Correlation between Ultrasound Peak Systolic Velocity and Angiography for Grading Internal Carotid Artery Stenosis
Abstract
1. Introduction
2. Materials and Methods
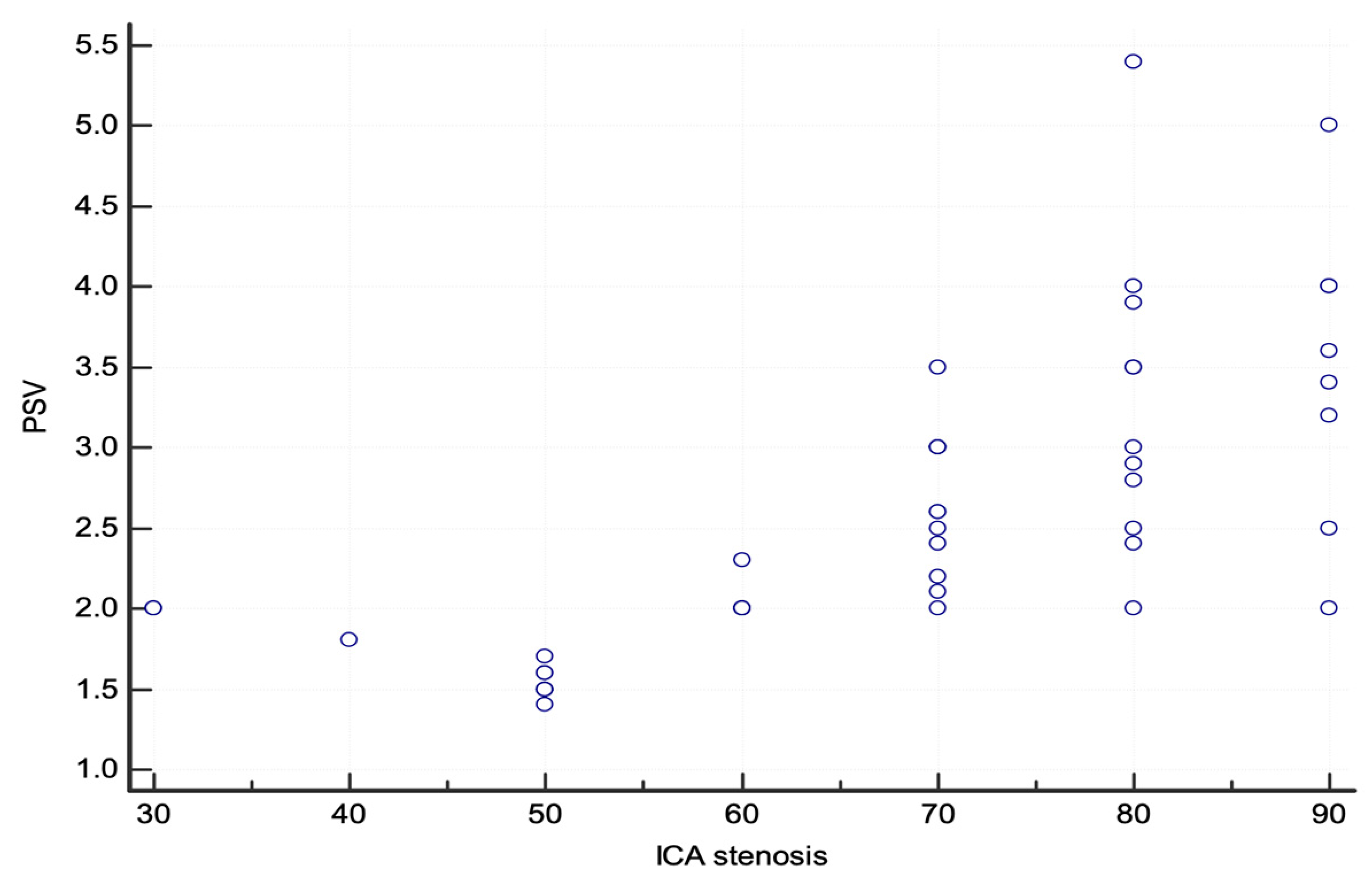
3. Results
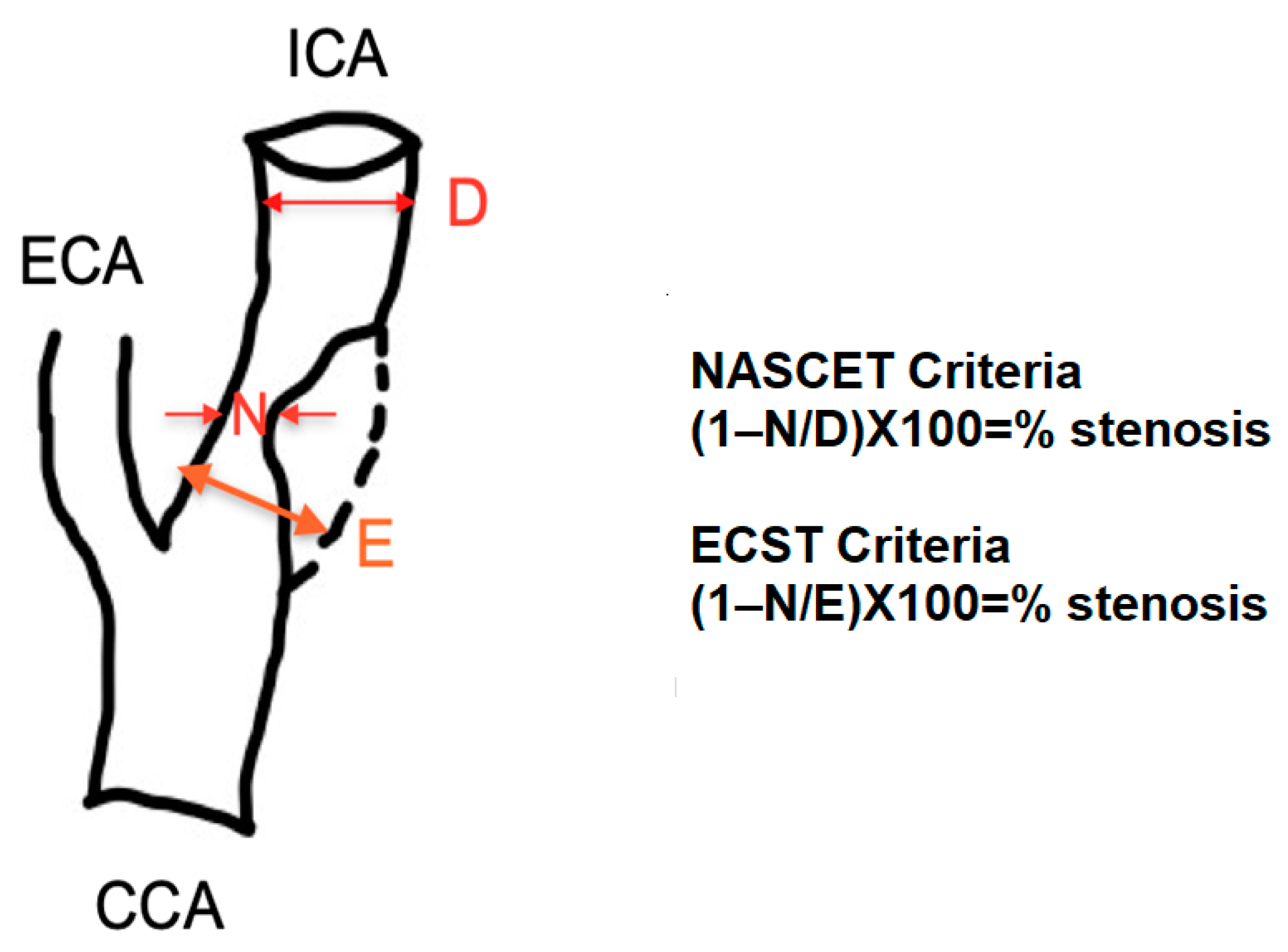
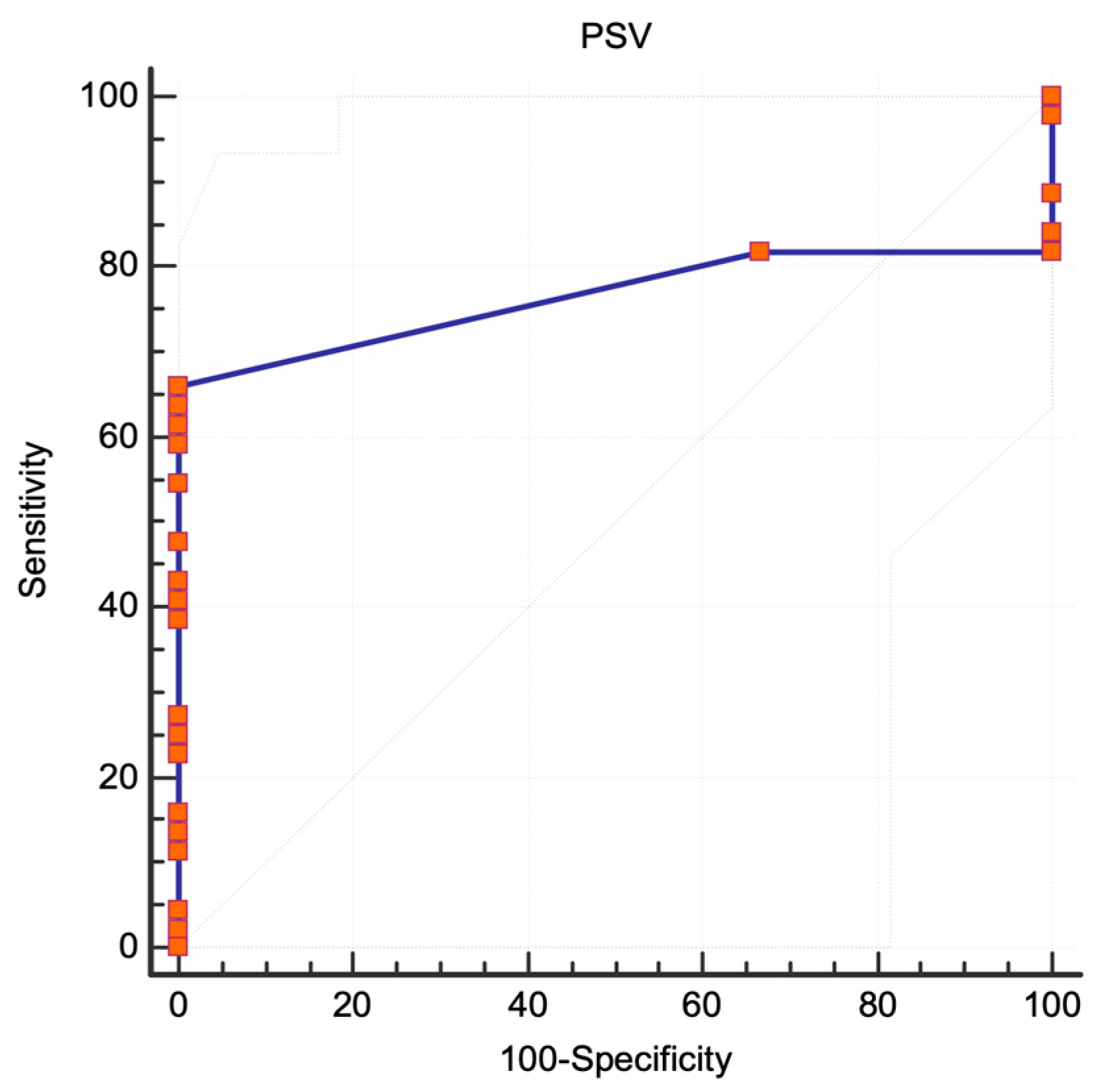
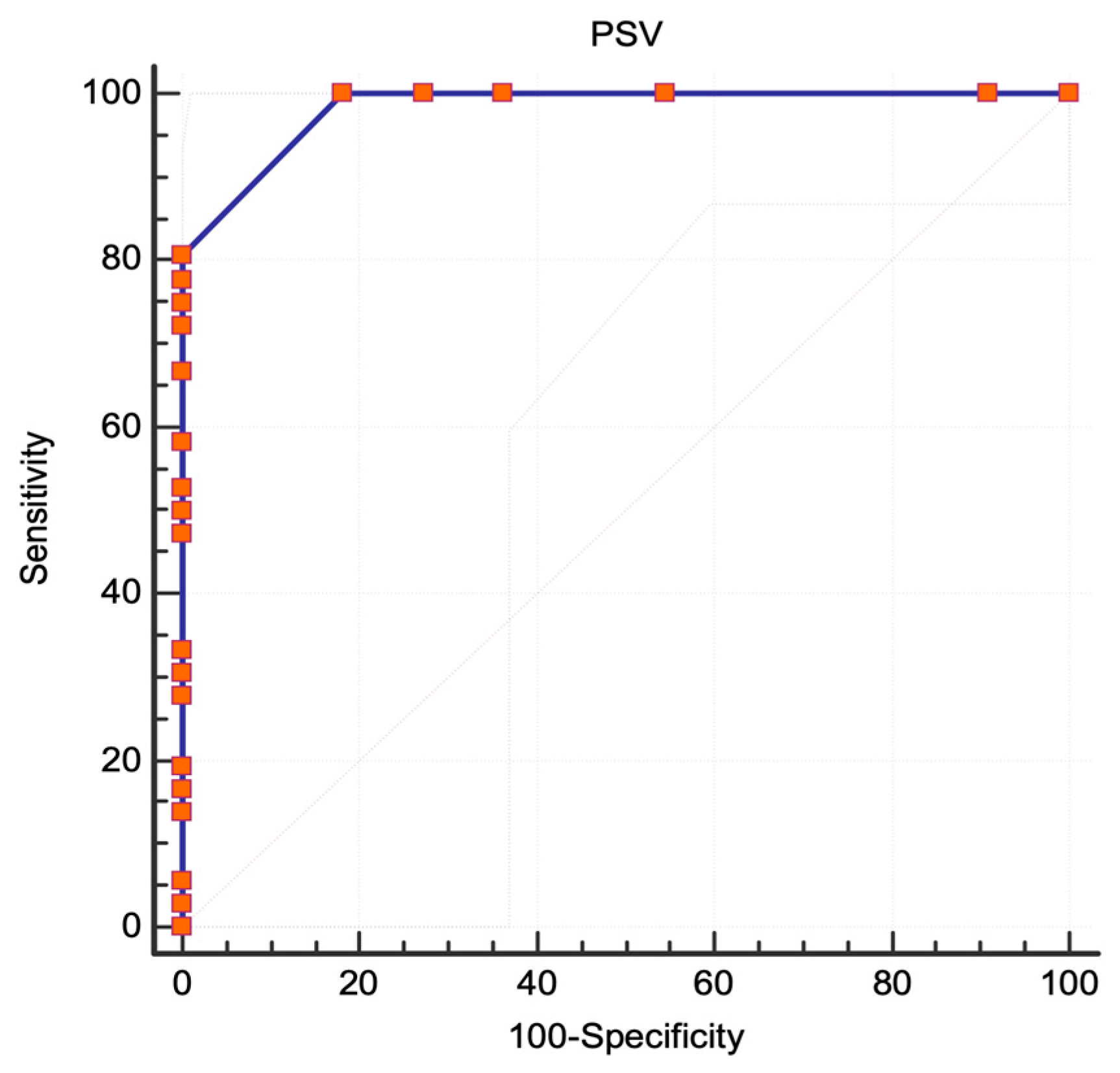
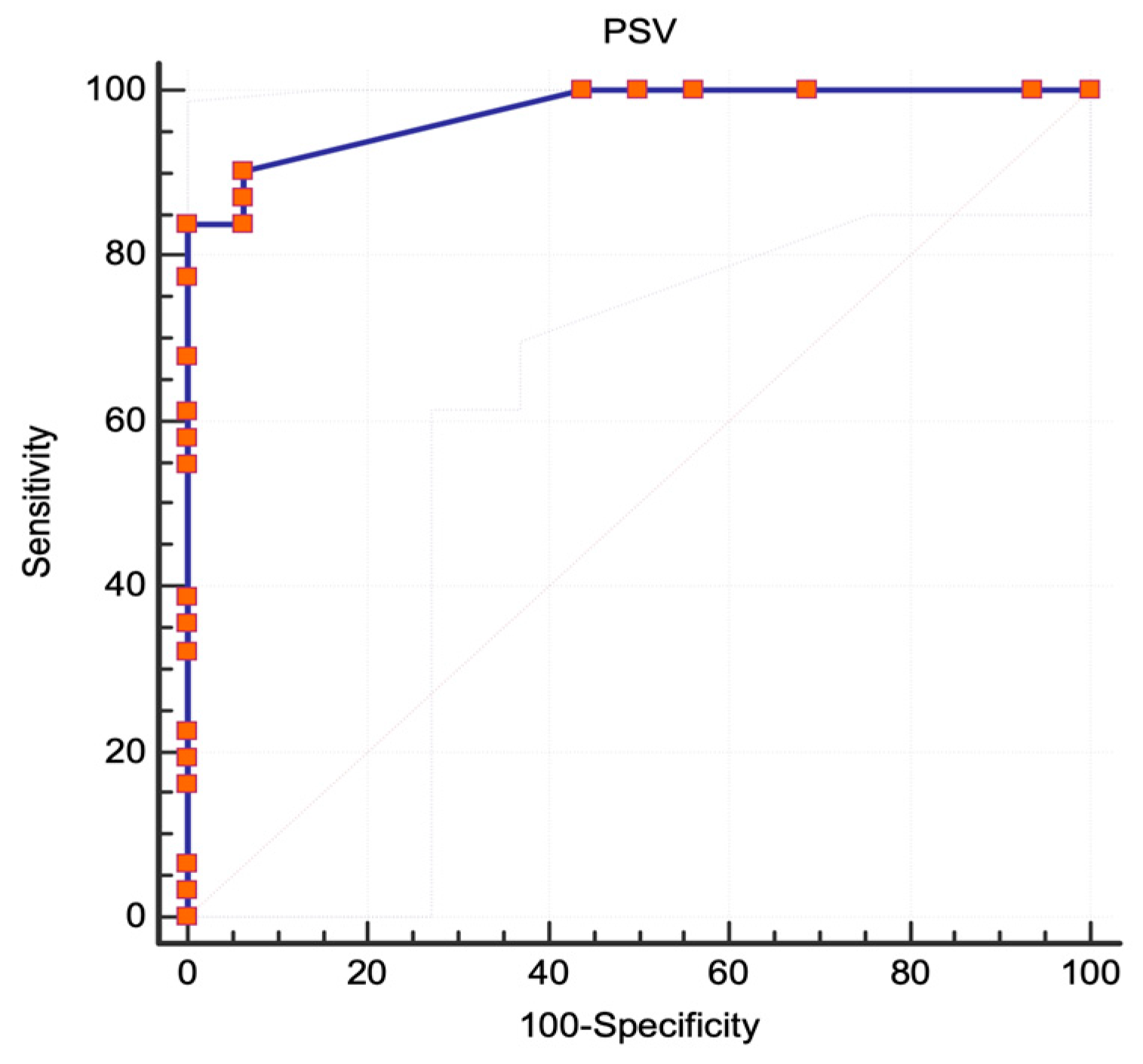
3.1. NASCET Criteria
3.2. ECST Criteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97, S6–S16. [Google Scholar] [CrossRef] [PubMed]
- Truelsen, B.; Piechowski-Jozwiak, T.; Bonita, R.; Mathersa, C.; Bogousslavsky, J.; Boysen, G. Stroke incidence and prevalence in Europe. Eur. Neurol. 2006, 13, 581–598. [Google Scholar] [CrossRef]
- Naylor, A.R.; Ricco, J.B.; de Borst, G.J.; Debus, S.; de Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor’s Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 1, 3–81. [Google Scholar] [CrossRef]
- Olinic, D.M.; Spinu, M.; Olinic, M.; Homorodean, C.; Tataru, D.A.; Liew, A.; Schernthaner, G.H.; Stanek, A.; Fowkes, G.; Catalano, M. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int. Angiol. 2018, 37, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Maroufi, S.F.; Rafiee Alavi, S.N.; Abbasi, M.H.; Famouri, A.; Mahya, N.; Armaghan, S.; Allahdadian, S.; Shahidi, A.; Nazarian, H.; Esmaeili, S.; et al. Comparison of Doppler Ultrasound and Digital Subtraction Angiography in extracranial stenosis. Ann. Med. Surg. 2021, 74, 103202. