Transforaminal Endoscopic Lumbar Lateral Recess Decompression for Octogenarian Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Surgical Procedure
2.2.1. Transforaminal Approach
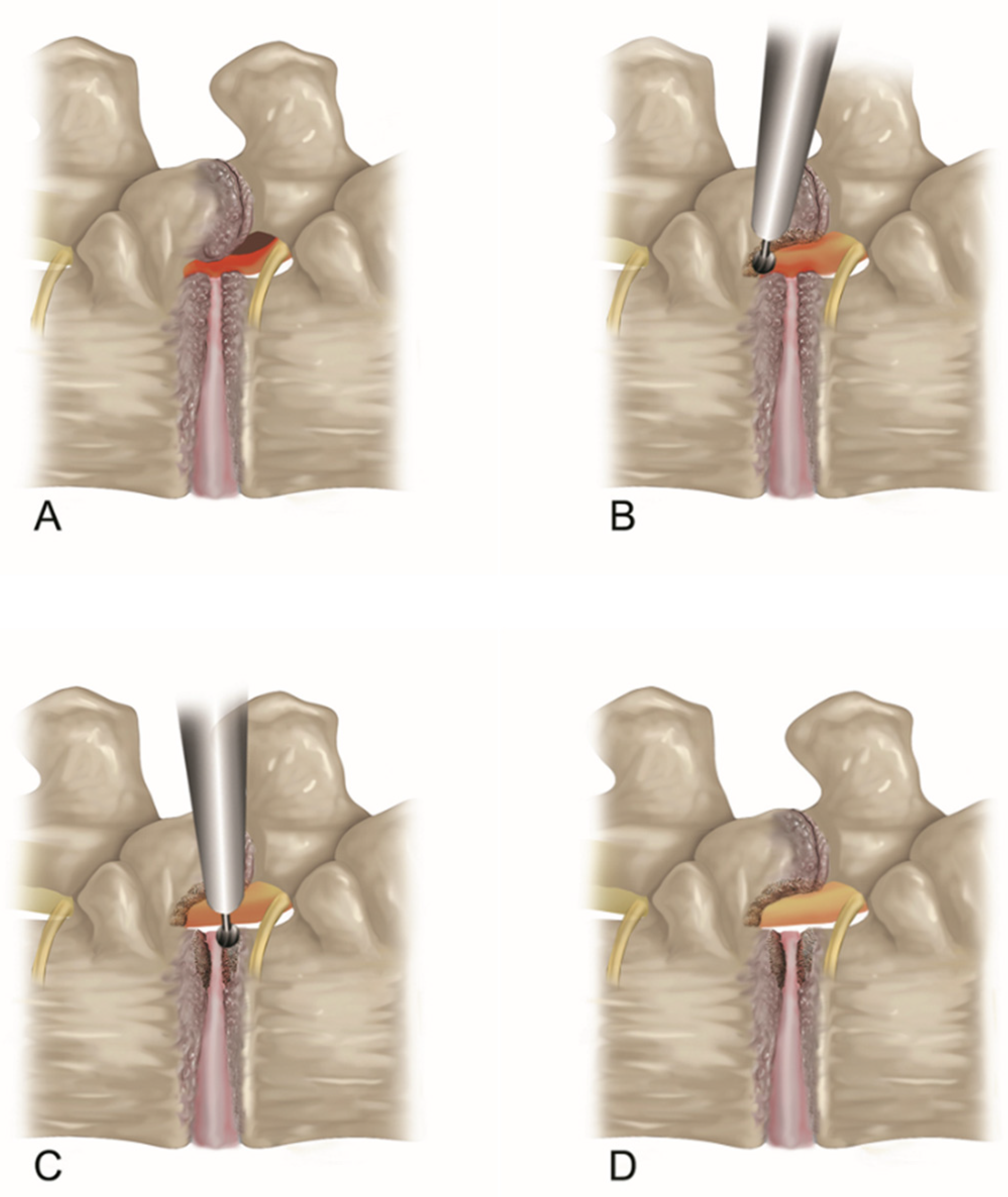
