1317. [Google Scholar] [CrossRef]
- Kampaktsis, P.N.; Kokkinidis, D.G.; Wong, S.C.; Vavuranakis, M.; Skubas, N.J.; Devereux, R.B. The role and clinical implications of diastolic dysfunction in aortic stenosis. Heart 2017, 103, 1481–1487. [Google Scholar] [CrossRef]
- Luçon, A.; Oger, E.; Bedossa, M.; Boulmier, D.; Verhoye, J.P.; Eltchaninoff, H.; Iung, B.; Leguerrier, A.; Laskar, M.; Leprince, P.; et al. Prognostic Implications of Pulmonary Hypertension in Patients With Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Implantation. Circ. Cardiovasc. Interv. 2014, 7, 240–247. [Google Scholar] [CrossRef]
- Faggiano, P.; Antonini-Canterin, F.; Ribichini, F.; D’aloia, A.; Ferrero, V.; Cervesato, E.; Pavan, D.; Burelli, C.; Nicolosi, G. Pulmonary artery hypertension in adult patients with symptomatic valvular aortic stenosis. Am. J. Cardiol. 2008, 85, 204–208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riley, J.M.; Fradin, J.J.; Russ, D.H.; Warner, E.D.; Brailovsky, Y.; Rajapreyar, I. Post-Capillary Pulmonary Hypertension: Clinical Review. J. Clin. Med. 2024, 13, 625. https://doi.org/10.3390/jcm13020625
Riley JM, Fradin JJ, Russ DH, Warner ED, Brailovsky Y, Rajapreyar I. Post-Capillary Pulmonary Hypertension: Clinical Review. Journal of Clinical Medicine. 2024; 13(2):625. https://doi.org/10.3390/jcm13020625
Chicago/Turabian StyleRiley, Joshua M., James J. Fradin, Douglas H. Russ, Eric D. Warner, Yevgeniy Brailovsky, and Indranee Rajapreyar. 2024. "Post-Capillary Pulmonary Hypertension: Clinical Review" Journal of Clinical Medicine 13, no. 2: 625. https://doi.org/10.3390/jcm13020625
APA StyleRiley, J. M., Fradin, J. J., Russ, D. H., Warner, E. D., Brailovsky, Y., & Rajapreyar, I. (2024). Post-Capillary Pulmonary Hypertension: Clinical Review. Journal of Clinical Medicine, 13(2), 625. https://doi.org/10.3390/jcm13020625