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.G.; Eliasziw, M.; Barr, H.W.K.; Clagett, G.P.; Barnes, R.W.; Wallace, M.C.; Taylor, D.W.; Haynes, R.B.; Finan, J.W.; Hachinski, V.C.; et al. The North American Symptomatic Carotid Endarterectomy Trial: Surgical Results in 1415 Patients. Stroke 1999, 30, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998, 351, 1379–1387. [CrossRef]
- Walker, M.D.; Marler, J.R.; Goldstein, M. The Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995, 273, 1421–1428. [Google Scholar] [CrossRef]
- Halliday, A.; Mansfield, A.; Marro, J.; Peto, C.; Peto, R.; Potter, J.; Thomas, D. Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomised controlled trial. Lancet 2004, 363, 1491–1502. [Google Scholar] [CrossRef]
- Economopoulos, K.P.; Sergentanis, T.N.; Tsivgoulis, G.; Mariolis, A.D.; Stefanadis, C. Carotid artery stenting versus carotid endarterectomy: A comprehensive meta-analysis of short-term and long-term outcomes. Stroke 2011, 42, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Chappell, F.M.; Wardlaw, J.M.; Brazzelli, M.; Best, J.J.K. Doppler ultrasound, CT angiography, MR angiography, and contrast-enhanced MR angiography versus intra-arterial angiography for moderate and severe carotid stenosis in symptomatic patients. Cochrane Database Syst. Rev. 2017, 2, CD007423. [Google Scholar] [CrossRef]
