The Clinical Utility of the Saliva Proteome in Rare Diseases: A Pilot Study for Biomarker Discovery in Primary Sclerosing Cholangitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Solvents and Materials
2.2. Study Design and Patient Characteristics
2.3. Salivary Sample Collection and Processing
2.4. LC-MS/MS Analysis
2.5. Data Processing and Statistical Analysis
3. Results
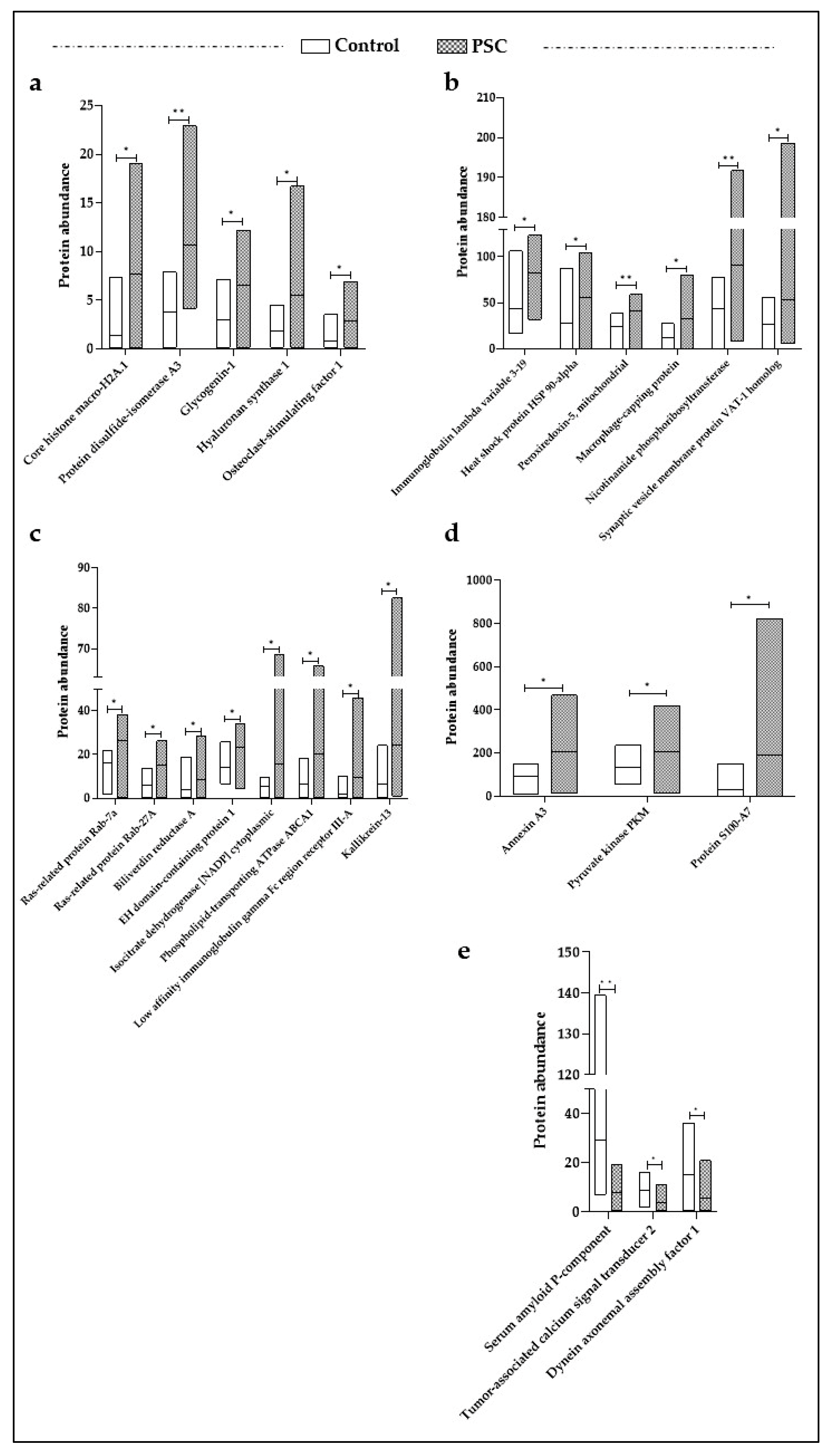
3.1. Differentially Expressed Proteins (DEPs) in PSC Patients
3.2. Functional Network Analysis for DEPs
3.3. Correlation between DEPs and Clinical Features of PSC Patients
3.4. ROC Curves Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karlsen, T.H.; Folseraas, T.; Thorburn, D.; Vesterhus, M. Primary Sclerosing Cholangitis—A Comprehensive Review. J. Hepatol. 2017, 67, 1298–1323. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, K.; Weersma, R.K.; Van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.M.; Poen, A.C.; Van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-Based Epidemiology, Malignancy Risk, and Outcome of Primary Sclerosing Cholangitis: Boonstra et Al. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef]
- Weismüller, T.J.; Trivedi, P.J.; Bergquist, A.; Imam, M.; Lenzen, H.; Ponsioen, C.Y.; Holm, K.; Gotthardt, D.; Färkkilä, M.A.; Marschall, H.-U.; et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology 2017, 152, 1975–1984.e4. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Kodra, Y.; Rocchetti, A.; Manno, V.; Minelli, G.; Gerussi, A.; Ronca, V.; Malinverno, F.; Cristoferi, L.; Floreani, A.; et al. Primary Sclerosing Cholangitis: Burden of Disease and Mortality Using Data from the National Rare Diseases Registry in Italy. Int. J. Environ. Res. Public Health 2020, 17, 3095. [Google Scholar] [CrossRef]
- Lindor, K.D.; Kowdley, K.V.; Harrison, M.E. American College of Gastroenterology ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am. J. Gastroenterol. 2015, 110, 646–659. [Google Scholar] [CrossRef]
- Dyson, J.K.; Beuers, U.; Jones, D.E.J.; Lohse, A.W.; Hudson, M. Primary Sclerosing Cholangitis. Lancet 2018, 391, 2547–2559. [Google Scholar] [CrossRef]
- Chapman, R.; Fevery, J.; Kalloo, A.; Nagorney, D.M.; Boberg, K.M.; Shneider, B.; Gores, G.J. American Association for the Study of Liver Diseases Diagnosis and Management of Primary Sclerosing Cholangitis. Hepatology 2010, 51, 660–678. [Google Scholar] [CrossRef]
- Abdalian, R.; Heathcote, E.J. Sclerosing Cholangitis: A Focus on Secondary Causes. Hepatology 2006, 44, 1063–1074. [Google Scholar] [CrossRef]
