Deep Infiltrating Endometriosis in Adolescence: Early Diagnosis and Possible Prevention of Disease Progression
Abstract
1. Introduction
2. Search Methodology
3. Pathogenesis of DIE in Adolescents
4. Prevalence of DIE in Adolescents
5. DIE Symptoms
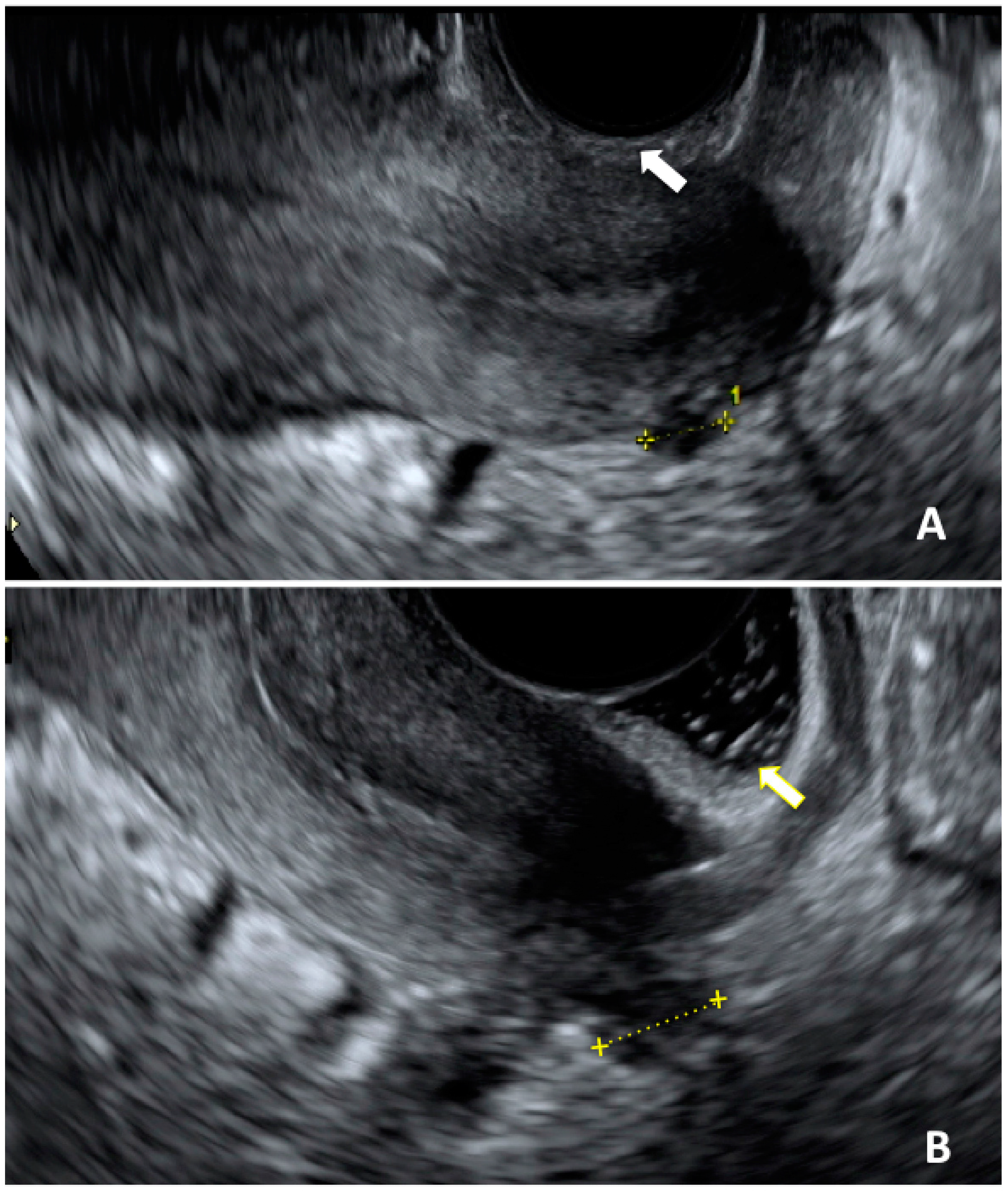
6. The Role of Imaging in Adolescence DIE Diagnosis
7. Adenomyosis and DIE in Adolescence
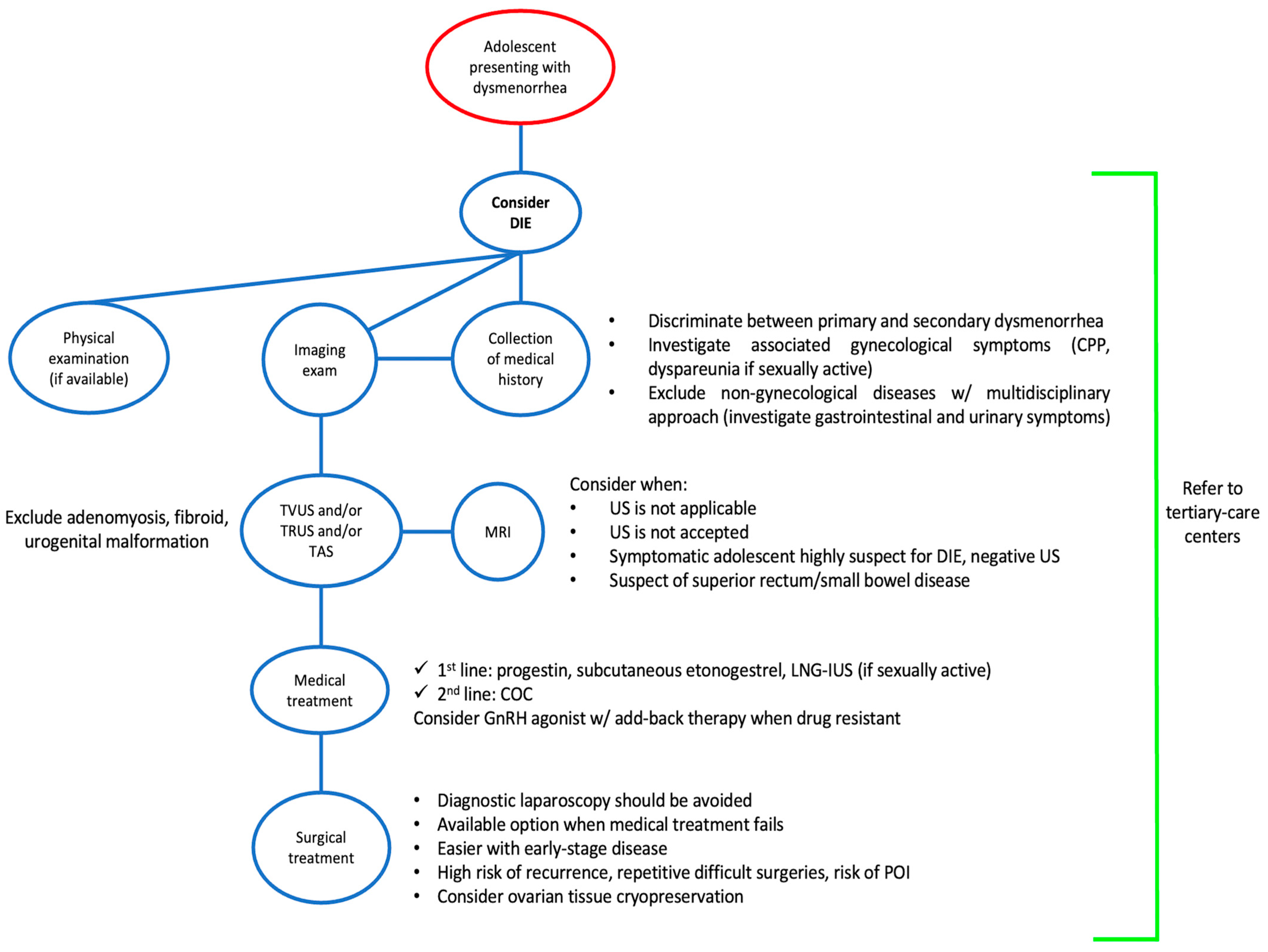
8. Management
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- D’alterio, M.N.; D’ancona, G.; Raslan, M.; Tinelli, R.; Daniilidis, A.; Angioni, S. Management Challenges of Deep Infiltrating Endometriosis. Int. J. Fertil. Steril. 2021, 15, 88. [Google Scholar] [CrossRef]
- Treloar, S.A.; Bell, T.A.; Nagle, C.M.; Purdie, D.M.; Green, A.C. Early menstrual characteristics associated with subsequent diagnosis of endometriosis. Am. J. Obstet. Gynecol. 2010, 202, 534.e1–534.e6. [Google Scholar] [CrossRef] [PubMed]
- Banikarim, C.; Chacko, M.R.; Kelder, S.H. Prevalence and impact of dysmenorrhea on Hispanic female adolescents. Arch. Pediatr. Adolesc. Med. 2000, 154, 1226–1229. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Chapron, C.; Lafay-Pillet, M.C.; Monceau, E.; Borghese, B.; Ngô, C.; Souza, C.; de Ziegler, D. Questioning patients about their adolescent history can identify markers associated with deep infiltrating endometriosis. Fertil. Steril. 2011, 95, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Zannoni, L.; Giorgi, M.; Spagnolo, E.; Montanari, G.; Villa, G.; Seracchioli, R. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J. Pediatr. Adolesc. Gynecol. 2014, 27, 258–265. [Google Scholar] [CrossRef]
- Laufer, M.R. Helping “adult gynecologists” diagnose and treat adolescent endometriosis: Reflections on my 20 years of personal experience. J. Pediatr. Adolesc. Gynecol. 2011, 24 (Suppl. S5), S13–S17. [Google Scholar] [CrossRef]
- Geysenbergh, B.; Dancet, E.A.F.; D’hooghe, T. Detecting Endometriosis in Adolescents: Why Not Start from Self-Report Screening Questionnaires for Adult Women? Gynecol. Obstet. Investig. 2017, 82, 322–328. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front. Endocrinol. 2021, 12, 745548. [Google Scholar] [CrossRef]
- Brosens, I.A.; Brosens, J.J. Redefining endometriosis: Is deep endometriosis a progressive disease? Hum. Reprod. 2000, 15, 1–3. [Google Scholar] [CrossRef]
- Gargett, C.E.; Schwab, K.E.; Brosens, J.J.; Puttemans, P.; Benagiano, G.; Brosens, I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol. Hum. Reprod. 2014, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Gargett, C.E.; Guo, S.W.; Puttemans, P.; Gordts, S.; Brosens, J.J.; Benagiano, G. Origins and Progression of Adolescent Endometriosis. Reprod. Sci. 2016, 23, 1282–1288. [Google Scholar] [CrossRef]
- Benagiano, G.; Guo, S.W.; Puttemans, P.; Gordts, S.; Brosens, I. Progress in the diagnosis and management of adolescent endometriosis: An opinion. Reprod. Biomed. Online 2018, 36, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Kondo, W.; Bourdel, N.; Tamburro, S.; Cavoli, D.; Jardon, K.; Rabischong, B.; Botchorishvili, R.; Pouly, J.; Mage, G.; Canis, M. Complications after surgery for deeply infiltrating pelvic endometriosis. BJOG 2011, 118, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Martire, F.G.; Zupi, E.; Lazzeri, L.; Morosetti, G.; Conway, F.; Centini, G.; Solima, E.; Pietropolli, A.; Piccione, E.; Exacoustos, C. Transvaginal Ultrasound Findings After Laparoscopic Rectosigmoid Segmental Resection for Deep Infiltrating Endometriosis. J. Ultrasound Med. 2021, 40, 1219–1228. [Google Scholar] [CrossRef]