- Grant, E.G.; Benson, C.B.; Moneta, G.L.; Alexandrov, A.V.; Baker, J.D.; Bluth, E.I.; Carroll, B.A.; Eliasziw, M.; Gocke, J.; Hertzberg, B.S.; et al. Carotid artery stenosis: Grey-scale and Doppler US diagnosis—Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003, 229, 340–346. [Google Scholar] [CrossRef]
- Oates, C.; Naylor, A.R.; Hartshorne, T.; Charles, S.M.; Humphries, K.; Aslam, M. Reporting carotid ultrasound investigations in the United Kingdom. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Anacleto, A.; Filho, C.M.; Ledesma, S.; Aldrovani, M.; Wolosker, N. Peak systolic velocity for calcified plaques fails to estimate carotid stenosis degree. Ann. Vasc. Surg. 2019, 59, 1–4. [Google Scholar] [CrossRef]
- Tessler, F.N.; Kimme-Smith, C.; Sutherland, M.L.; Schiller, V.L.; Perrella, R.P.; Grant, E.G. Inter- and intra-observer variability of Doppler peak velocity measurements: An in-vitro study. Ultrasound Med. Biol. 1990, 16, 653–657. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extra-cranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Ober, M.-C.; Lazăr, F.-L.; Achim, A.; Tirinescu, D.C.; Leibundgut, G.; Homorodean, C.; Olinic, M.; Onea, H.L.; Spînu, M.; Tătaru, D.; et al. Interventional Management of a Rare Combination of Nutcracker and Wilkie Syndromes. J. Pers. Med. 2022, 12, 1461. [Google Scholar] [CrossRef]
- Fawcett, T. An Introduction to ROC Analysis. Pattern Recognit. Lett. 2006, 8, 861–874. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Perkins, N.J.; Liu, A.; Bondell, H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology 2005, 16, 73–81. [Google Scholar] [CrossRef]