- Nallagangula, K.S.; Lakshmaiah, V.; Muninarayana, C.; Deepa, K.; Shashidhar, K. A Proteomic Approach of Biomarker Candidate Discovery for Alcoholic Liver Cirrhosis. J. Circ. Biomark. 2018, 7, 184945441878841. [Google Scholar] [CrossRef]
- Niu, L.; Thiele, M.; Geyer, P.E.; Rasmussen, D.N.; Webel, H.E.; Santos, A.; Gupta, R.; Meier, F.; Strauss, M.; Kjaergaard, M.; et al. Noninvasive Proteomic Biomarkers for Alcohol-Related Liver Disease. Nat. Med. 2022, 28, 1277–1287. [Google Scholar] [CrossRef]
- Lankisch, T.O.; Metzger, J.; Negm, A.A.; Vosskuhl, K.; Schiffer, E.; Siwy, J.; Weismüller, T.J.; Schneider, A.S.; Thedieck, K.; Baumeister, R.; et al. Bile Proteomic Profiles Differentiate Cholangiocarcinoma from Primary Sclerosing Cholangitis and Choledocholithiasis. Hepatology 2011, 53, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, U.; Lourdusamy, V.; Gk Venkatesh, P.; Willard, B.; Sanaka, M.R.; Parsi, M.A. Bile Proteomics for Differentiation of Malignant from Benign Biliary Strictures: A Pilot Study. Gastroenterol. Rep. 2015, 3, 136–143. [Google Scholar] [CrossRef]
- Metzger, J.; Negm, A.A.; Plentz, R.R.; Weismüller, T.J.; Wedemeyer, J.; Karlsen, T.H.; Dakna, M.; Mullen, W.; Mischak, H.; Manns, M.P.; et al. Urine Proteomic Analysis Differentiates Cholangiocarcinoma from Primary Sclerosing Cholangitis and Other Benign Biliary Disorders. Gut 2013, 62, 122–130. [Google Scholar] [CrossRef]
- Vesterhus, M.; Holm, A.; Hov, J.R.; Nygård, S.; Schrumpf, E.; Melum, E.; Thorbjørnsen, L.W.; Paulsen, V.; Lundin, K.; Dale, I.; et al. Novel Serum and Bile Protein Markers Predict Primary Sclerosing Cholangitis Disease Severity and Prognosis. J. Hepatol. 2017, 66, 1214–1222. [Google Scholar] [CrossRef]
- Chuang, Y.-H.; Lian, Z.-X.; Tsuneyama, K.; Chiang, B.-L.; Ansari, A.A.; Coppel, R.L.; Eric Gershwin, M. Increased Killing Activity and Decreased Cytokine Production in NK Cells in Patients with Primary Biliary Cirrhosis. J. Autoimmun. 2006, 26, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.W.; Seidler, S.; Gassler, N.; Nattermann, J.; Luedde, T.; Trautwein, C.; Tacke, F. Interleukin-8 Is Activated in Patients with Chronic Liver Diseases and Associated with Hepatic Macrophage Accumulation in Human Liver Fibrosis. PLoS ONE 2011, 6, e21381. [Google Scholar] [CrossRef]
- Umemura, T.; Sekiguchi, T.; Joshita, S.; Yamazaki, T.; Fujimori, N.; Shibata, S.; Ichikawa, Y.; Komatsu, M.; Matsumoto, A.; Shums, Z.; et al. Association between Serum Soluble CD 14 and IL-8 Levels and Clinical Outcome in Primary Biliary Cholangitis. Liver Int. 2017, 37, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhu, B.; Qu, Y.; Zhang, W. Abnormal Expression of ERα in Cholangiocytes of Patients With Primary Biliary Cholangitis Mediated Intrahepatic Bile Duct Inflammation. Front. Immunol. 2019, 10, 2815. [Google Scholar] [CrossRef]
