Preparing Enteral Formulas for Adult Patients with Phenylketonuria: A Minor Necessity but Major Challenge—A Case Report
Abstract
:1. Introduction
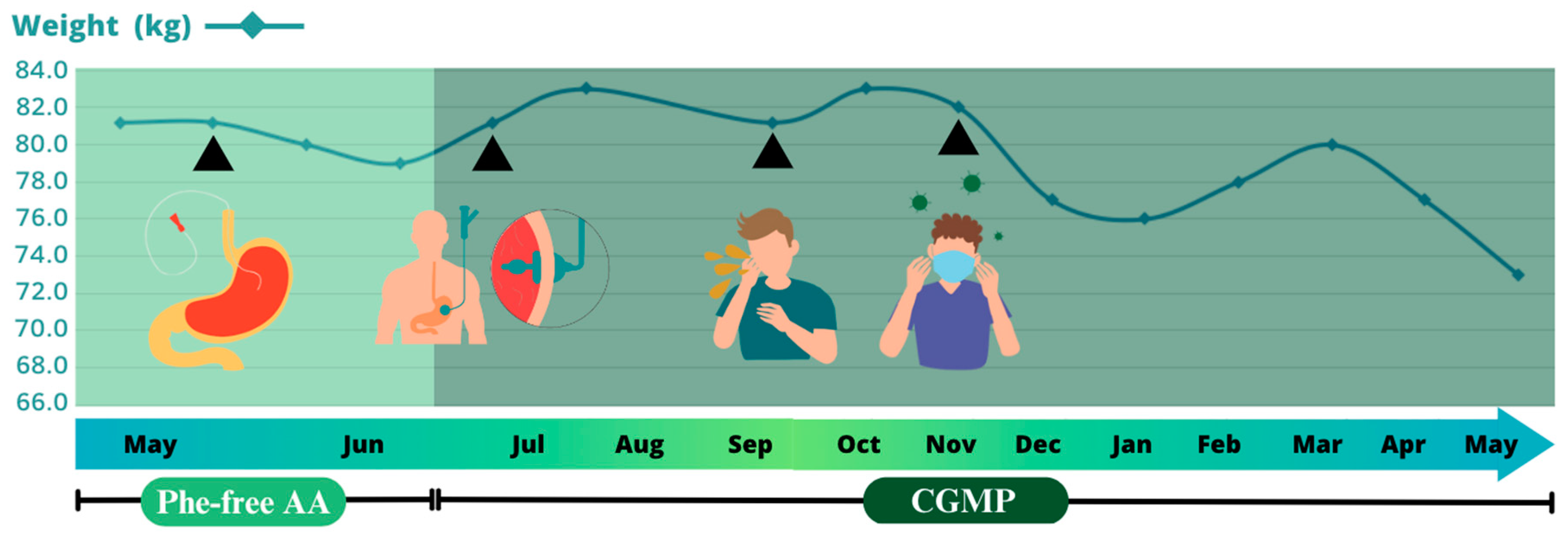
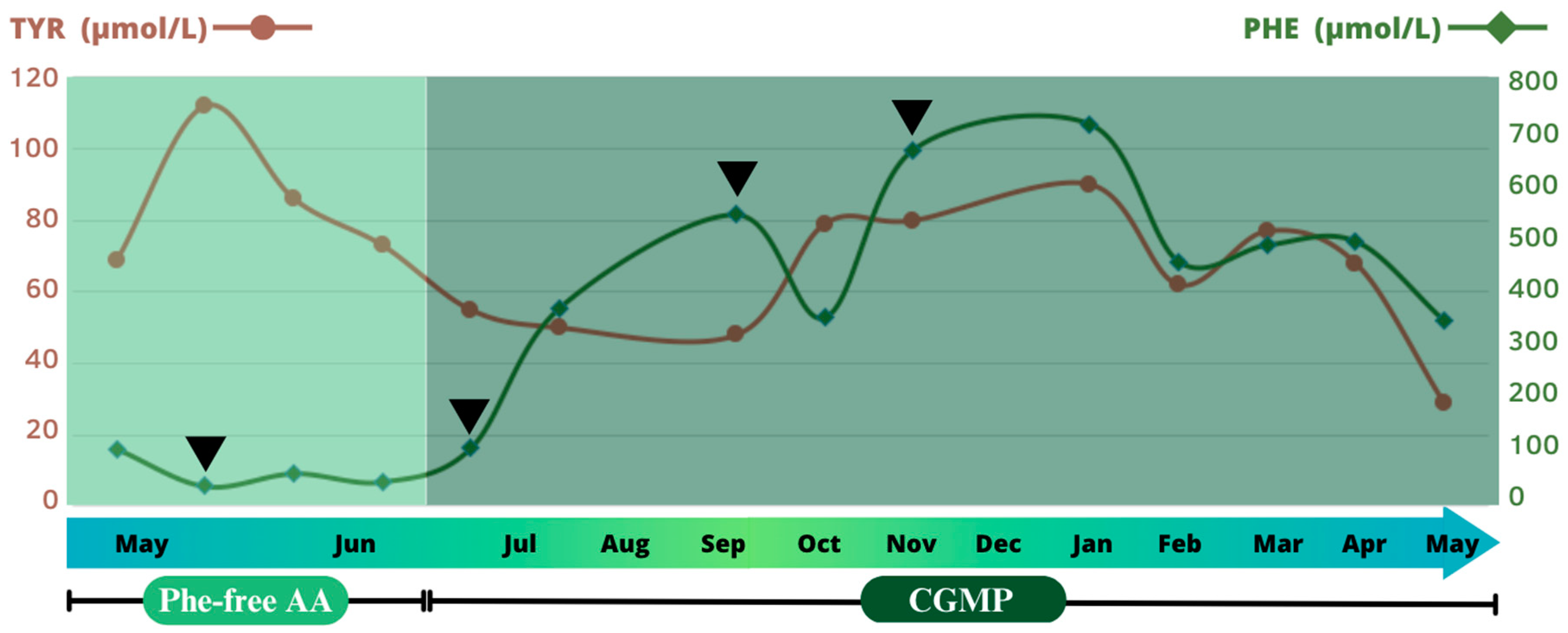
2. Case Report
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenneson, A.; Singh, R.H. Natural history of children and adults with phenylketonuria in the NBS-PKU Connect registry. Mol. Genet. Metab. 2021, 134, 243–249. [Google Scholar] [CrossRef]
- Brown, B.; Roehl, K.; Betz, M. Enteral nutrition formula selection: Current evidence and implications for practice. Nutr. Clin. Pract. 2015, 30, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Escuro, A.A.; Hummell, A.C. Enteral Formulas in Nutrition Support Practice: Is There a Better Choice for Your Patient? Nutr. Clin. Pract. 2016, 31, 709–722. [Google Scholar] [CrossRef]
- Jaulent, P.; Charriere, S.; Feillet, F.; Douillard, C.; Fouilhoux, A.; Thobois, S. Neurological manifestations in adults with phenylketonuria: New cases and review of the literature. J. Neurol. 2020, 267, 531–542. [Google Scholar] [CrossRef] [PubMed]
- van Spronsen, F.J.; van Wegberg, A.M.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 2017, 5, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Vockley, J.; Andersson, H.C.; Antshel, K.M.; Braverman, N.E.; Burton, B.K.; Frazier, D.M.; Mitchell, J.; Smith, W.E.; Thompson, B.H.; Berry, S.A. Phenylalanine hydroxylase deficiency: Diagnosis and management guideline. Genet. Med. 2014, 16, 188–200. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Rahman, S.; Keller, M.; Zschocke, J.; Abdenur, J.; Ali, H.; Artuch, R.; Ballabio, A.; Barshop, B.; Baumgartner, M.; et al. An international classification of inherited metabolic disorders (ICIMD). J. Inherit. Metab. Dis. 2021, 44, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Preston, F.; Daly, A.; Ashmore, C.; Holden, C.; Macdonald, A. Home enteral tube feeding in children with inherited metabolic disorders: A review of long-term carer knowledge and technique. J. Hum. Nutr. Diet. 2012, 25, 520–525. [Google Scholar] [CrossRef]
- Bérat, C.M.; Roda, C.; Brassier, A.; Bouchereau, J.; Wicker, C.; Servais, A.; Dubois, S.; Assoun, M.; Belloche, C.; Barbier, V.; et al. Enteral tube feeding in patients receiving dietary treatment for metabolic diseases: A retrospective analysis in a large French cohort. Mol. Genet. Metab. Rep. 2021, 26, 100655. [Google Scholar] [CrossRef]