- Barnett, H.J.; Taylor, D.W.; Eliasziw, M.; Fox, A.J.; Ferguson, G.G.; Haynes, R.B.; Rankin, R.N.; Clagett, G.P.; Hachinski, V.C.; Sackett, D.L.; et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N. Engl. J. Med. 1998, 20, 1415–1425. [Google Scholar] [CrossRef]
- Muller, M.D.; Lyrer, P.; Brown, M.M.; Bonati, L.H. Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis. Cochrane Database Syst. Rev. 2020, 2, CD000515. [Google Scholar] [CrossRef]
- Yadav, J.S.; Wholey, M.H.; Kuntz, R.E.; Fayad, P.; Katzen, B.T.; Mishkel, G.J.; Bajwa, T.K.; Whitlow, P.; Strickman, N.E.; Jaff, M.R.; et al. Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N. Engl. J. Med. 2004, 15, 1493–1501. [Google Scholar] [CrossRef]
- Tokunaga, K.; Koga, M.; Yoshimura, S.; Arihiro, S.; Suzuki, R.; Nagatsuka, K.; Toyoda, K. Optimal Peak Systolic Velocity Thresholds for Predicting Internal Carotid Artery Stenosis Greater than or Equal to 50%, 60%, 70%, and 80%. J. Stroke Cerebrovasc. Dis. 2016, 4, 921–926. [Google Scholar] [CrossRef]
- Winter, W.K.; Zorach, B.B.; Arpin, P.A.; Nelson, J.; Mackey, W.C. Progression of moderate-to-severe carotid disease. J. Vasc. Surg. 2016, 6, 1505–1510. [Google Scholar] [CrossRef][Green Version]
- Koga, M.; Kimura, K.; Minematsu, K.; Yamaguchi, T. Diagnosis of internal carotid artery stenosis greater than 70% with power Doppler duplex sonography. Am. J. Neuroradiol. 2001, 22, 413–417. [Google Scholar]
