Impact of Toxoplasma gondii and Human Microbiome on Suicidal Behavior: A Systematic Review
Abstract
1. Introduction
1.1. Toxoplasmosis and Suicidal Behavior
1.2. Gut–Brain Axis
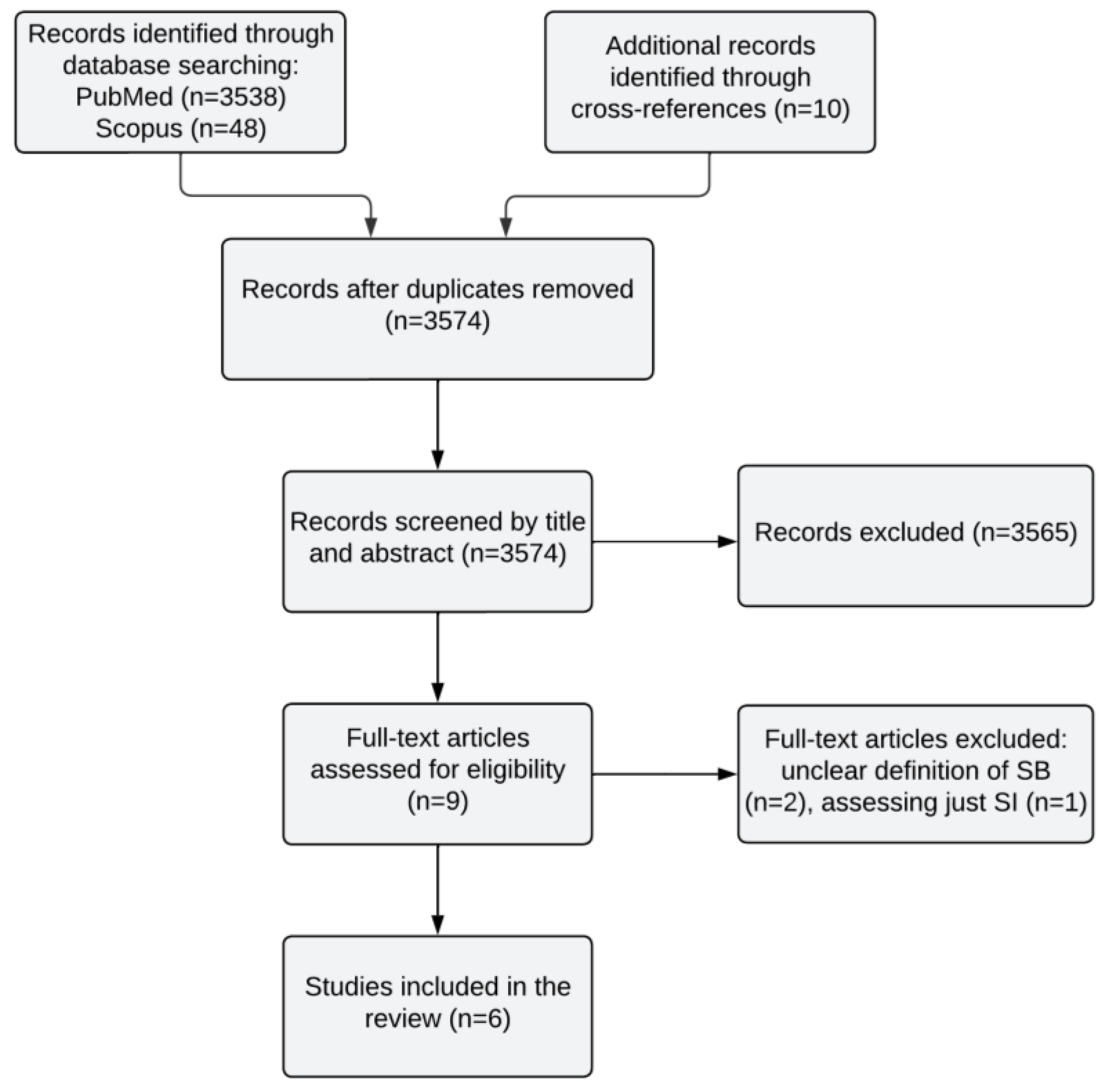
2. Methods
Search Strategy
- Studies, including case–control, cohort, and cross–sectional, both pre mortem and post mortem;
- Studies published in English;
- Studies published up to 9 November 2023, with no further time limitation;
- Studies investigating a potential association between T. gondii infection or microbiome or microbiota and suicidal behavior;
- Studies including individuals with suicidal behavior meeting the criteria of SBD as defined by DSM-5;
- Participants with self-harm were included only if the intent to die or an expectation of the lethality of a suicide attempt was identifiable in the study’s definition of self-harm;
- Studies that assessed suicide attempts using standardized methods such as the Columbia Suicide Severity Rating Scale (C-SSRS);
- Post mortem studies including death cases defined as suicide.
- Systematic reviews or meta-analyses;
- Published in a language other than English;
- Studies that did not explore a potential association between T. gondii infection or microbiome or microbiota and suicidal behavior;
- Studies exclusively involving individuals with suicidal ideations;
- Studies with unclear definitions of suicidal behavior, or those assessing suicidal behavior with self-rating questionnaires or unstandardized assessment tools;
- Studies including individuals engaging in self-harm without the intent to die.