- Daly, A.; Evans, S.; Gerrard, A.; Santra, S.; Vijay, S.; MacDonald, A. The Nutritional Intake of Patients with Organic Acidaemias on Enteral Tube Feeding: Can We Do Better? JIMD Rep. 2016, 28, 29. [Google Scholar] [CrossRef]
- MacDonald, A.; Van Wegberg, A.M.J.; Ahring, K.; Beblo, S.; Bélanger-Quintana, A.; Burlina, A.; Campistol, J.; Coşkun, T.; Feillet, F.; Giżewska, M.; et al. PKU dietary handbook to accompany PKU guidelines. Orphanet J. Rare Dis. 2020, 15, 171. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.; Evans, S.; Pinto, A.; Ashmore, C.; Macdonald, A. Protein Substitutes in PKU; Their Historical Evolution. Nutrients 2021, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Acosta, P.B.; Kennedy, B. Osmolality of enteral formulas for maternal phenylketonuria. J. Inherit. Metab. Dis. 1986, 9, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, P.W.; Vanderveen, T.W.; Morrison, J.I.; Hohenwarter, M.W. Gastrointestinal Disorders Caused by Medication and Electrolyte Solution Osmolality during Enteral Nutrition. J. Parenter. Enter. Nutr. 1983, 7, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.H.; Cunningham, A.C.; Mofidi, S.; Douglas, T.D.; Frazier, D.M.; Hook, D.G.; Jeffers, L.; McCune, H.; Moseley, K.D.; Ogata, B.; et al. Updated, web-based nutrition management guideline for PKU: An evidence and consensus based approach. Mol. Genet. Metab. 2016, 118, 72–83. [Google Scholar] [CrossRef]
- Robertson, L.; Adam, S.; Ellerton, C.; Ford, S.; Hill, M.; Randles, G.; Woodall, A.; Young, C.; Macdonald, A. Dietetic Management of Adults with Phenylketonuria (PKU) in the UK: A Care Consensus Document. Nutrients 2022, 14, 576. [Google Scholar] [CrossRef]
- Smith, J.L.; Heymsfield, S.B. Enteral nutrition support: Formula preparation from modular ingredients. J. Parenter. Enteral Nutr. 1983, 7, 280–288. [Google Scholar] [CrossRef]
- Ireton-Jones, C. Adjusted body weight, con: Why adjust body weight in energy-expenditure calculations? Nutr. Clin. Pract. 2005, 20, 474–479. [Google Scholar] [CrossRef]
- Van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Austin, P.; Boeykens, K.; Chourdakis, M.; Cuerda, C.; Jonkers-Schuitema, C.; Lichota, M.; Nyulasi, I.; Schneider, S.M.; Stanga, Z.; et al. ESPEN practical guideline: Home enteral nutrition. Clin. Nutr. 2022, 41, 468–488. [Google Scholar] [CrossRef]