- Jahromi, A.S.; Cina, C.S.; Liu, Y.; Clase, C.M. Sensitivity and specificity of colour duplex ultrasound measurement in the estimation of internal carotid artery stenosis: A systematic review and meta-analysis. J. Vasc. Surg. 2005, 41, 962–972. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Pendlebury, S.T.; Wardlaw, J.; Warlow, C.P. Critical appraisal of the design and reporting of studies of imaging and measurement of carotid stenosis. Stroke 2000, 31, 1444–1450. [Google Scholar] [CrossRef][Green Version]
- Mead, G.E.; Lewis, S.C.; Wardlaw, J.M. Variability in Doppler ultrasound influences referral of patients for carotid surgery. Eur. J. Ultrasound 2000, 12, 137–143. [Google Scholar] [CrossRef]
- Netuka, D.; Belsan, T.; Broulikova, K.; Mandys, V.; Charvat, F.; Malik, J.; Coufalova, L.; Bradac, O.; Ostry, S.; Benes, V. Detection of carotid artery stenosis using histological specimens: A comparison of CT angiography, magnetic resonance angiography, digital subtraction angiography and Doppler ultrasonography. Acta Neurochir. 2016, 8, 1505–1514. [Google Scholar] [CrossRef]
- Hathout, G.M.; Fink, J.R.; El-saden, S.M.; Grant, E.G. Sonographic NASCET index: A new doppler parameter for assessment of internal carotid artery stenosis. AJNR Am. J. Neuroradiol. 2005, 26, 68–75. [Google Scholar] [PubMed]