- Martire, F.G.; Russo, C.; Selntigia, A.; Siciliano, T.; Lazzeri, L.; Piccione, E.; Zupi, E.; Exacoustos, C. Transvaginal ultrasound evaluation of the pelvis and symptoms after laparoscopic partial cystectomy for bladder endometriosis. J. Turk. Ger. Gynecol. Assoc. 2022, 23, 145–153. [Google Scholar] [CrossRef]
- Gordts, S.; Koninckx, P.; Brosens, I. Pathogenesis of deep endometriosis. Fertil. Steril. 2017, 108, 872–885.e1. [Google Scholar] [CrossRef]
- Balun, J.; Dominick, K.; Cabral, M.D.; Taubel, D. Endometriosis in adolescents: A narrative review. Pediatr. Med. 2019, 2, 33. [Google Scholar] [CrossRef]
- Brosens, I.; Benagiano, G. Is neonatal uterine bleeding involved in the pathogenesis of endometriosis as a source of stem cells? Fertil. Steril. 2013, 100, 622–623. [Google Scholar] [CrossRef]
- Brosens, I.; Benagiano, G. Clinical significance of neonatal menstruation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 196, 57–59. [Google Scholar] [CrossRef]
- Olive, D.L.; Henderson, D.Y. Endometriosis and mullerian anomalies. Obstet. Gynecol. 1987, 69 Pt 1, 412–415. [Google Scholar]
- Liakopoulou, M.K.; Tsarna, E.; Eleftheriades, A.; Arapaki, A.; Toutoudaki, K.; Christopoulos, P. Medical and Behavioral Aspects of Adolescent Endometriosis: A Review of the Literature. Children 2022, 9, 384. [Google Scholar] [CrossRef]
- Mendiola, J.; Sánchez-Ferrer, M.L.; Jiménez-Velázquez, R.; Cánovas-López, L.; Hernández-Peñalver, A.I.; Corbalán-Biyang, S.; Carmona-Barnosi, A.; Prieto-Sánchez, M.T.; Nieto, A.; Torres-Cantero, A.M. Endometriomas and deep infiltrating endometriosis in adulthood are strongly associated with anogenital distance, a biomarker for prenatal hormonal environment. Hum. Reprod. 2016, 31, 2377–2383. [Google Scholar] [CrossRef]
- Donnez, J. Introduction: From pathogenesis to therapy, deep endometriosis remains a source of controversy. Fertil. Steril. 2017, 108, 869–871. [Google Scholar] [CrossRef]
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Amro, B.; Aristondo, M.E.R.; Alsuwaidi, S.; Almaamari, B.; Hakim, Z.; Tahlak, M.; Wattiez, A.; Koninckx, P.R. New Understanding of Diagnosis, Treatment and Prevention of Endometriosis. Int. J. Environ. Res. Public. Health 2022, 19, 6725. [Google Scholar] [CrossRef]
- Savilova, A.M.; Farkhat, K.N.; Yushina, M.N.; Rudimova, Y.V.; Makiyan, Z.N.; Adamyan, L.V. Characteristics of Multipotent Mesenchymal Stromal Cells Isolated from the Endometrium and Endometriosis Lesions of Women with Malformations of the Internal Reproductive Organs. Bull. Exp. Biol. Med. 2017, 162, 539–544. [Google Scholar] [CrossRef]
- Gargett, C.E.; Masuda, H. Adult stem cells in the endometrium. Mol. Hum. Reprod. 2010, 16, 818–834. [Google Scholar] [CrossRef]
- Du, H.; Taylor, H.S. Stem cells and reproduction. Curr. Opin. Obstet. Gynecol. 2010, 22, 235–241. [Google Scholar] [CrossRef]
- Sasson, I.E.; Taylor, H.S. Stem cells and the pathogenesis of endometriosis. Ann. N. Y. Acad. Sci. 2008, 1127, 106–115. [Google Scholar] [CrossRef]
- Ibrahim, M.G.; Chiantera, V.; Frangini, S.; Younes, S.; Köhler, C.; Taube, E.T.; Plendl, J.; Mechsner, S. Ultramicro-trauma in the endometrial-myometrial junctional zone and pale cell migration in adenomyosis. Fertil. Steril. 2015, 104, 1475–1483.e3. [Google Scholar] [CrossRef] [PubMed]
- Tapmeier, T.T.; Becker, C.M. Is pale the way to go to understand adenomyosis? Fertil. Steril. 2015, 104, 1378. [Google Scholar] [CrossRef][Green Version]
- Koninckx, P.R.; Barlow, D.; Kennedy, S. Implantation versus infiltration: The Sampson versus the endometriotic disease theory. Gynecol. Obstet. Investig. 1999, 47 (Suppl. S1), 3–10. [Google Scholar] [CrossRef]