- Kreymann, K.G.; Berger, M.M.; Deutz, N.E.P.; Hiesmayr, M.; Jolliet, P.; Kazandjiev, G.; Nitenberg, G.; van den Berghe, G.; Wernerman, J.; Ebner, C.; et al. ESPEN Guidelines on Enteral Nutrition: Intensive care. Clin. Nutr. 2006, 25, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. J. Parenter. Enteral Nutr. 2022, 46, 12–41. [Google Scholar] [CrossRef]
- Evans, S.; Shelton, F.; Holden, C.; Daly, A.; Hopkins, V.; MacDonald, A. Monitoring of home safety issues in children on enteral feeds with inherited metabolic disorders. Arch. Dis. Child. 2010, 95, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Zemanova, M.; Chrastina, P.; Sebron, V.; Prochazkova, D.; Jahnova, H.; Sanakova, P.; Prochazkova, L.; Tesarova, B.; Zeman, J. Extremely low birthweight neonates with phenylketonuria require special dietary management. Acta Paediatr. 2021, 110, 2994–2999. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.; Lotz-Havla, A.; Dokoupil, K.; Maier, E.M. Management of three preterm infants with phenylketonuria. Nutrition 2020, 71, 110619. [Google Scholar] [CrossRef]
- Ballhausen, D.; Egli, D.; Bickle-Graz, M.; Bianchi, N.; Bonafé, L. Born at 27 weeks of gestation with classical PKU: Challenges of dietetic management in a very preterm infant. Pediatr. Rep. 2011, 3, 103–107. [Google Scholar] [CrossRef]
- Cole, D.E.C.; Landry, D.A. Parenteral nutrition in a premature infant with phenylketonuria. J. Parenter. Enter. Nutr. 1984, 8, 42–44. [Google Scholar] [CrossRef]
- Salvarinova-Zivkovic, R.; Hartnett, C.; Sinclair, G.; Dix, D.; Horvath, G.; Lillquist, Y.; Stockler-Ipsiroglu, S. The use of parenteral nutrition for the management of PKU patient undergoing chemotherapy for lymphoma: A case report. Mol. Genet. Metab. 2012, 105, 571–574. [Google Scholar] [CrossRef]
- Angelis, A.; Tordrup, D.; Kanavos, P. Socio-economic burden of rare diseases: A systematic review of cost of illness evidence. Health Policy 2015, 119, 964–979. [Google Scholar] [CrossRef]
- van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Primers 2021, 7, 36. [Google Scholar] [CrossRef]
- Lee, P.J.; Amos, A.; Robertson, L.; Fitzgerald, B.; Hoskin, R.; Lilburn, M.; Weetch, E.; Murphy, G. Adults with late diagnosed PKU and severe challenging behaviour: A randomised placebo-controlled trial of a phenylalanine-restricted diet. J. Neurol. Neurosurg. Psychiatry 2009, 80, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.R.; Meskell, R.J.; Foy, J.; Garner, A.E. Determining the osmolality of over-concentrated and supplemented infant formulas. J. Hum. Nutr. Diet. 2013, 26, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.J.; Pinto, A.; Daly, A.; Macdonald, A.; Azevedo, L.; Rocha, J.C.; Borges, N. The Use of Glycomacropeptide in Patients with Phenylketonuria: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1794. [Google Scholar] [CrossRef] [PubMed]