- von Reutern, G.M.; Goertler, M.W.; Bornstein, N.M.; Del Sette, M.; Evans, D.H.; Hetzel, A.; Kaps, M.; Perren, F.; Razumovky, A.; von Reutern, M.; et al. Grading carotid stenosis using ultrasonic methods. Stroke 2012, 3, 916–921. [Google Scholar] [CrossRef]
- van Everdingen, K.J.; van der Grond, J.; Kappelle, L.J. Overestimation of a stenosis in the internal carotid artery by duplex sonography caused by an increase in volume flow. J. Vasc. Surg. 1998, 27, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Homorodean, C.; Leucuta, D.C.; Ober, M.; Homorodean, R.; Spinu, M.; Olinic, M.; Tataru, D.; Olinic, D.M. Intravascular ultrasound insights into the unstable features of the coronary atherosclerotic plaques: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2022, 52, e13671. [Google Scholar] [CrossRef]
- Shaalan, W.E.; Wahlgren, C.M.; Desai, T.; Piano, G.; Skelly, C.; Bassiouny, H.S. Reappraisal of velocity criteria for carotid bulb/internal carotid artery stenosis utilizing high-resolution B-mode ultrasound validated with computed tomography angiography. J. Vasc. Surg. 2008, 48, 104–112. [Google Scholar] [CrossRef]
- Kurosaki, Y.; Yoshida, K.; Fukumitsu, R.; Sadamasa, N.; Handa, A.; Chin, M.; Yamagata, S. Carotid artery plaque assessment using quantitative expansive remodeling evaluation and MRI plaque signal intensity. J. Neurosurg. 2016, 3, 736–742. [Google Scholar] [CrossRef]