3. Results
3.1. Toxoplasmosis and Suicidal Behavior
3.2. Microbiome and Suicidal Behavior
4. Discussion
4.1. Toxoplasmosis
4.2. Microbiome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Mental Health: Suicide Data; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. World Health Statistics 2023: Monitoring Health for the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- United Nations Department of Economic. World Population Prospects: The 1998 Revision; UN: New York, NY, USA, 1999; Volume 180. [Google Scholar]
- Astraud, L.-P.; Bridge, J.A.; Jollant, F. Thirty Years of Publications in Suicidology: A Bibliometric Analysis. Arch. Suicide Res. 2021, 25, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Klonsky, E.D.; May, A.M.; Saffer, B.Y. Suicide, Suicide Attempts, and Suicidal Ideation. Annu. Rev. Clin. Psychol. 2016, 12, 307–330. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.C.; Ribeiro, J.D.; Fox, K.R.; Bentley, K.H.; Kleiman, E.M.; Huang, X.; Musacchio, K.M.; Jaroszewski, A.C.; Chang, B.P.; Nock, M.K. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol. Bull. 2017, 143, 187–232. [Google Scholar] [CrossRef] [PubMed]
- CDC. Definitions: Self-Directed Violence. Injury Prevention and Control: Division of Violence; CDC: Atlanta, GA, USA, 2015. [Google Scholar]
- Crosby, A.; Ortega, L.; Melanson, C. Self-Directed Violence Surveillance: Uniform Definitions and Recommended Data Elements; CDC: Atlanta, GA, USA, 2011. [Google Scholar]
- Oquendo, M.A.; Sullivan, G.M.; Sudol, K.; Baca-Garcia, E.; Stanley, B.H.; Sublette, M.E.; Mann, J.J. Toward a Biosignature for Suicide. Am. J. Psychiatry 2014, 171, 1259–1277. [Google Scholar] [CrossRef] [PubMed]
- Baldini, V.; Di Stefano, R.; Rindi, L.V.; O Ahmed, A.; Koola, M.M.; Solmi, M.; Papola, D.; De Ronchi, D.; Barbui, C.; Ostuzzi, G. Association between adverse childhood experiences and suicidal behavior in schizophrenia spectrum disorders: A systematic review and meta-analysis. Psychiatry Res. 2023, 329, 115488. [Google Scholar] [CrossRef] [PubMed]
- Calati, R.; Romano, D.; Lopez-Castroman, J.; Turolla, F.; Zimmermann, J.; Madeddu, F.; Preti, E. BOrderliNe symptoms and suIcide-related outcomes: ProTOcol for a systematic review/meta-analysis and an individual patient data meta-analysis (BONITO study). BMJ Open 2022, 12, e056492. [Google Scholar] [CrossRef]
- Miller, J.N.; Black, D.W. Bipolar Disorder and Suicide: A Review. Curr. Psychiatry Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Xu, Y.E.; Barron, D.A.; Sudol, K.; Zisook, S.; Oquendo, M.A. Suicidal behavior across a broad range of psychiatric disorders. Mol. Psychiatry 2023, 28, 2764–2810. [Google Scholar] [CrossRef]
- Yuodelis-Flores, C.; Ries, R.K. Addiction and suicide: A review. Am. J. Addict. 2015, 24, 98–104. [Google Scholar] [CrossRef]
- Carter, G.; Spittal, M.J. Spittal, Suicide Risk Assessment; Hogrefe Publishing: Göttingen, Germany, 2018. [Google Scholar]
- Hawton, K.; van Heeringen, K. Suicide. Lancet 2009, 373, 1372–1381. [Google Scholar] [CrossRef]
- Mann, J.J.; Rizk, M.M. A Brain-Centric Model of Suicidal Behavior. Am. J. Psychiatry 2020, 177, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Docherty, A.R.; Mullins, N.; Ashley-Koch, A.E.; Qin, X.; Coleman, J.R.; Shabalin, A.; Kang, J.; Murnyak, B.; Wendt, F.; Adams, M.; et al. GWAS Meta-Analysis of Suicide Attempt: Identification of 12 Genome-Wide Significant Loci and Implication of Genetic Risks for Specific Health Factors. Am. J. Psychiatry 2023, 180, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Punzi, G.; Ursini, G.; Chen, Q.; Radulescu, E.; Tao, R.; Huuki, L.A.; Di Carlo, P.; Collado-Torres, L.; Shin, J.H.; Catanesi, R.; et al. Genetics and Brain Transcriptomics of Completed Suicide. Am. J. Psychiatry 2022, 179, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; Schultz, C.C.; Koch, K.; Schachtzabel, C.; Sauer, H.; Schlösser, R.G. Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. J. Psychiatr. Res. 2012, 46, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.; de la Cruz, F.; Köhler, S.; Pereira, F.; Richard-Devantoy, S.; Turecki, G.; Bär, K.-J.; Jollant, F. Connectomics-Based Functional Network Alterations in both Depressed Patients with Suicidal Behavior and Healthy Relatives of Suicide Victims. Sci. Rep. 2019, 9, 14330. [Google Scholar] [CrossRef] [PubMed]
- Olié, E.; Jollant, F.; Deverdun, J.; de Champfleur, N.M.; Cyprien, F.; Le Bars, E.; Mura, T.; Bonafé, A.; Courtet, P. The experience of social exclusion in women with a history of suicidal acts: A neuroimaging study. Sci. Rep. 2017, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Das, P.; Outhred, T.; Gessler, D.; Mann, J.J.; Bryant, R. Cognitive and emotional impairments underpinning suicidal activity in patients with mood disorders: An fMRI study. Acta Psychiatr. Scand. 2019, 139, 454–463. [Google Scholar] [CrossRef]
- Verrocchio, M.C.; Carrozzino, D.; Marchetti, D.; Andreasson, K.; Fulcheri, M.; Bech, P. Mental Pain and Suicide: A Systematic Review of the Literature. Front. Psychiatry 2016, 7, 108. [Google Scholar] [CrossRef]
- Dombrovski, A.Y.; Hallquist, M.N. The decision neuroscience perspective on suicidal behavior: Evidence and hypotheses. Curr. Opin. Psychiatry 2017, 30, 7–14. [Google Scholar] [CrossRef]
- Richard-Devantoy, S.; Olié, E.; Guillaume, S.; Courtet, P. Decision-making in unipolar or bipolar suicide attempters. J. Affect. Disord. 2016, 190, 128–136. [Google Scholar] [CrossRef]
- Sastre-Buades, A.; Alacreu-Crespo, A.; Courtet, P.; Baca-Garcia, E.; Barrigon, M.L. Decision-making in suicidal behavior: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 131, 642–662. [Google Scholar] [CrossRef] [PubMed]
- Lübbert, M.; Bahlmann, L.; Sobanski, T.; Schulz, A.; Kastner, U.W.; Walter, M.; Jollant, F.; Wagner, G. Investigating the Clinical Profile of Suicide Attempters Who Used a Violent Suicidal Means. J. Clin. Med. 2022, 11, 7170. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.E.; Gay, P.; d’Acremont, M.; Van der Linden, M. A German Adaptation of the UPPS Impulsive Behavior Scale: Psychometric Properties and Factor Structure. Swiss J. Psychol. 2008, 67, 107–112. [Google Scholar] [CrossRef]