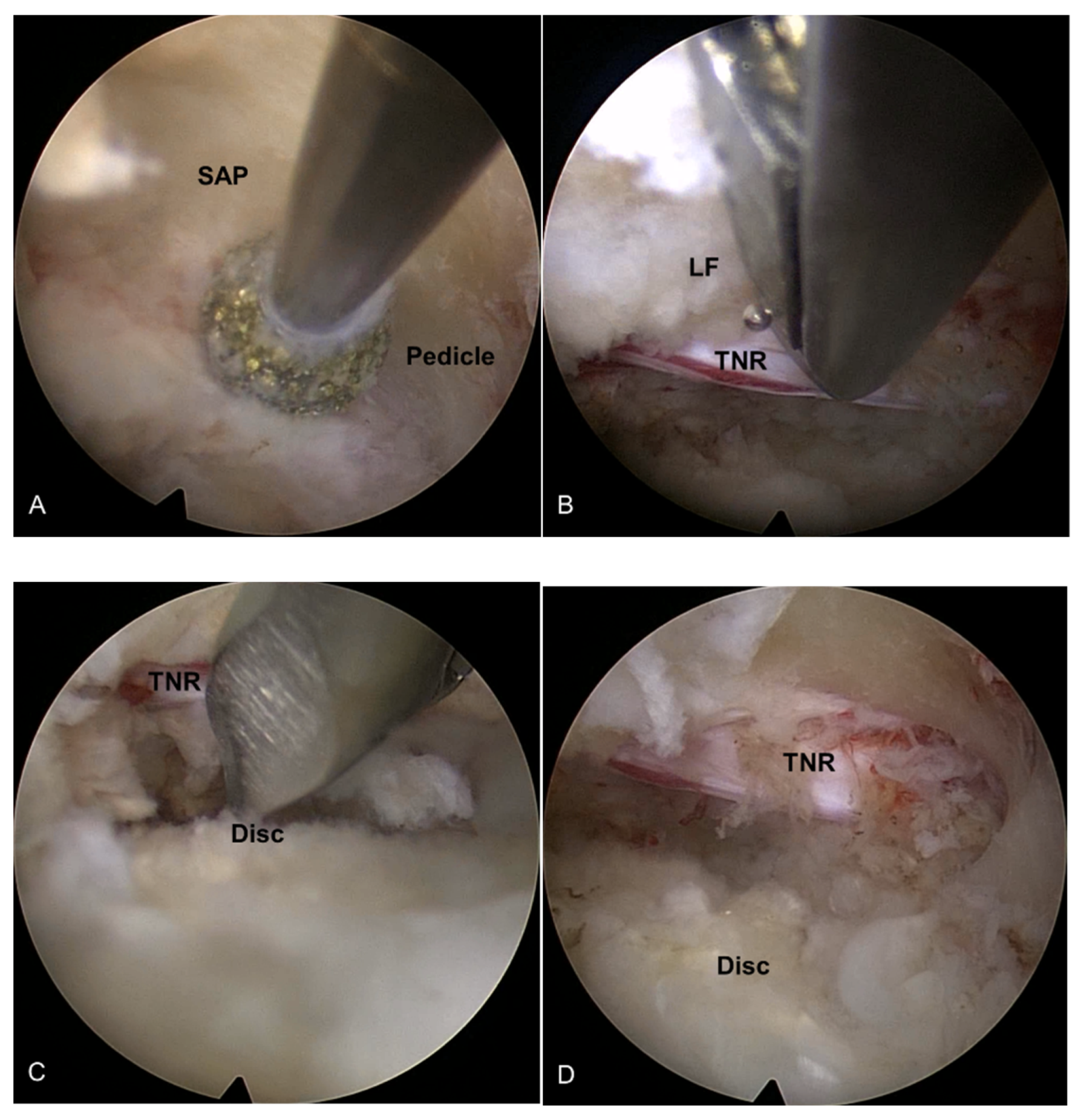
2.2.2. Endoscopic Bone Work
2.2.3. Endoscopic Soft Tissue Decompression