- Sinaii, N.; Cleary, S.D.; Ballweg, M.L.; Nieman, L.K.; Stratton, P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: A survey analysis. Hum. Reprod. 2002, 17, 2715–2724. [Google Scholar] [CrossRef]
- Solnik, M.J. Chronic pelvic pain and endometriosis in adolescents. Curr. Opin. Obstet. Gynecol. 2006, 18, 511–518. [Google Scholar] [CrossRef]
- Sasamoto, N.; Ngo, L.; Vitonis, A.F.; Dillon, S.T.; Sieberg, C.B.; A Missmer, S.; A Libermann, T.; Terry, K.L. Plasma proteomic profiles of pain subtypes in adolescents and young adults with endometriosis. Hum. Reprod. 2023, 38, 1509–1519. [Google Scholar] [CrossRef]
- Han, S.J.; O’Malley, B.W. The dynamics of nuclear receptors and nuclear receptor coregulators in the pathogenesis of endometriosis. Hum. Reprod. Update 2014, 20, 467. [Google Scholar] [CrossRef]
- Knox, B.; Ong, Y.C.; Bakar, M.A.; Grover, S.R. A longitudinal study of adolescent dysmenorrhoea into adulthood. Eur. J. Pediatr. 2019, 178, 1325–1332. [Google Scholar] [CrossRef]
- Karadadas, E.; Hortu, I.; Ak, H.; Ergenoglu, A.M.; Karadadas, N.; Aydin, H.H. Evaluation of complement system proteins C3a, C5a and C6 in patients of endometriosis. Clin. Biochem. 2020, 81, 15–19. [Google Scholar] [CrossRef]
- Borghese, B.; Zondervan, K.T.; Abrao, M.S.; Chapron, C.; Vaiman, D. Recent insights on the genetics and epigenetics of endometriosis. Clin. Genet. 2017, 91, 254–264. [Google Scholar] [CrossRef]
- Sapkota, Y.; Attia, J.; Gordon, S.D.; Henders, A.K.; Holliday, E.G.; Rahmioglu, N.; MacGregor, S.; Martin, N.G.; McEvoy, M.; Morris, A.P.; et al. Genetic burden associated with varying degrees of disease severity in endometriosis. Mol. Hum. Reprod. 2015, 21, 594. [Google Scholar] [CrossRef]
- Baranov, V.S.; Ivaschenko, T.E.; Liehr, T.; Yarmolinskaya, M.I. Systems genetics view of endometriosis: A common complex disorder. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 185, 59–65. [Google Scholar] [CrossRef]
- Treloar, S.A.; O’Connor, D.T.; O’Connor, V.M.; Martin, N.G. Genetic influences on endometriosis in an Australian twin sample. Fertil. Steril. 1999, 71, 701–710. [Google Scholar] [CrossRef]
- Kennedy, S.; Hadfield, R.; Mardon, H.; Barlow, D. Age of onset of pain symptoms in non-twin sisters concordant for endometriosis. Hum. Reprod. 1996, 11, 403–405. [Google Scholar] [CrossRef][Green Version]
- Hadfield, R.M.; Mardon, H.J.; Barlow, D.H.; Kennedy, S.H. Endometriosis in monozygotic twins. Fertil. Steril. 1997, 68, 941–942. [Google Scholar] [CrossRef]
- Rahmioglu, N.; Nyholt, D.R.; Morris, A.P.; Missmer, S.A.; Montgomery, G.W.; Zondervan, K.T. Genetic variants underlying risk of endometriosis: Insights from meta-analysis of eight genome-wide association and replication datasets. Hum. Reprod. Update 2014, 20, 702. [Google Scholar] [CrossRef]
- Rahmioglu, N.; Morris, A.P.; Nyholt, D.R.; Montgomery, G.W.; Becker, C.M.; Missmer, S.A.; Zondervan, K.T. Beyond endometriosis GWAS: From Genomics to Phenomics to the Patient. Semin. Reprod. Med. 2016, 34, 242. [Google Scholar] [CrossRef] [PubMed]
- Rahmioglu, N.; MacGregor, S.; Drong, A.W.; Hedman, K.; Harris, H.R.; Randall, J.C.; Prokopenko, I.; Nyholt, D.R.; Morris, A.P.; Montgomery, G.W.; et al. Genome-wide enrichment analysis between endometriosis and obesity-related traits reveals novel susceptibility loci. Hum. Mol. Genet. 2015, 24, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Rahmioglu, N.; Mortlock, S.; Ghiasi, M.; Møller, P.L.; Stefansdottir, L.; Galarneau, G.; Turman, C.; Danning, R.; Law, M.H.; Sapkota, Y.; et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat. Genet. 2023, 55, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Iwai, K.; Niiro, E.; Morioka, S.; Yamada, Y. Fetal programming theory: Implication for the understanding of endometriosis. Hum. Immunol. 2014, 75, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W. Epigenetics of endometriosis. Mol. Hum. Reprod. 2009, 15, 587–607. [Google Scholar] [CrossRef] [PubMed]