- Perrain, R.; Dardennes, R.; Jollant, F. Risky decision-making in suicide attempters, and the choice of a violent suicidal means: An updated meta-analysis. J. Affect. Disord. 2021, 280, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Baleón, C.; Gutiérrez-Mondragón, L.F.; Gonzalez, A.I.C.; Rentería, M.E. Neuroimaging Studies of Suicidal Behavior and Non-suicidal Self-Injury in Psychiatric Patients: A Systematic Review. Front. Psychiatry 2018, 9, 500. [Google Scholar] [CrossRef]
- Johnston, J.N.; Campbell, D.; Caruncho, H.J.; Henter, I.D.; Ballard, E.D.; A Zarate, C. Suicide Biomarkers to Predict Risk, Classify Diagnostic Subtypes, and Identify Novel Therapeutic Targets: 5 Years of Promising Research. Int. J. Neuropsychopharmacol. 2022, 25, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Courtet, P.; Giner, L.; Seneque, M.; Guillaume, S.; Olie, E.; Ducasse, D. Neuroinflammation in suicide: Toward a comprehensive model. World J. Biol. Psychiatry 2016, 17, 564–586. [Google Scholar] [CrossRef]
- Brisch, R.; Wojtylak, S.; Saniotis, A.; Steiner, J.; Gos, T.; Kumaratilake, J.; Henneberg, M.; Wolf, R. The role of microglia in neuropsychiatric disorders and suicide. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 929–945. [Google Scholar] [CrossRef]
- Chen, X.; Pu, J.; Liu, Y.; Tian, L.; Chen, Y.; Gui, S.; Xu, S.; Song, X.; Xie, P. Increased C-reactive protein concentrations were associated with suicidal behavior in patients with depressive disorders: A meta-analysis. Psychiatry Res. 2020, 292, 113320. [Google Scholar] [CrossRef]
- Savitz, J.; Yolken, R.H. Therapeutic Implications of the Microbial Hypothesis of Mental Illness. Curr. Top. Behav. Neurosci. 2023, 61, 315–351. [Google Scholar]
- Bertolote, J.M.; Fleischmann, A.; De Leo, D.; Wasserman, D. Psychiatric Diagnoses and Suicide: Revisiting the Evidence. Crisis 2004, 25, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Refisch, A.; Sen, Z.D.; Klassert, T.E.; Busch, A.; Besteher, B.; Danyeli, L.V.; Helbing, D.; Schulze-Späte, U.; Stallmach, A.; Bauer, M.; et al. Microbiome and immuno-metabolic dysregulation in patients with major depressive disorder with atypical clinical presentation. Neuropharmacology 2023, 235, 109568. [Google Scholar] [CrossRef] [PubMed]
- Refisch, A.; Walter, M. The importance of the human microbiome for mental health. Nervenarzt 2023, 94, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Pereira, J.S.; Rea, K.; Nolan, Y.M.; O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Depression’s Unholy Trinity: Dysregulated Stress, Immunity, and the Microbiome. Annu. Rev. Psychol. 2020, 71, 49–78. [Google Scholar] [CrossRef] [PubMed]
- Halonen, S.K.; Weiss, L.M. Toxoplasmosis. Handb. Clin. Neurol. 2013, 114, 125–145. [Google Scholar] [PubMed]
- Stanić, Ž.; Fureš, R. Toxoplasmosis: A Global Zoonosis. Veterinaria 2020, 69, 31–42. [Google Scholar]
- Guimarães, E.V.; de Carvalho, L.; Barbosa, H.S. Interaction and cystogenesis of Toxoplasma gondii within skeletal muscle cells in vitro. Mem. Inst. Oswaldo Cruz 2009, 104, 170–174. [Google Scholar] [CrossRef][Green Version]
- Robert-Gangneux, F.; Dardé, M.-L. Epidemiology of and Diagnostic Strategies for Toxoplasmosis. Clin. Microbiol. Rev. 2012, 25, 264–296. [Google Scholar] [CrossRef]
- Kamal, A.M.; Kamal, A.M.; Abd El-Fatah, A.S.; Rizk, M.M.; Hassan, E.E. Latent Toxoplasmosis is Associated with Depression and Suicidal Behavior. Arch. Suicide Res. 2022, 26, 819–830. [Google Scholar] [CrossRef]
- Dasa, T.T.; Geta, T.G.; Yalew, A.Z.; Abebe, R.M.; Kele, H.U. Toxoplasmosis infection among pregnant women in Africa: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0254209. [Google Scholar] [CrossRef] [PubMed]
- McConkey, G.A.; Martin, H.L.; Bristow, G.C.; Webster, J.P. Toxoplasma gondii infection and behaviour-location, location, location? J. Exp. Biol. 2013, 216, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Berdoy, M.; Webster, J.P.; Macdonald, D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 1591–1594. [Google Scholar] [CrossRef]
- Vyas, A.; Kim, S.-K.; Giacomini, N.; Boothroyd, J.C.; Sapolsky, R.M. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc. Natl. Acad. Sci. USA 2007, 104, 6442–6447. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.J.; Cassidy, K.A.; Stahler, E.E.; Brandell, E.E.; Anton, C.B.; Stahler, D.R.; Smith, D.W. Parasitic infection increases risk-taking in a social, intermediate host carnivore. Commun. Biol. 2022, 5, 1180. [Google Scholar] [CrossRef] [PubMed]
- Poirotte, C.; Kappeler, P.M.; Ngoubangoye, B.; Bourgeois, S.; Moussodji, M.; Charpentier, M.J. Morbid attraction to leopard urine in Toxoplasma-infected chimpanzees. Curr. Biol. 2016, 26, R98–R99. [Google Scholar] [CrossRef] [PubMed]
- Gohardehi, S.; Sharif, M.; Sarvi, S.; Moosazadeh, M.; Alizadeh-Navaei, R.; Hosseini, S.A.; Amouei, A.; Pagheh, A.; Sadeghi, M.; Daryani, A. The potential risk of toxoplasmosis for traffic accidents: A systematic review and meta-analysis. Exp. Parasitol. 2018, 191, 19–24. [Google Scholar] [CrossRef]
- Samojłowicz, D.; Twarowska-Małczyńska, J.; Borowska-Solonynko, A.; Poniatowski, A.; Sharma, N.; Olczak, M. Presence of Toxoplasma gondii infection in brain as a potential cause of risky behavior: A report of 102 autopsy cases. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 305–317. [Google Scholar] [CrossRef]
- Soleymani, E.; Faizi, F.; Heidarimoghadam, R.; Davoodi, L.; Mohammadi, Y. Association of T. gondii infection with suicide: A systematic review and meta-analysis. BMC Public Health 2020, 20, 766. [Google Scholar] [CrossRef]
- Sutterland, A.L.; Kuin, A.; Kuiper, B.; van Gool, T.; Leboyer, M.; Fond, G.; de Haan, L. Driving us mad: The association of Toxoplasma gondii with suicide attempts and traffic accidents—A systematic review and meta-analysis. Psychol. Med. 2019, 49, 1608–1623. [Google Scholar] [CrossRef]
- Postolache, T.T.; Wadhawan, A.; Rujescu, D.; Hoisington, A.J.; Dagdag, A.; Baca-Garcia, E.; Lowry, C.A.; Okusaga, O.O.; Brenner, L.A. Toxoplasma gondii, Suicidal Behavior, and Intermediate Phenotypes for Suicidal Behavior. Front. Psychiatry 2021, 12, 665682. [Google Scholar] [CrossRef] [PubMed]
- Amouei, A.; Moosazadeh, M.; Chegeni, T.N.; Sarvi, S.; Mizani, A.; Pourasghar, M.; Teshnizi, S.H.; Hosseininejad, Z.; Dodangeh, S.; Pagheh, A.; et al. Evolutionary puzzle of Toxoplasma gondii with suicidal ideation and suicide attempts: An updated systematic review and meta-analysis. Transbound. Emerg. Dis. 2020, 67, 1847–1860. [Google Scholar] [CrossRef] [PubMed]