- Staikov, I.N.; Arnold, M.; Mattle, H.P.; Remonda, L.; Sturzenegger, M.; Baumgartner, R.W.; Schroth, G. Comparison of the ECST, CC, and NASCET grading methods and ultrasound for assessing carotid stenosis. European Carotid Surgery Trial. North American Symptomatic Carotid Endarterectomy Trial. J. Neurol. 2000, 9, 681–686. [Google Scholar] [CrossRef]
- Alexandrov, A.V.; Brodie, D.S.; McLean, A.; Hamilton, P.; Murphy, J.; Burns, P.N. Correlation of peak systolic velocity and angiographic measurement of carotid stenosis revisited. Stroke 1997, 2, 339–342. [Google Scholar] [CrossRef]

| PSV (cm/s) | Sensitivity | 95% CI | Specificity | 95% CI |
|---|---|---|---|---|
| NASCET protocol, ICA stenosis ≥ 60% | ||||
| 170 | 100.00 | 90.3–100.0 | 63.64 | 30.8–89.1 |
| 180 * | 100.00 | 90.3–100.0 | 72.73 | 39.0–94.0 |
| 190 | 100.00 | 90.3–100.0 | 81.82 | 48.2–97.7 |
| 200 | 80.56 | 64.0–91.8 | 100.00 | 71.5–100.0 |
| 210 | 77.78 | 60.8–89.9 | 100.00 | 71.5–100.0 |
| 220 | 75.00 | 57.8–87.9 | 100.00 | 71.5–100.0 |
| NASCET protocol, ICA stenosis ≥ 70% | ||||
| 180 | 100.00 | 88.8–100.0 | 50.00 | 24.7–75.3 |
| 190 | 100.00 | 88.8–100.0 | 56.25 | 29.9–80.2 |
| 200 * | 90.32 | 74.2–98.0 | 93.75 | 69.8–99.8 |
| 210 | 87.10 | 70.2–96.4 | 93.75 | 69.8–99.8 |
| 220 | 83.87 | 66.3–94.5 | 93.75 | 69.8–99.8 |
| 230 | 83.87 | 66.3–94.5 | 100.00 | 79.4–100.0 |
| ECST protocol, ICA stenosis ≥ 80% | ||||
| 160 | 100.00 | 90.3–100.0 | 63.64 | 30.8–89.1 |
| 170 | 100.00 | 90.3–100.0 | 72.73 | 39.0–94.0 |
| 180 * | 100.00 | 90.3–100.0 | 81.82 | 48.2–97.7 |