- Liu, G.; Wang, X.; Yang, T.; Yan, Y.; Xiang, T.; Yang, L.; Luo, X. High Interleukin-8 Levels Associated With Decreased Survival in Patients With Cirrhosis Following Transjugular Intrahepatic Portosystemic Shunt. Front. Med. 2022, 9, 829245. [Google Scholar] [CrossRef]
- Holm, M.; Joenväärä, S.; Saraswat, M.; Tohmola, T.; Saarela, T.; Tenca, A.; Arola, J.; Renkonen, R.; Färkkilä, M. Quantitative Bile and Serum Proteomics for the Screening and Differential Diagnosis of Primary Sclerosing Cholangitis. PLoS ONE 2022, 17, e0272810. [Google Scholar] [CrossRef]
- Ceccherini, E.; Signore, G.; Tedeschi, L.; Vozzi, F.; Di Giorgi, N.; Michelucci, E.; Cecchettini, A.; Rocchiccioli, S. Proteomic Modulation in TGF-β-Treated Cholangiocytes Induced by Curcumin Nanoparticles. Int. J. Mol. Sci. 2023, 24, 10481. [Google Scholar] [CrossRef]
- Comelli, L.; Rocchiccioli, S.; Smirni, S.; Salvetti, A.; Signore, G.; Citti, L.; Trivella, M.G.; Cecchettini, A. Characterization of Secreted Vesicles from Vascular Smooth Muscle Cells. Mol. Biosyst. 2014, 10, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgi, N.; Cecchettini, A.; Michelucci, E.; Signore, G.; Ceccherini, E.; Ferro, F.; Elefante, E.; Tani, C.; Baldini, C.; Rocchiccioli, S. Salivary Proteomics Markers for Preclinical Sjögren’s Syndrome: A Pilot Study. Biomolecules 2022, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Finamore, F.; Ucciferri, N.; Signore, G.; Cecchettini, A.; Ceccherini, E.; Vitiello, M.; Poliseno, L.; Rocchiccioli, S. Proteomics Pipeline for Phosphoenrichment and Its Application on a Human Melanoma Cell Model. Talanta 2020, 220, 121381. [Google Scholar] [CrossRef] [PubMed]
- Arbelaiz, A.; Azkargorta, M.; Krawczyk, M.; Santos-Laso, A.; Lapitz, A.; Perugorria, M.J.; Erice, O.; Gonzalez, E.; Jimenez-Agüero, R.; Lacasta, A.; et al. Serum Extracellular Vesicles Contain Protein Biomarkers for Primary Sclerosing Cholangitis and Cholangiocarcinoma. Hepatology 2017, 66, 1125–1143. [Google Scholar] [CrossRef] [PubMed]
- Rupp, C.; Bode, K.A.; Leopold, Y.; Sauer, P.; Gotthardt, D.N. Pathological Features of Primary Sclerosing Cholangitis Identified by Bile Proteomic Analysis. Biochim. Biophys. Acta Mol. Basis. Dis. 2018, 1864, 1380–1389. [Google Scholar] [CrossRef]
- Kan, M.; Chiba, T.; Konno, R.; Kouchi, Y.; Mishima, T.; Kawashima, Y.; Kishimoto, T.; Ohtsuka, M.; Ohara, O.; Kato, N. Bile Proteome Analysis by High-Precision Mass Spectrometry to Examine Novel Biomarkers of Primary Sclerosing Cholangitis. J. Hepato Biliary Pancreat. 2022, 30, 914–923. [Google Scholar] [CrossRef]