- Baumann, C.; Olson, M.; Wang, K.; Fazleabas, A.; De La Fuente, R. Arginine methyltransferases mediate an epigenetic ovarian response to endometriosis. Reproduction 2015, 150, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Houshdaran, S.; Nezhat, C.R.; Vo, K.C.; Zelenko, Z.; Irwin, J.C.; Giudice, L.C. Aberrant Endometrial DNA Methylome and Associated Gene Expression in Women with Endometriosis. Biol. Reprod. 2016, 95, 93. [Google Scholar] [CrossRef]
- Koukoura, O.; Sifakis, S.; Spandidos, D.A. DNA methylation in endometriosis (Review). Mol. Med. Rep. 2016, 13, 2939–2948. [Google Scholar] [CrossRef] [PubMed]
- Zidan, H.E.; Rezk, N.A.; Alnemr, A.A.A.; Abd el Ghany, A.M. COX-2 gene promoter DNA methylation status in eutopic and ectopic endometrium of Egyptian women with endometriosis. J. Reprod. Immunol. 2015, 112, 63–67. [Google Scholar] [CrossRef]
- Koninckx, P.P. The physiopathology of endometriosis: Pollution and dioxin. Gynecol. Obstet. Investig. 1999, 47 (Suppl. S1), 47–50. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Osteen, K.G. Dioxin-like PCBs and Endometriosis. Syst. Biol. Reprod. Med. 2010, 56, 132. [Google Scholar] [CrossRef]
- Wood, D.H.; Yochmowitz, M.G.; Salmon, Y.L.; Eason, R.L.; Boster, R.A. Proton irradiation and endometriosis. Aviat. Space Environ. Med. 1983, 54, 718–724. [Google Scholar]
- Fanton, J.W.; Golden, J.G. Radiation-induced endometriosis in Macaca mulatta. Radiat. Res. 1991, 126, 141–146. [Google Scholar] [CrossRef]
- Birnbaum, L.S.; Cummings, A.M. Dioxins and endometriosis: A plausible hypothesis. Environ. Health Perspect. 2002, 110, 15. [Google Scholar] [CrossRef] [PubMed]
- Gubbels, A.; Spivack, L.; Lindheim, S.R.; Bhagavath, B. Adolescent Endometriosis. Obstet. Gynecol. Surv. 2020, 75, 483–496. [Google Scholar] [CrossRef]
- Hirsch, M.; Dhillon-Smith, R.; Cutner, A.S.; Yap, M.; Creighton, S.M. The Prevalence of Endometriosis in Adolescents with Pelvic Pain: A Systematic Review. J. Pediatr. Adolesc. Gynecol. 2020, 33, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Donnez, J. Deep endometriosis: Definition, diagnosis, and treatment. Fertil. Steril. 2012, 98, 564–571. [Google Scholar] [CrossRef] [PubMed]
- DiVasta, A.D.; Vitonis, A.F.; Laufer, M.R.; Missmer, S.A. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs. adulthood. Am. J. Obstet. Gynecol. 2018, 218, 324.e1–324.e11. [Google Scholar] [CrossRef]
- Suvitie, P.A.; Hallamaa, M.K.; Matomäki, J.M.; Mäkinen, J.I.; Perheentupa, A.H. Prevalence of Pain Symptoms Suggestive of Endometriosis Among Finnish Adolescent Girls (TEENMAPS Study). J. Pediatr. Adolesc. Gynecol. 2016, 29, 97–103. [Google Scholar] [CrossRef]
- Sasamoto, N.; Shafrir, A.L.; Wallace, B.M.; Vitonis, A.F.; Fraer, C.J.; Gallagher, J.S.; DePari, M.; Ghiasi, M.; Laufer, M.R.; Sieberg, C.B.; et al. Trends in pelvic pain symptoms over 2 years of follow-up among adolescents and young adults with and without endometriosis. Pain 2023, 164, 613–624. [Google Scholar] [CrossRef]
- Bush, D.; Brick, E.; East, M.C.; Johnson, N. Endometriosis education in schools: A New Zealand model examining the impact of an education program in schools on early recognition of symptoms suggesting endometriosis. Aust. N. Z. J. Obstet. Gynaecol. 2017, 57, 452–457. [Google Scholar] [CrossRef]