| 190 | 80.56 | 64.0–91.8 | 100.00 | 71.5–100.0 |
| 200 | 77.78 | 60.8–89.9 | 100.00 | 71.5–100.0 |
| 210 | 75.00 | 57.8–87.9 | 100.00 | 71.5–100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tătaru, D.-A.; Olinic, M.; Homorodean, C.; Ober, M.-C.; Spînu, M.; Lazăr, F.-L.; Onea, L.; Olinic, D.-M. Correlation between Ultrasound Peak Systolic Velocity and Angiography for Grading Internal Carotid Artery Stenosis. J. Clin. Med. 2024, 13, 517. https://doi.org/10.3390/jcm13020517
Tătaru D-A, Olinic M, Homorodean C, Ober M-C, Spînu M, Lazăr F-L, Onea L, Olinic D-M. Correlation between Ultrasound Peak Systolic Velocity and Angiography for Grading Internal Carotid Artery Stenosis. Journal of Clinical Medicine. 2024; 13(2):517. https://doi.org/10.3390/jcm13020517
Chicago/Turabian StyleTătaru, Dan-Alexandru, Maria Olinic, Călin Homorodean, Mihai-Claudiu Ober, Mihail Spînu, Florin-Leontin Lazăr, Laurențiu Onea, and Dan-Mircea Olinic. 2024. "Correlation between Ultrasound Peak Systolic Velocity and Angiography for Grading Internal Carotid Artery Stenosis" Journal of Clinical Medicine 13, no. 2: 517. https://doi.org/10.3390/jcm13020517
APA StyleTătaru, D.-A., Olinic, M., Homorodean, C., Ober, M.-C., Spînu, M., Lazăr, F.-L., Onea, L., & Olinic, D.-M. (2024). Correlation between Ultrasound Peak Systolic Velocity and Angiography for Grading Internal Carotid Artery Stenosis. Journal of Clinical Medicine, 13(2), 517. https://doi.org/10.3390/jcm13020517