- Maitre, Y.; Micheneau, P.; Delpierre, A.; Mahalli, R.; Guerin, M.; Amador, G.; Denis, F. Did the Brain and Oral Microbiota Talk to Each Other? A Review of the Literature. J. Clin. Med. 2020, 9, 3876. [Google Scholar] [CrossRef] [PubMed]
- Hooks, K.B.; O’malley, M.A. Dysbiosis and Its Discontents. mBio 2017, 8, e01492-17. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Kubo, C.; Koga, Y.; Yu, X.-N. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J. Physiol. 2004, 558 Pt 1, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Zhang, H.; Chen, X.; Zhang, Y.; Wu, J.; Zhao, L.; Wang, D.; Pu, J.; Ji, P.; et al. Toward a Deeper Understanding of Gut Microbiome in Depression: The Promise of Clinical Applicability. Adv. Sci. 2022, 9, e2203707. [Google Scholar] [CrossRef]
- Radjabzadeh, D.; Bosch, J.A.; Uitterlinden, A.G.; Zwinderman, A.H.; Ikram, M.A.; van Meurs, J.B.J.; Luik, A.I.; Nieuwdorp, M.; Lok, A.; van Duijn, C.M.; et al. Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 2022, 13, 1877–2013. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Dinan, T.G. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Ahn, J.; Hayes, R.B. Environmental Influences on the Human Microbiome and Implications for Noncommunicable Disease. Annu. Rev. Public Health 2021, 42, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, P.; Li, Y.; Wu, J.; Tan, X.; Zhou, J.; Sun, Z.; Chen, X.; Zhang, G.; Zhang, H.; et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci. Adv. 2020, 6, eaba8555. [Google Scholar] [CrossRef] [PubMed]
- Le Port, A.; Gueguen, A.; Kesse-Guyot, E.; Melchior, M.; Lemogne, C.; Nabi, H.; Goldberg, M.; Zins, M.; Czernichow, S. Association between Dietary Patterns and Depressive Symptoms Over Time: A 10-Year Follow-Up Study of the GAZEL Cohort. PLoS ONE 2012, 7, e51593. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Stubbs, B.; Firth, J.; Smith, L.; Haro, J.M.; Koyanagi, A. Fast food consumption and suicide attempts among adolescents aged 12–15 years from 32 countries. J. Affect. Disord. 2020, 266, 63–70. [Google Scholar] [CrossRef]
- Yun, J.-Y.; Yun, Y.H. Health-promoting behavior to enhance perceived meaning and control of life in chronic disease patients with role limitations and depressive symptoms: A network approach. Sci. Rep. 2023, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Research Methods & Reporting-Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement-David Moher and colleagues introduce PRISMA, an update of the QUOROM guidelines for reporting systematic reviews and meta-analyses. Ann. Intern. Med. 2009, 338, 332. [Google Scholar]
- De Bles, N.J.; van der Does, J.E.; Kortbeek, L.M.; Hofhuis, A.; van Grootheest, G.; Vollaard, A.M.; Giltay, E.J. Toxoplasma gondii seropositivity in patients with depressive and anxiety disorders. Brain Behav. Immun. Health 2021, 11, 100197. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Esquivel, C.; Estrada-Martínez, S.; Ramos-Nevárez, A.; Pérez-Álamos, A.R.; Beristain-García, I.; Alvarado-Félix, O.; Cerrillo-Soto, S.M.; Sifuentes-Álvarez, A.; Alvarado-Félix, G.A.; Guido-Arreola, C.A.; et al. Association between Toxoplasma gondii Exposure and Suicidal Behavior in Patients Attending Primary Health Care Clinics. Pathogens 2021, 10, 677. [Google Scholar] [CrossRef]
- Alvarado-Esquivel, C.; Estrada-Martínez, S.; Pérez-Álamos, A.R.; Beristain-García, I.; Alvarado-Félix, O.; Alvarado-Félix, G.A.; Sifuentes-Álvarez, A. Toxoplasma gondii Infection and Suicidal Behavior in People with Alcohol Consumption. Pathogens 2021, 10, 734. [Google Scholar] [CrossRef]
- Bahceci, I.; Bahceci, B.; Senturk, S.; E Yildiz, I.; A Yazici, Z. Correlation of Suicidal Thoughts and Toxoplasmosis in Patients with Depression. Cureus 2021, 13, e13369. [Google Scholar] [CrossRef]
- Lindgren, M.; Holm, M.; Markkula, N.; Härkänen, T.; Dickerson, F.; Yolken, R.H.; Suvisaari, J. Exposure to common infections and risk of suicide and self-harm: A longitudinal general population study. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.G.; Mortensen, P.B.; Norgaard-Pedersen, B.; Postolache, T.T. Toxoplasma gondii Infection and Self-directed Violence in Mothers. Arch. Gen. Psychiatry 2012, 69, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Okusaga, O.; Langenberg, P.; Sleemi, A.; Vaswani, D.; Giegling, I.; Hartmann, A.M.; Konte, B.; Friedl, M.; Groer, M.W.; Yolken, R.H.; et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with schizophrenia. Schizophr. Res. 2011, 133, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Yagmur, F.; Yazar, S.; Temel, H.O.; Cavusoglu, M. May Toxoplasma gondii increase suicide attempt-preliminary results in Turkish subjects? Forensic Sci. Int. 2010, 199, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Sari, S.A.; Kara, A. Association of Suicide Attempt with Seroprevalence of Toxoplasma gondii in Adolescents. J. Nerv. Ment. Dis. 2019, 207, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Yucel, H.; Acikel, S.B.; Senel, S. An investigation into the association between latent toxoplasmosis and suicide attempts among adolescents. J. Infect. Dev. Ctries. 2020, 14, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Esquivel, C.; Carrillo-Oropeza, D.; Pacheco-Vega, S.J.; Hernández-Tinoco, J.; Salcedo-Jaquez, M.; Sánchez-Anguiano, L.F.; Ortiz-Jurado, M.N.; Alarcón-Alvarado, Y.; Liesenfeld, O.; Beristain-García, I. Toxoplasma gondii exposure in patients suffering from mental and behavioral disorders due to psychoactive substance use. BMC Infect. Dis. 2015, 15, 172. [Google Scholar] [CrossRef]
- Fond, G.; Boyer, L.; Gaman, A.; Laouamri, H.; Attiba, D.; Richard, J.-R.; Delavest, M.; Houenou, J.; Le Corvoisier, P.; Charron, D.; et al. Treatment with anti-toxoplasmic activity (TATA) for toxoplasma positive patients with bipolar disorders or schizophrenia: A cross-sectional study. J. Psychiatr. Res. 2015, 63, 58–64. [Google Scholar] [CrossRef]
- Sapmaz, Y.; Şen, S.; Özkan, Y.; Kandemir, H. Relationship between Toxoplasma gondii seropositivity and depression in children and adolescents. Psychiatry Res. 2019, 278, 263–267. [Google Scholar] [CrossRef]