- Martire, F.G.; Lazzeri, L.; Conway, F.; Siciliano, T.; Pietropolli, A.; Piccione, E.; Solima, E.; Centini, G.; Zupi, E.; Exacoustos, C. Adolescence and endometriosis: Symptoms, ultrasound signs and early diagnosis. Fertil. Steril. 2020, 114, 1049–1057. [Google Scholar] [CrossRef]
- Martire, F.G.; Russo, C.; Selntigia, A.; Nocita, E.; Soreca, G.; Lazzeri, L.; Zupi, E.; Exacoustos, C. Early noninvasive diagnosis of endometriosis: Dysmenorrhea and specific ultrasound findings are important indicators in young women. Fertil. Steril. 2023, 119, 455–464. [Google Scholar] [CrossRef]
- Millischer, A.E.; Santulli, P.; Da Costa, S.; Bordonne, C.; Cazaubon, E.; Marcellin, L.; Chapron, C. Adolescent endometriosis: Prevalence increases with age on magnetic resonance imaging scan. Fertil. Steril. 2023, 119, 626–633. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yang, J.; Wang, S.; Lang, J. Adolescent Endometriosis in China: A Retrospective Analysis of 63 Cases. J. Pediatr. Adolesc. Gynecol. 2012, 25, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Stochino-Loi, E.; Millochau, J.C.; Angioni, S.; Touleimat, S.; Abo, C.; Chanavaz-Lacheray, I.; Hennetier, C.; Roman, H. Relationship between Patient Age and Disease Features in a Prospective Cohort of 1560 Women Affected by Endometriosis. J. Minim. Invasive Gynecol. 2020, 27, 1158–1166. [Google Scholar] [CrossRef]
- Janssen, E.B.; Rijkers, A.C.M.; Hoppenbrouwers, K.; Meuleman, C.; D’Hooghe, T.M. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: A systematic review. Hum. Reprod. Update 2013, 19, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, L.; Andersson, K.L.; Angioni, S.; Arena, A.; Arena, S.; Bartiromo, L.; Berlanda, N.; Bonin, C.; Candiani, M.; Centini, G.; et al. How to Manage Endometriosis in Adolescence: The Endometriosis Treatment Italian Club Approach. J. Minim. Invasive Gynecol. 2023, 30, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Greene, R.; Stratton, P.; Cleary, S.D.; Ballweg, M.L.; Sinaii, N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil. Steril. 2009, 91, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Martire, F.G.; Piccione, E.; Exacoustos, C.; Zupi, E. Endometriosis and Adolescence: The Impact of Dysmenorrhea. J. Clin. Med. 2023, 12, 5624. [Google Scholar] [CrossRef]
- Takeda, T.; Tadakawa, M.; Koga, S.; Nagase, S.; Yaegashi, N. Relationship between dysmenorrhea and posttraumatic stress disorder in Japanese high school students 9 months after the Great East Japan Earthquake. J. Pediatr. Adolesc. Gynecol. 2013, 26, 355–357. [Google Scholar] [CrossRef]
- Li, R.; Li, B.; Kreher, D.A.; Benjamin, A.R.; Gubbels, A.; Smith, S.M. Association between dysmenorrhea and chronic pain: A systematic review and meta-analysis of population-based studies. Am. J. Obstet. Gynecol. 2020, 223, 350–371. [Google Scholar] [CrossRef]
- Centini, G.; Lazzeri, L.; Dores, D.; Pianigiani, L.; Iannone, P.; Luisi, S.; Petraglia, F.; Zupi, E. Chronic Pelvic Pain and Quality of Life in Women with and without Endometriosis. J. Endometr. Pelvic Pain Disord. 2013, 5, 27–33. [Google Scholar] [CrossRef]
- Hailemeskel, S.; Demissie, A.; Assefa, N. Primary dysmenorrhea magnitude, associated risk factors, and its effect on academic performance: Evidence from female university students in Ethiopia. Int. J. Women’s Health 2016, 8, 489. [Google Scholar] [CrossRef]
- Moumane, K.; Idri, A. Mobile applications for endometriosis management functionalities: Analysis and potential. Sci. Afr. 2023, 21, e01833. [Google Scholar] [CrossRef]
- Mona Lisa Smile EU Project. Available online: https://www.monalisasmile.eu (accessed on 14 January 2024).