- Wang, C.; Yu, J.; Huo, L.; Wang, L.; Feng, W.; Wang, C. Human Protein-Disulfide Isomerase Is a Redox-Regulated Chaperone Activated by Oxidation of Domain A′. J. Biol. Chem. 2012, 287, 1139–1149. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, E.; El Mourabit, H.; Jager, M.; Clavel, M.; Moog, S.; Vaquero, J.; Ledent, T.; Cadoret, A.; Gautheron, J.; Fouassier, L.; et al. Cholangiopathy Aggravation Is Caused by VDR Ablation and Alleviated by VDR-Independent Vitamin D Signaling in ABCB4 Knockout Mice. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166067. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Okada, H.; Omatsu, T.; Mizushima, K.; Handa, O.; Kokura, S.; Ichikawa, H.; Fujiwake, H.; Yoshikawa, T. Identification of Inflammation-related Proteins in a Murine Colitis Model by 2D Fluorescence Difference Gel Electrophoresis and Mass Spectrometry. J. Gastro. Hepatol. 2010, 25, S144–S148. [Google Scholar] [CrossRef]
- Dubuisson, M.; Vander Stricht, D.; Clippe, A.; Etienne, F.; Nauser, T.; Kissner, R.; Koppenol, W.H.; Rees, J.-F.; Knoops, B. Human Peroxiredoxin 5 Is a Peroxynitrite Reductase. FEBS Lett. 2004, 571, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.; Breton, J.; Picot, C.R.; Quesniaux, V.; Bouton, C.; Drapier, J.-C. Signaling Events Leading to Peroxiredoxin 5 Up-Regulation in Immunostimulated Macrophages. Free Radic. Biol. Med. 2009, 47, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Seong, J.B.; Kim, B.; Kim, S.; Kim, M.H.; Park, Y.-H.; Lee, Y.; Lee, H.J.; Hong, C.-W.; Lee, D.-S. Macrophage Peroxiredoxin 5 Deficiency Promotes Lung Cancer Progression via ROS-Dependent M2-like Polarization. Free Radic. Biol. Med. 2021, 176, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Trussoni, C.E.; Krishnan, A.; Bronk, S.F.; Lorenzo Pisarello, M.J.; O’Hara, S.P.; Splinter, P.L.; Gao, Y.; Vig, P.; Revzin, A.; et al. Macrophages Contribute to the Pathogenesis of Sclerosing Cholangitis in Mice. J. Hepatol. 2018, 69, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Govaere, O.; Cockell, S.; Van Haele, M.; Wouters, J.; Van Delm, W.; Van Den Eynde, K.; Bianchi, A.; Van Eijsden, R.; Van Steenbergen, W.; Monbaliu, D.; et al. High-throughput Sequencing Identifies Aetiology-dependent Differences in Ductular Reaction in Human Chronic Liver Disease. J. Pathol. 2019, 248, 66–76. [Google Scholar] [CrossRef]