- Okusaga, O.; Duncan, E.; Langenberg, P.; Brundin, L.; Fuchs, D.; Groer, M.W.; Giegling, I.; Stearns-Yoder, K.A.; Hartmann, A.M.; Konte, B.; et al. Combined Toxoplasma gondii seropositivity and high blood kynurenine—Linked with nonfatal suicidal self-directed violence in patients with schizophrenia. J. Psychiatr. Res. 2016, 72, 74–81. [Google Scholar] [CrossRef]
- Ansari-Lari, M.; Farashbandi, H.; Mohammadi, F. Association of Toxoplasma gondii infection with schizophrenia and its relationship with suicide attempts in these patients. Trop. Med. Int. Health 2017, 22, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Esquivel, C.; Sánchez-Anguiano, L.F.; Arnaud-Gil, C.A.; López-Longoria, J.C.; Molina-Espinoza, L.F.; Estrada-Martínez, S.; Salas-Martínez, C. Toxoplasma gondii infection and suicide attempts: A case-control study in psychiatric outpatients. J. Nerv. Ment. Dis. 2013, 201, 948–952. [Google Scholar] [CrossRef]
- Alvarado-Esquivel, C.; Mendoza-Larios, L.A.; García-Dolores, F.; Sánchez-Anguiano, L.F.; Antuna-Salcido, E.I.; Hernández-Tinoco, J.; Rocha-Salais, A.; Segoviano-Mendoza, M.A.; Sifuentes-Álvarez, A. Association between Toxoplasma gondii Infection in Brain and a History of Depression in Suicide Decedents: A Cross-Sectional Study. Pathogens 2021, 10, 1313. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Larios, L.A.; García-Dolores, F.; Sánchez-Anguiano, L.F.; Hernández-Tinoco, J.; Alvarado-Esquivel, C. Association between Suicide and Toxoplasma gondii Seropositivity. Pathogens 2021, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Bak, J.; Shim, S.-H.; Kwon, Y.-J.; Lee, H.-Y.; Kim, J.S.; Yoon, H.; Lee, Y.J. The Association between Suicide Attempts and Toxoplasma gondii Infection. Clin. Psychopharmacol. Neurosci. 2018, 16, 95–102. [Google Scholar] [CrossRef]
- Coryell, W.; Yolken, R.; Butcher, B.; Burns, T.; Dindo, L.; Schlechte, J.; Calarge, C. Toxoplasmosis Titers and past Suicide Attempts Among Older Adolescents Initiating SSRI Treatment. Arch. Suicide Res. 2016, 20, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Demirel, Ö.F.; Akgül, Ö.; Bulu, E.; Tanrıöver Aydın, E.; Uysal Cesur, N.; Aksoy Poyraz, C.; Öner, Y.A. Are bipolar disorder, major depression, and suicidality linked with Toxoplasma gondii? A seromolecular case-control study. Postgrad. Med. 2023, 135, 179–186. [Google Scholar] [CrossRef]
- Coryell, W.; Wilcox, H.; Evans, S.J.; Pandey, G.N.; Jones-Brando, L.; Dickerson, F.; Yolken, R. Latent infection, inflammatory markers and suicide attempt history in depressive disorders. J. Affect. Disord. 2020, 270, 97–101. [Google Scholar] [CrossRef]
- Ling, V.J.M.; Lester, D.; Mortensen, P.B.D.; Langenberg, P.W.; Postolache, T.T. Toxoplasma gondii Seropositivity and Suicide Rates in Women. J. Nerv. Ment. Dis. 2011, 199, 440–444. [Google Scholar] [CrossRef]
- Zhang, Y.; Träskman-Bendz, L.; Janelidze, S.; Langenberg, P.; Saleh, A.; Constantine, N.; Okusaga, O.; Bay-Richter, C.; Brundin, L.; Postolache, T.T. Toxoplasma gondii Immunoglobulin G Antibodies and Nonfatal Suicidal Self-Directed Violence. J. Clin. Psychiatry 2012, 73, 1069–1076. [Google Scholar] [CrossRef]
- Akgül, Ö.; Demirel, F.; Poyraz, C.A.; Aydin, E.T.; Uysal, N.; Bulu, E.; Sapmaz, B.; Çalişkan, R.; Öner, Y.A. Toxoplasma gondii infection by serological and molecular methods in schizophrenia patients with and without suicide attempts: An age-sex-matched case-control study. Int. J. Clin. Pract. 2021, 75, e14449. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.; Wilcox, H.C.; Adamos, M.; Katsafanas, E.; Khushalani, S.; Origoni, A.; Savage, C.; Schweinfurth, L.; Stallings, C.; Sweeney, K.; et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J. Psychiatr. Res. 2017, 87, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Arling, T.A.; Yolken, R.H.; Lapidus, M.; Langenberg, P.; Dickerson, F.B.; Zimmerman, S.A.; Balis, T.; Cabassa, J.A.; Scrandis, D.A.; Tonelli, L.H.; et al. Toxoplasma gondii Antibody Titers and History of Suicide Attempts in Patients with Recurrent Mood Disorders. J. Nerv. Ment. Dis. 2009, 197, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Sugden, K.; Moffitt, T.E.; Pinto, L.; Poulton, R.; Williams, B.S.; Caspi, A. Is Toxoplasma gondii Infection Related to Brain and Behavior Impairments in Humans? Evidence from a Population-Representative Birth Cohort. PLoS ONE 2016, 11, e0148435. [Google Scholar] [CrossRef] [PubMed]
- Samojłowicz, D.; Borowska-Solonynko, A.; Golab, E. Prevalence of Toxoplasma gondii parasite infection among people who died due to sudden death in the capital city of Warsaw and its vicinity. Prz. Epidemiol. 2013, 67, 29–33. [Google Scholar]
- Coccaro, E.F.; Lee, R.; Groer, M.W.; Can, A.; Coussons-Read, M.; Postolache, T.T. Toxoplasma gondii infection: Relationship with aggression in psychiatric subjects. J. Clin. Psychiatry 2016, 77, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Samojłowicz, D.; Borowska-Solonynko, A.; Kruczyk, M. New, previously unreported correlations between latent Toxoplasma gondii infection and excessive ethanol consumption. Forensic Sci. Int. 2017, 280, 49–54. [Google Scholar] [CrossRef]
- Fond, G.; Boyer, L.; Schürhoff, F.; Berna, F.; Godin, O.; Bulzacka, E.; Andrianarisoa, M.; Brunel, L.; Aouizerate, B.; Capdevielle, D.; et al. Latent toxoplasma infection in real-world schizophrenia: Results from the national FACE-SZ cohort. Schizophr. Res. 2018, 201, 373–380. [Google Scholar] [CrossRef]
- Burgdorf, K.S.; Trabjerg, B.B.; Pedersen, M.G.; Nissen, J.; Banasik, K.; Pedersen, O.B.; Sørensen, E.; Nielsen, K.R.; Larsen, M.H.; Erikstrup, C.; et al. Large-scale study of Toxoplasma and Cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav. Immun. 2019, 79, 152–158. [Google Scholar] [CrossRef]
- Islam, M.S.; Page-Hefley, S.; Hernandez, A.P.; Whelchel, L.; Crasto, C.; Viator, W.; Vasylyeva, T.L. Change in Urinary Inflammatory Biomarkers and Psychological Health with Gut Microbiome Modulation after Six Months of a Lifestyle Modification Program in Children. Nutrients 2023, 15, 4243. [Google Scholar] [CrossRef]
- Ahrens, A.P.; Sanchez-Padilla, D.E.; Drew, J.C.; Oli, M.W.; Roesch, L.F.W.; Triplett, E.W. Saliva microbiome, dietary, and genetic markers are associated with suicidal ideation in university students. Sci. Rep. 2022, 12, 14306. [Google Scholar] [CrossRef] [PubMed]