- Chapron, C.; Santulli, P.; De Ziegler, D.; Noel, J.-C.; Anaf, V.; Streuli, I.; Foulot, H.; Souza, C.; Borghese, B. Ovarian endometrioma: Severe pelvic pain is associated with deeply infiltrating endometriosis. Hum. Reprod. 2012, 27, 702–711. [Google Scholar] [CrossRef]
- Pino, I.; Belloni, G.M.; Barbera, V.; Solima, E.; Radice, D.; Angioni, S.; Arena, S.; Bergamini, V.; Candiani, M.; Maiorana, A.; et al. Better late than never but never late is better”, especially in young women. A multicenter Italian study on diagnostic delay for symptomatic endometriosis. Eur. J. Contracept. Reprod. Health Care 2023, 28, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Tsonis, O.; Barmpalia, Z.; Gkrozou, F.; Chandraharan, E.; Pandey, S.; Siafaka, V.; Paschopoulos, M. Endometriosis in adolescence: Early manifestation of the traditional disease or a unique variant? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 247, 238–243. [Google Scholar] [CrossRef]
- Shim, J.Y.; Laufer, M.R. Adolescent Endometriosis: An Update. J. Pediatr. Adolesc. Gynecol. 2020, 33, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Laufer, M.R.; Goitein, L.; Bush, M.; Cramer, D.W.; Emans, S.J. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy. J. Pediatr. Adolesc. Gynecol. 1997, 10, 199–202. [Google Scholar] [CrossRef]
- Marcellin, L.; Santulli, P.; Pinzauti, S.; Bourdon, M.; Lamau, M.C.; Borghese, B.; Petraglia, F.; Chapron, C. Age at menarche does not correlate with the endometriosis phenotype. PLoS ONE 2019, 14, e0219497. [Google Scholar] [CrossRef]
- Raimondo, D.; Raffone, A.; Renzulli, F.; Sanna, G.; Raspollini, A.; Bertoldo, L.; Maletta, M.; Lenzi, J.; Rovero, G.; Travaglino, A.; et al. Prevalence and Risk Factors of Central Sensitization in Women with Endometriosis. J. Minim. Invasive Gynecol. 2023, 30, 73–80.e1. [Google Scholar] [CrossRef]
- Miller, J.A.; Missmer, S.A.; Vitonis, A.F.; Sarda, V.; Laufer, M.R.; DiVasta, A.D. Prevalence of migraines in adolescents with endometriosis. Fertil. Steril. 2018, 109, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.P.; Vitonis, A.F.; Fadayomi, A.B.; Charlton, B.M.; Missmer, S.A.; DiVasta, A.D. Quality of Life in Adolescent and Young Adult Women With Dyspareunia and Endometriosis. J. Adolesc. Health 2020, 67, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Schomacker, M.L.; Hansen, K.E.; Ramlau-Hansen, C.H.; Forman, A. Is endometriosis associated with irritable bowel syndrome? A cross-sectional study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 65–69. [Google Scholar] [CrossRef]
- El-Matary, W.; Deora, V.; Grover, K. Barriers to clinical research in children with inflammatory bowel disease: The patients’ perspective. PLoS ONE 2018, 13, e0206965. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, P.; Agnihotri, A.; Das, P.; Mishra, A.; Verma, A.K.; Ahuja, A.; Sreenivas, V.; Khadgawat, R.; Gupta, S.D.; et al. Celiac disease: A disease with varied manifestations in adults and adolescents. J. Dig. Dis. 2013, 14, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.C.; Søreide, K. Systematic review of epidemiology, presentation, and management of Meckel’s diverticulum in the 21st century. Medicine 2018, 97, e12154. [Google Scholar] [CrossRef] [PubMed]
- Neri, B.; Russo, C.; Mossa, M.; Martire, F.G.; Selntigia, A.; Mancone, R.; Calabrese, E.; Rizzo, G.; Exacoustos, C.; Biancone, L. High Frequency of Deep Infiltrating Endometriosis in Patients with Inflammatory Bowel Disease: A Nested Case-Control Study. Dig. Dis. 2023, 41, 719–728. [Google Scholar] [CrossRef]
- Dun, E.C.; Kho, K.A.; Morozov, V.V.; Kearney, S.; Zurawin, J.L.; Nezhat, C.H. Endometriosis in adolescents. JSLS 2015, 19, e2015.00019. [Google Scholar] [CrossRef]
- Wright, K.N.; Laufer, M.R. Endometriomas in adolescents. Fertil. Steril. 2010, 94, 1529.e7–1529.e9. [Google Scholar] [CrossRef]
- Hudelist, G.; Ballard, K.; English, J.; Wright, J.; Banerjee, S.; Mastoroudes, H.; Thomas, A.; Singer, C.F.; Keckstein, J. Transvaginal sonography vs. clinical examination in the preoperative diagnosis of deep infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2011, 37, 480–487. [Google Scholar] [CrossRef]
- Hudelist, G.; Fritzer, N.; Staettner, S.; Tammaa, A.; Tinelli, A.; Sparic, R.; Keckstein, J. Uterine sliding sign: A simple sonographic predictor for presence of deep infiltrating endometriosis of the rectum. Ultrasound Obstet. Gynecol. 2013, 41, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2, CD009591. [Google Scholar] [CrossRef] [PubMed]