- Solverson, P.; Murali, S.G.; Litscher, S.J.; Blank, R.D.; Ney, D.M. Low bone strength is a manifestation of phenylketonuria in mice and is attenuated by a glycomacropeptide diet. PLoS ONE 2012, 7, e45165. [Google Scholar] [CrossRef] [PubMed]
- Garrison, C.M. Enteral Feeding Tube Clogging: What Are the Causes and What Are the Answers? A Bench Top Analysis. Nutr. Clin. Pract. 2018, 33, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Pinto, A.; Faria, A.; Teixeira, D.; van Wegberg, A.M.J.; Ahring, K.; Feillet, F.; Calhau, C.; Macdonald, A.; Moreira-Rosário, A.; et al. Is the phenylalanine-restricted diet a risk factor for overweight or obesity in patients with phenylketonuria (Pku)? A systematic review and meta-analysis. Nutrients 2021, 13, 3443. [Google Scholar] [CrossRef]
- Krenitsky, J. Adjusted body weight, pro: Evidence to support the use of adjusted body weight in calculating calorie requirements. Nutr. Clin. Pract. 2005, 20, 468–473. [Google Scholar] [CrossRef]
- Hillert, A.; Anikster, Y.; Belanger-Quintana, A.; Burlina, A.; Burton, B.K.; Carducci, C.; Chiesa, A.E.; Christodoulou, J.; Äorcevi, M.; Desviat, L.R.; et al. The Genetic Landscape and Epidemiology of Phenylketonuria. Am. J. Hum. Genet. 2020, 107, 234–250. [Google Scholar] [CrossRef]
- Kipnis, V.; Midthune, D.; Freedman, L.; Bingham, S.; Day, N.E.; Riboli, E.; Ferrari, P.; Carroll, R.J. Bias in dietary-report instruments and its implications for nutritional epidemiology. Public Health Nutr. 2002, 5, 915–923. [Google Scholar] [CrossRef]
- Illner, A.K.; Freisling, H.; Boeing, H.; Huybrechts, I.; Crispim, S.P.; Slimani, N. Review and evaluation of innovative technologies for measuring diet in nutritional epidemiology. Int. J. Epidemiol. 2012, 41, 1187–1203. [Google Scholar] [CrossRef]
- Araújo, A.C.M.F.; Araújo, W.M.C.; Marquez, U.M.L.; Akutsu, R.; Nakano, E.Y. Table of Phenylalanine Content of Foods: Comparative Analysis of Data Compiled in Food Composition Tables. JIMD Rep. 2017, 34, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Blau, N.; Hennermann, J.B.; Langenbeck, U.; Lichter-Konecki, U. Diagnosis, classification, and genetics of phenylketonuria and tetrahydrobiopterin (BH4) deficiencies. Mol. Genet. Metab. 2011, 104, S2–S9. [Google Scholar] [CrossRef] [PubMed]