- Meng, F.; Wang, K.; Aoyama, T.; Grivennikov, S.I.; Paik, Y.; Scholten, D.; Cong, M.; Iwaisako, K.; Liu, X.; Zhang, M.; et al. Interleukin-17 Signaling in Inflammatory, Kupffer Cells, and Hepatic Stellate Cells Exacerbates Liver Fibrosis in Mice. Gastroenterology 2012, 143, 765–776.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiao, Q.; Liu, M.; He, T.; Shi, J.; Bai, X.; Zhang, Y.; Li, Y.; Cai, W.; Han, S.; et al. IL-17 Promotes Scar Formation by Inducing Macrophage Infiltration. Am. J. Pathol. 2018, 188, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sun, X.; Pan, B.; Cao, S.; Cao, J.; Che, D.; Liu, F.; Zhang, S.; Yu, Y. IL-17 Induces Macrophages to M2-like Phenotype via NF-κB. Cancer Manag. Res. 2018, 10, 4217–4228. [Google Scholar] [CrossRef]
- Starr, A.E.; Deeke, S.A.; Ning, Z.; Chiang, C.-K.; Zhang, X.; Mottawea, W.; Singleton, R.; Benchimol, E.I.; Wen, M.; Mack, D.R.; et al. Proteomic Analysis of Ascending Colon Biopsies from a Paediatric Inflammatory Bowel Disease Inception Cohort Identifies Protein Biomarkers That Differentiate Crohn’s Disease from UC. Gut 2017, 66, 1573–1583. [Google Scholar] [CrossRef]
- de La Motte, C.A.; Hascall, V.C.; Calabro, A.; Yen-Lieberman, B.; Strong, S.A. Mononuclear Leukocytes Preferentially Bind via CD44 to Hyaluronan on Human Intestinal Mucosal Smooth Muscle Cells after Virus Infection or Treatment with Poly(I.C). J. Biol. Chem. 1999, 274, 30747–30755. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Tryndyak, V.P.; Bagnyukova, T.V.; Melnyk, S.; Montgomery, B.; Ross, S.A.; Latendresse, J.R.; Rusyn, I.; Beland, F.A. Hepatic Epigenetic Phenotype Predetermines Individual Susceptibility to Hepatic Steatosis in Mice Fed a Lipogenic Methyl-Deficient Diet. J. Hepatol. 2009, 51, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Rappa, F.; Greco, A.; Podrini, C.; Cappello, F.; Foti, M.; Bourgoin, L.; Peyrou, M.; Marino, A.; Scibetta, N.; Williams, R.; et al. Immunopositivity for Histone MacroH2A1 Isoforms Marks Steatosis-Associated Hepatocellular Carcinoma. PLoS ONE 2013, 8, e54458. [Google Scholar] [CrossRef]
- Minghui, Z.; Kunhua, H.; Yunwen, B.; Hongmei, L.; Jing, L.; Shaowen, W.; Longqiaozi, S.; Chaohui, D. Analysis of Differentially Expressed Proteins Involved in Autoimmune Cirrhosis and Normal Serum by iTRAQ Proteomics. Proteom. Clin. Apps 2019, 13, 1700153. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lou, D.; Chen, J.; Shi, K.; Wang, Y.; Zhu, Q.; Liu, F.; Zhang, Y. Deep Dive on the Proteome of Salivary Extracellular Vesicles: Comparison between Ultracentrifugation and Polymer-Based Precipitation Isolation. Anal. Bioanal. Chem. 2021, 413, 365–375. [Google Scholar] [CrossRef]

| Number of Proteins | p Value | |
|---|---|---|