- Margueritte, F.; Fauconnier, A. Adolescent endometriosis: Should we really use magnetic resonance imaging scans in this population? Fertil. Steril. 2023, 120 Pt 2, 706. [Google Scholar] [CrossRef] [PubMed]
- Güdücü, N.; Sidar, G.; Işçi, H.; Yiǧiter, A.B.; Dünder, I. The Utility of Transrectal Ultrasound in Adolescents When Transabdominal or Transvaginal Ultrasound Is Not Feasible. J. Pediatr. Adolesc. Gynecol. 2013, 26, 265–268. [Google Scholar] [CrossRef]
- Ohba, T.; Mizutani, H.; Maeda, T.; Matsuura, K.; Okamura, H. Evaluation of endometriosis in uterosacral ligaments by transrectal ultrasonography. Hum. Reprod. 1996, 11, 2014–2017. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Osuga, Y.; Yano, T.; Momoeda, M.; Yoshino, O.; Hirota, Y.; Kugu, K.; Nishii, O.; Tsutsumi, O.; Taketani, Y. Characteristic images of deeply infiltrating rectosigmoid endometriosis on transvaginal and transrectal ultrasonography. Hum. Reprod. 2003, 18, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Copley, S.; Morris, J.; Lindsell, D.; Golding, S.; Kennedy, S. A systematic review of the accuracy of ultrasound in the diagnosis of endometriosis. Ultrasound Obstet. Gynecol. 2002, 20, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Back, S.J.; Maya, C.L.; Zewdneh, D.; Epelman, M. Emergent ultrasound evaluation of the pediatric female pelvis. Pediatr. Radiol. 2017, 47, 1134–1143. [Google Scholar] [CrossRef]
- Torres, A.; Horodeńska, M.; Woźniakowska, E.; Borowik, J. Anxiety Connected with Pelvic Ultrasound in Adolescents and Their Caregivers: Comparison of Transabdominal and Transrectal Approaches. J. Pediatr. Adolesc. Gynecol. 2023, 36, 284–290. [Google Scholar] [CrossRef]
- Zannoni, L.; Del Forno, S.; Paradisi, R.; Seracchioli, R. Endometriosis in Adolescence: Practical Rules for an Earlier Diagnosis. Pediatr. Ann. 2016, 45, e332–e335. [Google Scholar] [CrossRef]
- Alborzi, S.; Rasekhi, A.; Shomali, Z.; Madadi, G.; Alborzi, M.; Kazemi, M.; Nohandani, A.H. Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine 2018, 97, e9536. [Google Scholar] [CrossRef]
- Bazot, M.; Bharwani, N.; Huchon, C.; Kinkel, K.; Cunha, T.M.; Guerra, A.; Manganaro, L.; Buñesch, L.; Kido, A.; Togashi, K.; et al. European society of urogenital radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur. Radiol. 2017, 27, 2765–2775. [Google Scholar] [CrossRef]
- Kinkel, K.; Frei, K.A.; Balleyguier, C.; Chapron, C. Diagnosis of endometriosis with imaging: A review. Eur. Radiol. 2006, 16, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, S.; Saba, L.; Pascual, M.A.; Ajossa, S.; Rodriguez, I.; Mais, V.; Alcazar, J.L. Transvaginal ultrasound vs. magnetic resonance imaging for diagnosing deep infiltrating endometriosis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Smorgick, N.; As-Sanie, S.; Marsh, C.A.; Smith, Y.R.; Quint, E.H. Advanced stage endometriosis in adolescents and young women. J. Pediatr. Adolesc. Gynecol. 2014, 27, 320–323. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Daguati, R.; Abbiati, A.; Fedele, L. Adenomyosis: Epidemiological factors. Best. Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Viganò, P.; Parazzini, F.; Somigliana, E.; Vercellini, P. Endometriosis: Epidemiology and aetiological factors. Best. Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 177–200. [Google Scholar] [CrossRef]
- Kunz, G.; Herbertz, M.; Beil, D.; Huppert, G.; Leyendecker, G. Adenomyosis as a disorder of the early and late human reproductive period. Reprod. Biomed. Online 2007, 15, 681–685. [Google Scholar] [CrossRef]
- Exacoustos, C.; Lazzeri, L.; Martire, F.G.; Russo, C.; Martone, S.; Centini, G.; Piccione, E.; Zupi, E. Ultrasound Findings of Adenomyosis in Adolescents: Type and Grade of the Disease. J. Minim. Invasive Gynecol. 2022, 29, 291–299.e1. [Google Scholar] [CrossRef]
- Dietrich, J.E. An update on adenomyosis in the adolescent. Curr. Opin. Obstet. Gynecol. 2010, 22, 388–392. [Google Scholar] [CrossRef]
- Exacoustos, C.; Morosetti, G.; Conway, F.; Camilli, S.; Martire, F.G.; Lazzeri, L.; Piccione, E.; Zupi, E. New Sonographic Classification of Adenomyosis: Do Type and Degree of Adenomyosis Correlate to Severity of Symptoms? J. Minim. Invasive Gynecol. 2020, 27, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Conway, F.; Morosetti, G.; Camilli, S.; Martire, F.G.; Sorrenti, G.; Piccione, E.; Zupi, E.; Exacoustos, C. Ulipristal acetate therapy increases ultrasound features of adenomyosis: A good treatment given in an erroneous diagnosis of uterine fibroids. Gynecol. Endocrinol. 2019, 35, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Ludovisi, M.; Moro, F.; Pasciuto, T.; Di Noi, S.; Giunchi, S.; Savelli, L.; Pascual, M.A.; Sladkevicius, P.; Alcazar, J.L.; Franchi, D.; et al. Imaging in gynecological disease (15): Clinical and ultrasound characteristics of uterine sarcoma. Ultrasound Obstet. Gynecol. 2019, 54, 676–687. [Google Scholar] [CrossRef]