| 5:00 A.M. | 10:00 A.M. | 4:00 P.M. | 10:00 P.M. | |
|---|---|---|---|---|
| PKU Sphere 15® powder (g) Vitaflo a Nestlé Health Science Company, Vevey, Switzerland | 27.0 (1 sachet) | 27.0 (1 sachet) | 27.0 (1 sachet) | 27.0 (1 sachet) |
| Fresubin® Original Fiber® (mL) Fresenius Kabi, Bad Homburg, Germany | 150.0 | 200.0 | 150.0 | - |
| Prozero® (mL) Vitaflo a Nestlé Health Science Company, Vevey, Switzerland | 150.0 | 200.0 | 200.0 | 300.0 |
| Duocal® (g) Nutricia N.V., Zoetermeer, The Netherlands | 50.0 | 40.0 | 40.0 | 50.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pané, A.; Carrasco-Serrano, M.; Milad, C.; Leyes, P.; Moreno-Lozano, P.J.; Ventura, R.; Milisenda, J.C.; García-García, F.J.; Garrabou, G.; García-Villoria, J.; et al. Preparing Enteral Formulas for Adult Patients with Phenylketonuria: A Minor Necessity but Major Challenge—A Case Report. J. Clin. Med. 2023, 12, 7452. https://doi.org/10.3390/jcm12237452
Pané A, Carrasco-Serrano M, Milad C, Leyes P, Moreno-Lozano PJ, Ventura R, Milisenda JC, García-García FJ, Garrabou G, García-Villoria J, et al. Preparing Enteral Formulas for Adult Patients with Phenylketonuria: A Minor Necessity but Major Challenge—A Case Report. Journal of Clinical Medicine. 2023; 12(23):7452. https://doi.org/10.3390/jcm12237452
Chicago/Turabian StylePané, Adriana, Marcos Carrasco-Serrano, Camila Milad, Pere Leyes, Pedro Juan Moreno-Lozano, Roser Ventura, José Cesar Milisenda, Francesc Josep García-García, Glòria Garrabou, Judit García-Villoria, and et al. 2023. "Preparing Enteral Formulas for Adult Patients with Phenylketonuria: A Minor Necessity but Major Challenge—A Case Report" Journal of Clinical Medicine 12, no. 23: 7452. https://doi.org/10.3390/jcm12237452
APA StylePané, A., Carrasco-Serrano, M., Milad, C., Leyes, P., Moreno-Lozano, P. J., Ventura, R., Milisenda, J. C., García-García, F. J., Garrabou, G., García-Villoria, J., López-Galera, R. M., Ribes, A., Grau-Junyent, J. M., Forga-Visa, M. d. T., Montserrat-Carbonell, C., & on behalf of PKU.CAT Consortium. (2023). Preparing Enteral Formulas for Adult Patients with Phenylketonuria: A Minor Necessity but Major Challenge—A Case Report. Journal of Clinical Medicine, 12(23), 7452. https://doi.org/10.3390/jcm12237452