- Kaszubinski, S.F.; Pechal, J.L.; Smiles, K.; Schmidt, C.J.; Jordan, H.R.; Meek, M.H.; Benbow, M.E. Dysbiosis in the Dead: Human Postmortem Microbiome Beta-Dispersion as an Indicator of Manner and Cause of Death. Front. Microbiol. 2020, 11, 555347. [Google Scholar] [CrossRef]
- Javan, G.T.; Finley, S.J.; Smith, T.; Miller, J.; Wilkinson, J.E. Cadaver Thanatomicrobiome Signatures: The Ubiquitous Nature of Clostridium Species in Human Decomposition. Front. Microbiol. 2017, 8, 2096. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H.; Vangelatos, A.; Gottel, N.; Osculati, A.; Visona, S.; Finley, S.J.; Gilbert, J.A.; Javan, G.T. Effects of Extended Postmortem Interval on Microbial Communities in Organs of the Human Cadaver. Front. Microbiol. 2020, 11, 569630. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Vasupanrajit, A.; Jirakran, K.; Klomkliew, P.; Chanchaem, P.; Tunvirachaisakul, C.; Plaimas, K.; Suratanee, A.; Payungporn, S. Adverse childhood experiences and reoccurrence of illness impact the gut microbiome, which affects suicidal behaviours and the phenome of major depression: Towards enterotypic phenotypes. Acta Neuropsychiatr. 2023, 35, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, L.; Gustafsson, A.; Lavant, E.; Suneson, K.; Brundin, L.; Westrin, Å.; Ljunggren, L.; Lindqvist, D. Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatr. Scand. 2019, 139, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pechal, J.L.; Schmidt, C.J.; Jordan, H.R.; Wang, W.W.; Benbow, M.E.; Sze, S.-H.; Tarone, A.M. Machine learning performance in a microbial molecular autopsy context: A cross-sectional postmortem human population study. PLoS ONE 2019, 14, e0213829. [Google Scholar] [CrossRef]
- Thompson, D.S.; Fowler, J.C.; Bradshaw, M.R.; Frueh, B.C.; Weinstein, B.L.; Petrosino, J.; Hadden, J.K.; Madan, A. Is the gut microbiota associated with suicidality? Non-significant finding among a large cohort of psychiatrically hospitalized individuals with serious mental illness. J. Affect. Disord. Rep. 2021, 6, 100266. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Flegr, J. How and why Toxoplasma makes us crazy. Trends Parasitol. 2013, 29, 156–163. [Google Scholar] [CrossRef]
- Petzold, J.; Hentschel, A.; Chen, H.; Kuitunen-Paul, S.; London, E.D.; Heinz, A.; Smolka, M.N. Value-based decision-making predicts alcohol use and related problems in young men. J. Psychopharmacol. 2023, 37, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Bigna, J.J.; Tochie, J.N.; Tounouga, D.N.; Bekolo, A.O.; Ymele, N.S.; Youda, E.L.; Sime, P.S.; Nansseu, J.R. Global, regional, and country seroprevalence of Toxoplasma gondii in pregnant women: A systematic review, modelling and meta-analysis. Sci. Rep. 2020, 10, 12102. [Google Scholar] [CrossRef]
- Mergl, R.; Koburger, N.; Heinrichs, K.; Székely, A.; Tóth, M.D.; Coyne, J.; Quintão, S.; Arensman, E.; Coffey, C.; Maxwell, M.; et al. What Are Reasons for the Large Gender Differences in the Lethality of Suicidal Acts? An Epidemiological Analysis in Four European Countries. PLoS ONE 2015, 10, e0129062. [Google Scholar] [CrossRef] [PubMed]
- Bergen, H.; Hawton, K.; Kapur, N.; Cooper, J.; Steeg, S.; Ness, J.; Waters, K. Shared characteristics of suicides and other unnatural deaths following non-fatal self-harm? A multicentre study of risk factors. Psychol. Med. 2012, 42, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Wyart, M.; Jaussent, I.; Ritchie, K.; Abbar, M.; Jollant, F.; Courtet, P. Iowa Gambling Task Performance in Elderly Persons with a Lifetime History of Suicidal Acts. Am. J. Geriatr. Psychiatry 2016, 24, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Jollant, F.; Bellivier, F.; Leboyer, M.; Astruc, B.; Torres, S.; Verdier, R.; Castelnau, D.; Malafosse, A.; Courtet, P. Impaired Decision Making in Suicide Attempters. Am. J. Psychiatry 2005, 162, 304–310. [Google Scholar] [CrossRef]
- Gorlyn, M.; Keilp, J.G.; Oquendo, M.A.; Burke, A.K.; Mann, J.J. Iowa Gambling Task performance in currently depressed suicide attempters. Psychiatry Res. 2013, 207, 150–157. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Pechal, J.L.; Schmidt, C.J.; Jordan, H.R.; Benbow, M.E. A large-scale survey of the postmortem human microbiome, and its potential to provide insight into the living health condition. Sci. Rep. 2018, 8, 5724. [Google Scholar] [CrossRef]
- Javan, G.T.; Finley, S.J.; Can, I.; Wilkinson, J.E.; Hanson, J.D.; Tarone, A.M. Human Thanatomicrobiome Succession and Time Since Death. Sci. Rep. 2016, 6, 29598. [Google Scholar] [CrossRef]
- Chait, Y.A.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci. Rep. 2020, 10, 17878. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Langgartner, D.; Vaihinger, C.A.; Haffner-Luntzer, M.; Kunze, J.F.; Weiss, A.-L.J.; Foertsch, S.; Bergdolt, S.; Ignatius, A.; Reber, S.O. The Role of the Intestinal Microbiome in Chronic Psychosocial Stress-Induced Pathologies in Male Mice. Front. Behav. Neurosci. 2018, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Lee, K.E.; Lee, H.J.; Kim, D.H. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-κB activation through gut microbiota disturbance. Sci. Rep. 2018, 8, 13897. [Google Scholar] [CrossRef] [PubMed]
- Turecki, G.; Brent, D.A.; Gunnell, D.; O’connor, R.C.; Oquendo, M.A.; Pirkis, J.; Stanley, B.H. Suicide and suicide risk. Nat. Rev. Dis. Prim. 2019, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Kreutzmann, J.; Havekes, R.; Abel, T.; Meerlo, P. Sleep deprivation and hippocampal vulnerability: Changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience 2015, 309, 173–190. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Han, Y. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef]
- Donegan, J.J.; Nemeroff, C.B. Nemeroff, Suicide and Inflammation. Adv. Exp. Med. Biol. 2023, 1411, 379–404. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Study | Country and Year | Design | SB Definition | Age in Years (Mean and First SD) | Number of Patients | Number of Control Participants | Gender (N) | Type of Biological Sample | Main Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Alvarado-Esquivel, Mendoza-Larios [89] | Mexico, 2021 | Cross-sectional | Decedents who died by suicide based on medico-legal autopsies. | 34.8 ± 17.4 | 87 suicide victims | - | P: (M: 67, F: 20) | Post mortem brain (prefrontal cortex and amygdala) | A history of depression was associated with T. gondii infection of the brain in suicide victims (OR: 12.00; 95% CI: 2.26–63.46; p = 0.003). |