- Russo, C.; Camilli, S.; Martire, F.G.; Di Giovanni, A.; Lazzeri, L.; Malzoni, M.; Zupi, E.; Exacoustos, C. Ultrasound features of highly vascularized uterine myomas (uterine smooth muscle tumors) and correlation with histopathology. Ultrasound Obstet. Gynecol. 2022, 60, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, G.D.; Gerancher, K.R. ACOG Committee Opinion No. 760: Dysmenorrhea and Endometriosis in the Adolescent. Obstet. Gynecol. 2018, 132, E249–E258. [Google Scholar] [CrossRef]
- Curtis, K.M.; Martins, S.L. Progestogen-only contraception and bone mineral density: A systematic review. Contraception 2006, 73, 470–487. [Google Scholar] [CrossRef]
- DiVasta, A.D.; Laufer, M.R.; Gordon, C.M. Bone density in adolescents treated with a GnRH agonist and add-back therapy for endometriosis. J. Pediatr. Adolesc. Gynecol. 2007, 20, 293–297. [Google Scholar] [CrossRef]
- Ding, D.; Wang, X.; Chen, Y.; Benagiano, G.; Liu, X.; Guo, S.W. Evidence in Support for the Progressive Nature of Ovarian Endometriomas. J. Clin. Endocrinol. Metab. 2020, 105, 2189–2202. [Google Scholar] [CrossRef]
- Afors, K.; Murtada, R.; Centini, G.; Fernandes, R.; Meza, C.; Castellano, J.; Wattiez, A. Employing laparoscopic surgery for endometriosis. Women’s Health 2014, 10, 431–443. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Meuleman, C.; Demeyere, S.; Lesaffre, E.; Cornillie, F.J. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil. Steril. 1991, 55, 759–765. [Google Scholar] [CrossRef]
- Canis, M.; Bourdel, N.; Houlle, C.; Gremeau, A.S.; Botchorishvili, R.; Matsuzaki, S. Endometriosis may not be a chronic disease: An alternative theory offering more optimistic prospects for our patients. Fertil. Steril. 2016, 105, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Centini, G.; Labanca, L.; Giorgi, M.; Martire, F.G.; Catania, F.; Zupi, E.; Lazzeri, L. The implications of the anatomy of the nerves and vessels in the treatment of rectosigmoid endometriosis. Clin Anat. 2023. [Google Scholar] [CrossRef]
- Somigliana, E.; Viganò, P.; Filippi, F.; Papaleo, E.; Benaglia, L.; Candiani, M.; Vercellini, P. Fertility preservation in women with endometriosis: For all, for some, for none? Hum. Reprod. 2015, 30, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Mifsud, J.M.; Pellegrini, L.; Cozzolino, M. Oocyte Cryopreservation in Women with Ovarian Endometriosis. J. Clin. Med. 2023, 12, 6767. [Google Scholar] [CrossRef]
- Ianieri, M.M.; Mautone, D.; Ceccaroni, M. Recurrence in Deep Infiltrating Endometriosis: A Systematic Review of the Literature. J. Minim. Invasive Gynecol. 2018, 25, 786–793. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martire, F.G.; Giorgi, M.; D’Abate, C.; Colombi, I.; Ginetti, A.; Cannoni, A.; Fedele, F.; Exacoustos, C.; Centini, G.; Zupi, E.; et al. Deep Infiltrating Endometriosis in Adolescence: Early Diagnosis and Possible Prevention of Disease Progression. J. Clin. Med. 2024, 13, 550. https://doi.org/10.3390/jcm13020550
Martire FG, Giorgi M, D’Abate C, Colombi I, Ginetti A, Cannoni A, Fedele F, Exacoustos C, Centini G, Zupi E, et al. Deep Infiltrating Endometriosis in Adolescence: Early Diagnosis and Possible Prevention of Disease Progression. Journal of Clinical Medicine. 2024; 13(2):550. https://doi.org/10.3390/jcm13020550
Chicago/Turabian StyleMartire, Francesco Giuseppe, Matteo Giorgi, Claudia D’Abate, Irene Colombi, Alessandro Ginetti, Alberto Cannoni, Francesco Fedele, Caterina Exacoustos, Gabriele Centini, Errico Zupi, and et al. 2024. "Deep Infiltrating Endometriosis in Adolescence: Early Diagnosis and Possible Prevention of Disease Progression" Journal of Clinical Medicine 13, no. 2: 550. https://doi.org/10.3390/jcm13020550
APA StyleMartire, F. G., Giorgi, M., D’Abate, C., Colombi, I., Ginetti, A., Cannoni, A., Fedele, F., Exacoustos, C., Centini, G., Zupi, E., & Lazzeri, L. (2024). Deep Infiltrating Endometriosis in Adolescence: Early Diagnosis and Possible Prevention of Disease Progression. Journal of Clinical Medicine, 13(2), 550. https://doi.org/10.3390/jcm13020550