| Biological Processes | ||
| natural killer cell degranulation | 2 | 3.9 × 10−5 |
| chaperone-mediated protein complex assembly | 2 | 4.3 × 10−4 |
| positive regulation of interferon-beta production | 2 | 1.5 × 10−3 |
| protein folding | 3 | 2.1 × 10−3 |
| protein secretion | 2 | 3.1 × 10−3 |
| endosomal transport | 2 | 4.9 × 10−3 |
| positive regulation of protein catabolic process | 2 | 7.1 × 10−3 |
| cholesterol homeostasis | 2 | 9.0 × 10−3 |
| Cellular Components | ||
| extracellular exosome | 20 | 5.5 × 10−14 |
| Molecular Functions | ||
| MHC class II protein complex binding | 2 | 7.8 × 10−4 |
| cadherin binding | 4 | 1.1 × 10−3 |
| NADP binding | 2 | 1.8 × 10−3 |
| GTP binding | 4 | 2.8 × 10−3 |
| oxidoreductase activity | 3 | 4.3 × 10−3 |
| calcium ion binding | 5 | 4.5 × 10−3 |
| Number of Proteins | p Value | |
|---|---|---|
| Innate immune system | 12 | 1.8 × 10−7 |
| Neutrophil degranulation | 9 | 1.7 × 10−7 |
| IL-17 signaling pathway | 3 | 7.3 × 10−4 |
| Metabolism of carbohydrates | 4 | 2.6 × 10−3 |
| Metabolism of vitamins and cofactors | 3 | 6.2 × 10−3 |
| Cellular responses to stress | 5 | 1.8 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccherini, E.; Michelucci, E.; Signore, G.; Coco, B.; Zari, M.; Bellini, M.; Brunetto, M.R.; Cecchettini, A.; Rocchiccioli, S. The Clinical Utility of the Saliva Proteome in Rare Diseases: A Pilot Study for Biomarker Discovery in Primary Sclerosing Cholangitis. J. Clin. Med. 2024, 13, 544. https://doi.org/10.3390/jcm13020544
Ceccherini E, Michelucci E, Signore G, Coco B, Zari M, Bellini M, Brunetto MR, Cecchettini A, Rocchiccioli S. The Clinical Utility of the Saliva Proteome in Rare Diseases: A Pilot Study for Biomarker Discovery in Primary Sclerosing Cholangitis. Journal of Clinical Medicine. 2024; 13(2):544. https://doi.org/10.3390/jcm13020544
Chicago/Turabian StyleCeccherini, Elisa, Elena Michelucci, Giovanni Signore, Barbara Coco, Michela Zari, Massimo Bellini, Maurizia Rossana Brunetto, Antonella Cecchettini, and Silvia Rocchiccioli. 2024. "The Clinical Utility of the Saliva Proteome in Rare Diseases: A Pilot Study for Biomarker Discovery in Primary Sclerosing Cholangitis" Journal of Clinical Medicine 13, no. 2: 544. https://doi.org/10.3390/jcm13020544
APA StyleCeccherini, E., Michelucci, E., Signore, G., Coco, B., Zari, M., Bellini, M., Brunetto, M. R., Cecchettini, A., & Rocchiccioli, S. (2024). The Clinical Utility of the Saliva Proteome in Rare Diseases: A Pilot Study for Biomarker Discovery in Primary Sclerosing Cholangitis. Journal of Clinical Medicine, 13(2), 544. https://doi.org/10.3390/jcm13020544