| Mendoza-Larios, Garcia-Dolores [90] | Mexico, 2021 | Case–control | Decedents who died by suicide and were studied after forensic examination. | P: 35.21 ± 17.48 HC: 31.82 ± 15.01 | 89 decedents who committed suicide | 58 decedents | P: (M: 68, F: 21) HC: (M: 48, F: 10) | Post mortem plasma | No association between T. gondii seropositivity and suicide (OR: 0.85; 95% CI: 0.28–2.60; p = 0.78). |
| Bak, Shim [91] | South Korea, 2018 | Case–control | SA according to Columbia Suicide Severity Rating Scale. | P: 43.75 ± 16.75 HC: 41.59 ± 11.54 | 155 inpatients and outpatients with depressive symptoms + SA | 135 HC | P: (M: 75, F: 80) HC: (M: 66, F: 69) | Plasma | Suicide attempters showed higher seroprevalence of T. gondii than healthy controls (OR: 2.49; 95% CI, 1.26–4.93; p = 0.011). |
| Coryell, Yolken [92] | USA, 2016 | Case–control | SA was defined as any self-harm that was intended to cause death regardless of premeditation or potential lethality. | P: 17.5 ± 1.7 PC: 19.0 ± 1.6 | 17 individuals with MDD + SA | 91 Individuals with MDD | P: (M: 4, F: 13) PC: (M: 26, F: 65) | Plasma | A significantly higher toxoplasmosis IgG titer among individuals who had recently begun a trial of SSRIs and had a history of suicide attempts (t = −2.67, df = 106, p = 0.009). |
| Demirel, Akgul [93] | Turkey, 2023 | Case–control | Structured clinical interview for DSM-5. | MDD: 39.90 ± 13.95 BD: 35.88 ± 12.38 HC: 38.30 ± 14.20 | 83 individuals with MDD + SA; 93 individuals with BD + SA | 78 individuals with MDD; 54 individuals with BD; 310 HC | MDD: (M: 71, F: 76) BD: (M: 73, F: 88) HC: (M: 149, F: 161) | Plasma | Stratified analyses of the binary outcome data indicated that T. gondii seropositivity was a predictor of individuals with a history of suicide attempts (OR = 17.17; 95% CI [8.12–36.28]; p < 0.001). |
| Coryell, Wilcox [94] | USA, 2020 | Cross-sectional | SA according to Columbia Suicide Severity Rating Scale. | P: 32.6 ± 13.1 PC: 38.2 ± 15.4 | 96 individuals with MDD + at leasr two SA | 128 individuals with MDD | P: (M: 33, F: 63) PC: (M: 37, F: 91) | Plasma | Toxoplasma gondii IgM levels were higher, and seropositivity more likely, in suicide attempters (X2 = 7.4, p = 0.01) |
| Ling, Lester [95] | WHO, Europe, 2011 | Ecological Study | Suicide rates by age group were obtained from the European Mortality Database. | Range 0–75+ | 432,974 individuals | Only females | Plasma | Positive relationship between rates of infection with T. gondii and suicide is apparent in women of postmenopausal age (t = 3.02, standardized beta, 0.62, p = 0.007) | |
| Zhang, Traskman-Bendz [96] | Sweden, 2012 | Cross-sectional | Situations in which a person has performed an actually or seemingly life-threatening behavior with the intent of jeopardizing his/her life or to give the appearance of such intent, but which has not resulted in death. | P: 38.4 ± 14.4 HC: 39.8 ± 14.2 | 54 individuals with mixed diagnosis + SA | 30 HC | P: (M: 23, F: 31) HC: (M: 11, F: 19) | Plasma | Seropositivity of T. gondii (OR = 7.12; 95% CI, 1.66–30.6; p = 0.008) and serointensity of T. gondii (OR = 2.01; 95% CI, 1.09–3.71; p = 0.03) were positively associated with a history of SA. |
| Akgul, Demirel [97] | Turkey, 2021 | Case–control | SA according to Suicide Behaviors Questionnaire—Revised and clinical interviews. | P: 47.51 ± 24.83 PC: 43.96 ± 18.33 HC: 42.27 ± 29.11 | 57 individuals with schizophrenia + SA | 60 individuals with schizophrenia 120 HC | P: (M: 34, F: 23) PC: (M: 32, F: 28) HC: (M: 53, F: 67) | Plasma | The relationship between the history of SA and seroprevalence of T gondii was found to be statistically significant (p < 0.05). The history of SA was not statistically associated (p = 0.831) with T gondii positivity by PCR. |
| Kamal, Kamal [46] | Egypt, 2022 | Cross-sectional Case–control | Columbia Suicide Severity Rating Scale (C-SSRS) | P: 32.39 ± 10.47 HC: 33.10 ± 11.03 | 384 depressed individuals | 400 HC | P: (M: 209, F: 175) HC: (M: 214,F: 186) | Serum | Seropositive depressed participants were more likely to have prior history of SA compared with seronegative participants (OR= 6.2, 95% CI: 3.4–11.2, p < 0.001). |
| Dickerson, Wilcox [98] | USA, 2017 | Cross-sectional | A suicide attempt was defined as a potentially self-injurious act committed with at least some intent to die as a result of the act. | P: 38.6 ± 13.0 PC: 36.5 ± 13.8 | 72 individuals with psychiatric diagnosis + SA | 90 patient controls | P: (M: 38, F: 34) PC: (M: 50, F: 40) | Plasma | Higher odds of a suicide attempt history in individuals who had elevated levels of IgM antibodies to T. gondii (OR = 2.41, 95% CI 1.02, 5.71, p = 0.046); A significant correlation between a lifetime history of suicide attempts and the level of IgM class antibodies (beta = 0.070, 95% CI 0.007, 0.134, p = 0.03). |
| Arling, Yolken [99] | USA, 2009 | Case–control | The Columbia Suicide History Form, a semi-structured questionnaire, was used to assess suicide attempt history. | P: 40.3 ± 9.8 PC: 43.4 ± 10.9 HC: 42.7 ± 11.0 | 99 individuals with MDD + SA | 119 individuals with mood disorders 39 HC | P: (M: 39, F: 60) PC: (M: 43, F: 76) HC: (M: 13, F: 26) | Plasma | A predictive association between titers of anti-T. gondii antibodies and history of suicide attempts (OR = 1.55; CI 1.14–2.12, p = 0.006) |
| Sugden, Moffitt [100] | USA, 2016 | Prospective cohort study | Self-reported suicide attempts: Behaviors was only considered a suicide attempt if it was accompanied by a self-reported intention to die. | Range 3–38 | 67 individuals with SA | 770 individuals without SA | M: 423, F: 414 | Plasma | Suicide attempts were marginally more frequent among individuals with T. gondii seropositivity (OR = 2.63, 95% CI 0.97–7.14, p = 0.06). |
| Samojlowicz, Borowska-Solonynko [101] | Poland, 2013 | Case–control | People who died as a result of suicide based on medico-legal autopsies. | P: median = 40 PC: median = 40 HC: median = 51 | 41 suicide victims | 42 traffic accident victims 86 HC | P: (M: 36, F: 5) TA: (M: 39, F: 3) HC: (M: 79, F: 7) | Post mortem Plasma | With respect to the prevalence of T. gondii infection, no statistically significant differences were found between the study and control group (p = 0.09). A statistically significant result was recorded in the 38–58 age group between suicide and control groups (p < 0.05). |
| Coccaro, Lee [102] | USA, 2016 | Cross-sectional | Behaviors was only considered a suicide attempt if it was accompanied by a self-reported intention to die. | P: 36.1 ± 8.3 PC: 33.7 ± 8.1 HC: 31.3 ±8.7 | 110 individuals with intermittent explosive disorder | 110 HC 138 psychiatric controls | P: (M: 70, F: 40) PC: (M: 81, F: 57) HC: (M: 64, F: 46) | Plasma | T. gondii seropositive status did not predict history of suicide attempt (beta = −0.26 ± 0.46, Wald X2= 0.31, p = 0.577) |
| Samojlowicz, Borowska-Solonynko [103] | Poland, 2017 | Case–control | Individuals who committed suicide based on medico-legal autopsies. | RB: median = 40 IRB: median = 50 HC: median = 56 | 126 indviduals with high-risk behavior, who committed suicide | 165 HC 96 individuals with inconclusively high-risk behavior; 51 individuals with risky behavior | RB: (M: 251, F: 26) IRB: (M: 86, F: 10) HC: (M: 140, F: 25) | Post mortem plasma | A strong correlation between latent T. gondii infection and suicide (X2 = 7.04, df = 1, p = 0.008). A strong positive association between T. gondii seropositivity and suicide under the influence of alcohol (p = 0.003, z = 2.95, q = 0.03). |
| Fond, Boyer [104] | France, 2018 | Cohort | Columbia Suicide Severity Rating Scale | 32.0 ± 8.6 | 250 individuals with schizophrenia | P: (M: 184, F: 66) | Plasma | No significant association of latent Toxoplasma infection with suicide behavior was found in the models (p > 0.05) | |
| Burgdorf, Trabjerg [105] | Denmark, 2019 | Case–control | First episode of deliberate self-violence was defined according to the International Classification of Diseases—8th to 10th revisions. Suicide cases were identified based on medico-legal autopsies. | 37.4 (no SD reported) | 655 individuals with SA or suicide | 2591 psychiatric controls 2724 traffic accident victims | P: (M: 278, F: 377) PC: (M: 1277, F: 1324) HC: (M: 1491, F: 1233) | Plasma | T. gondii infection was not statistically significantly associated with attempting or committing suicide (OR, 1.31; 95% CI, 1.10–1.56) |
| Study | Country and Year | Design | SB Definition | Age in Years (Mean and First SD) | Number of Patients | Number of Control Participants | Gender (N) | Type of Biological Sample | Main Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Javan, Finley [109] | USA, 2017 | Case–control | Post mortem samples from the Alabama Department of Forensic Sciences in Montgomery, AL and The Office of the District One Medical Examiner in Pensacola, FL, USA. | P: 43 ± 21.4 HC: 42.8 ± 15.2 | 5 suicide victims | 40 corpses | P: (M: 4, F: 1) HC: (M: 24, F: 16) | Post mortem liver and spleen tissues | Statistically significant difference in Chao1 richness between two 16S rRNA gene regions. In comparisons of gender and manner of death (accident, homicide, natural, suicide, or undetermined), statistically significant differences were observed (ANOVA; p < 0.001). |
| Lutz, Vangelatos [110] | Italy, 2020 | Case–control | Suicide based on medico-legal routine autopsy. | Range 15–90 years. | 40 suicide victims | M: 26, F: 14 | Microbiota of organ tissues including brain, heart, liver, spleen, prostate, and uterus (post mortem) | Death by suicide showed a strong negative association with amplicon sequence variants (ASVs) in the family Peptostreptococcaceae (>20 log2 fold change relative to other causes of death) | |
| Zhang, Pechal [113] | USA, 2019 | Cross-sectional | Suicide based on medico-legal routine autopsy. | 43.9, ranged from 18–88 years. | 23 suicide cases | 265 death by accident, homicide, or natural causes. | M: 105, F: 83 | Post mortem sample: by swabbing the external auditory canal, eyes, nose, mouth, umbilicus, and rectum with DNA-free sterile cotton-tipped applicators | Suicide cases had significantly higher Actinomyces sp. counts than homicides or natural or accidental deaths (p < 0.01) |
| Ohlsson, Gustafsson [112] | USA, 2019 | Cross-sectional | SB was assessed by means of the Suicide Assessment Scale (SUAS). | P: 38.5 ± 14.5 PC: 34.5 ± 11.5 HC: 34.4 ± 11.4 | 54 individuals with depressive symptomes + SA | 13 MDD 17 HC | P: (M: 24, F:3 0) P: (M: 6, F: 7) HC: (M: 9, F: 8) | Blood plasma | The rSA group had significantly higher I-FABP and lower zonulin levels compared to both HCs and the nsMDD group (all p < 0.001). SUAS scores correlated significantly and positively with I-FABP and negatively with zonulin (r = −0.51, p < 0.001). |
| Thompson, Fowler [114] | USA, 2021 | Cohort | Columbia Suicide Severity Rating Scale. | 37.0 ± 13.8 | 100 psychiatric inpatients | M: 53, F: 47 | Stool | No significant relationship between gut microbiota variance and SI and suicide-related behaviors in a cohort of individuals with mental disorder (p > 0.05). | |
| Maes, Vasupanrajit [111] | Thailand, 2023 | Cross-sectional | Columbia Suicide Severity Rating Scale. | Range: 19–58 years. | 32 individuals with MDD | 37 healthy controls | - | Stool | The enterotype dysbiosis index of MDD based on microbiota phyla, genera, and species was associated with the recurrence of MDD and suicidal behavior (r = 0.471, p < 0.001). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerekidze, A.; Li, M.; Refisch, A.; Shameya, J.; Sobanski, T.; Walter, M.; Wagner, G. Impact of Toxoplasma gondii and Human Microbiome on Suicidal Behavior: A Systematic Review. J. Clin. Med. 2024, 13, 593. https://doi.org/10.3390/jcm13020593
Zerekidze A, Li M, Refisch A, Shameya J, Sobanski T, Walter M, Wagner G. Impact of Toxoplasma gondii and Human Microbiome on Suicidal Behavior: A Systematic Review. Journal of Clinical Medicine. 2024; 13(2):593. https://doi.org/10.3390/jcm13020593
Chicago/Turabian StyleZerekidze, Ani, Meng Li, Alexander Refisch, Justina Shameya, Thomas Sobanski, Martin Walter, and Gerd Wagner. 2024. "Impact of Toxoplasma gondii and Human Microbiome on Suicidal Behavior: A Systematic Review" Journal of Clinical Medicine 13, no. 2: 593. https://doi.org/10.3390/jcm13020593
APA StyleZerekidze, A., Li, M., Refisch, A., Shameya, J., Sobanski, T., Walter, M., & Wagner, G. (2024). Impact of Toxoplasma gondii and Human Microbiome on Suicidal Behavior: A Systematic Review. Journal of Clinical Medicine, 13(2), 593. https://doi.org/10.3390/jcm13020593