2.2.4. The Target Point of the Decompression
2.3. Outcome Evaluation and Statistical Analysis
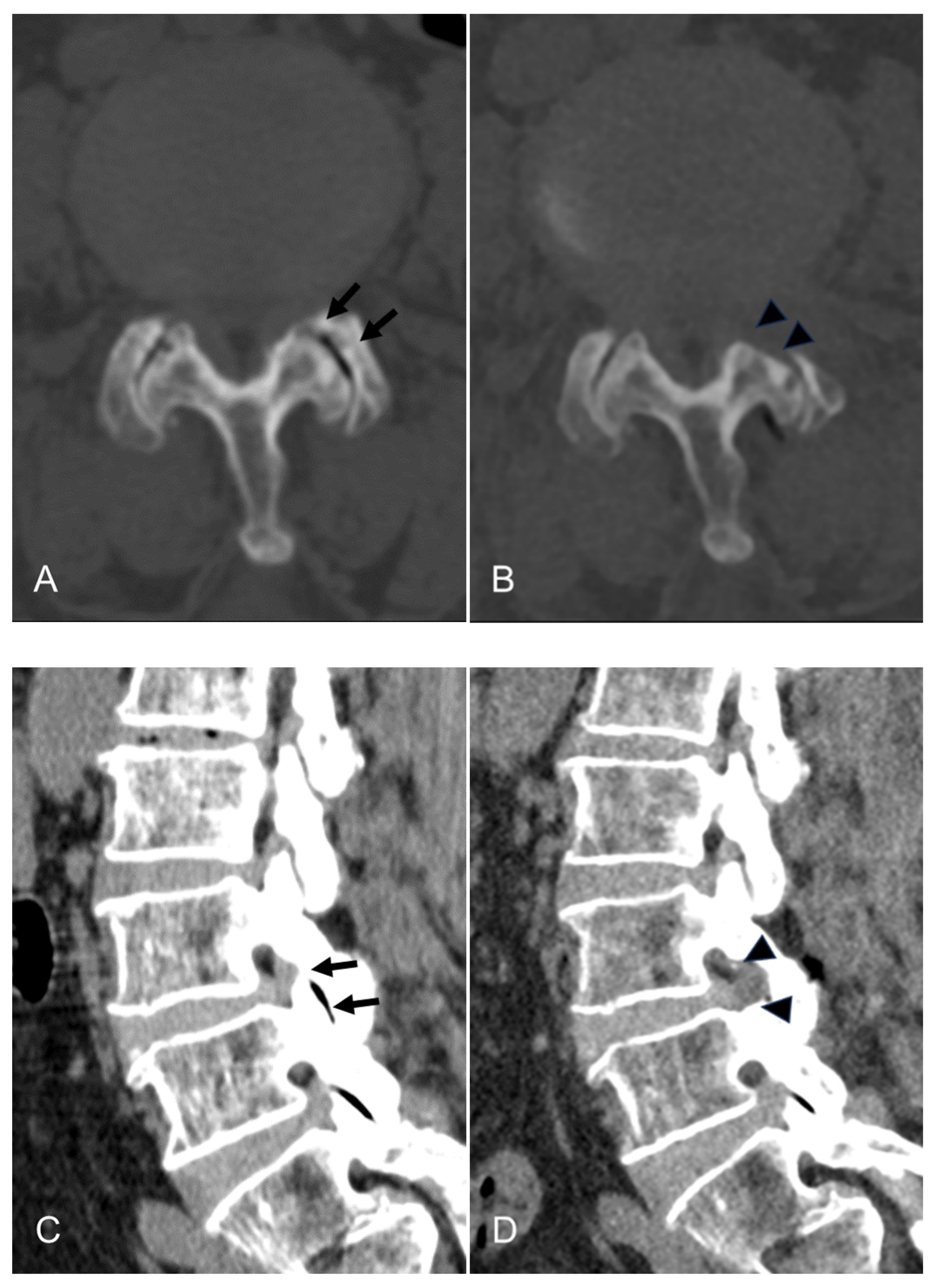
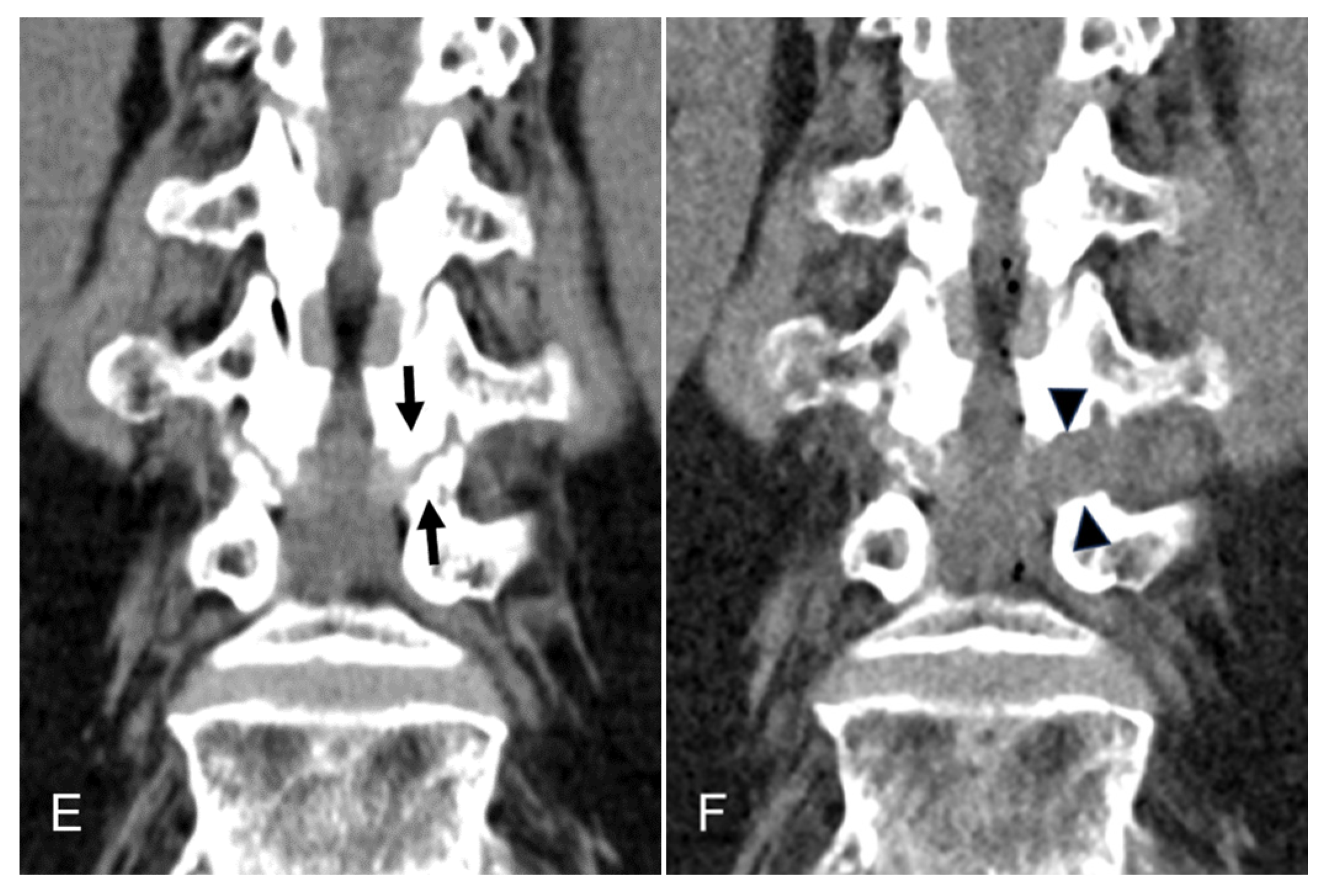
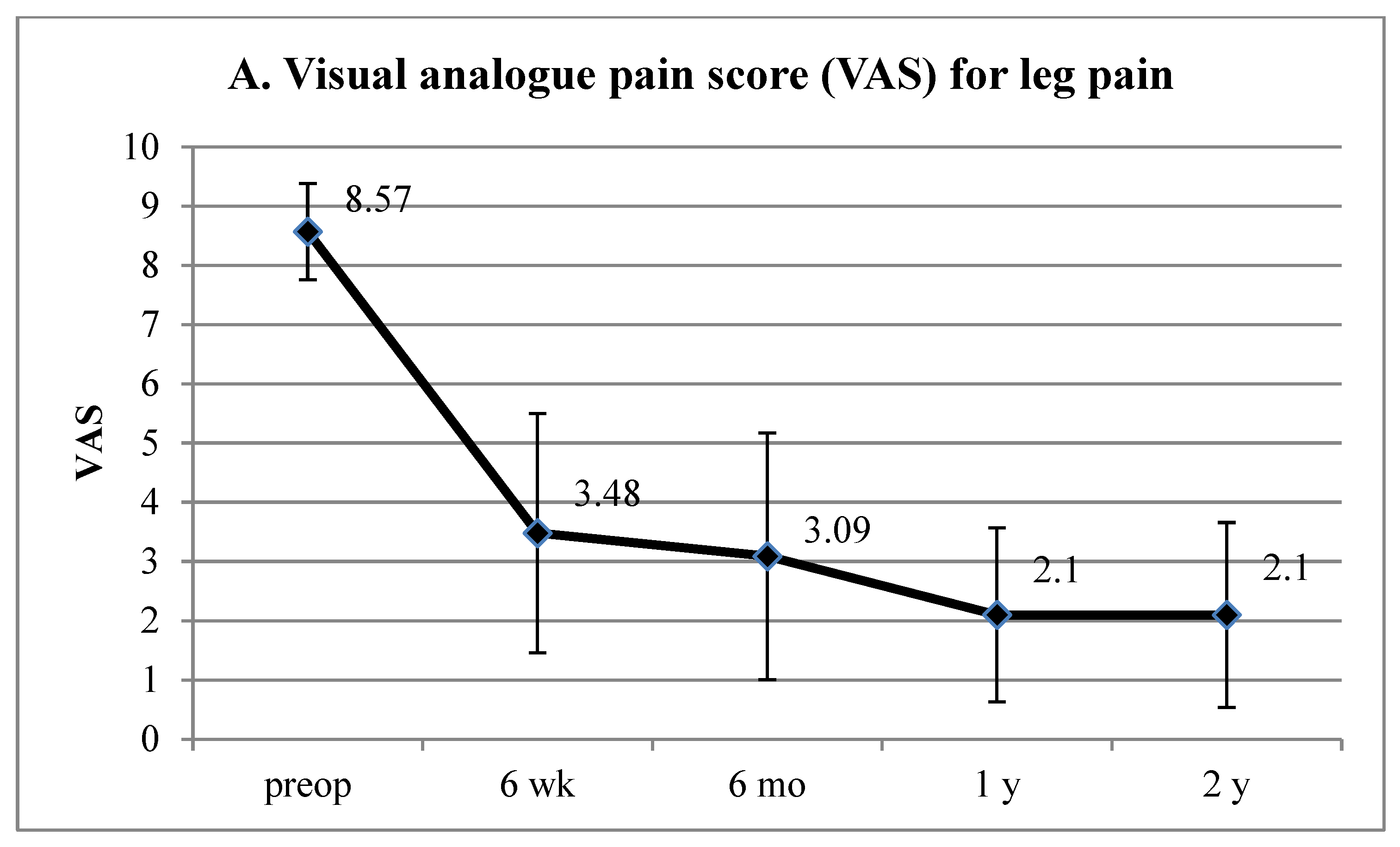
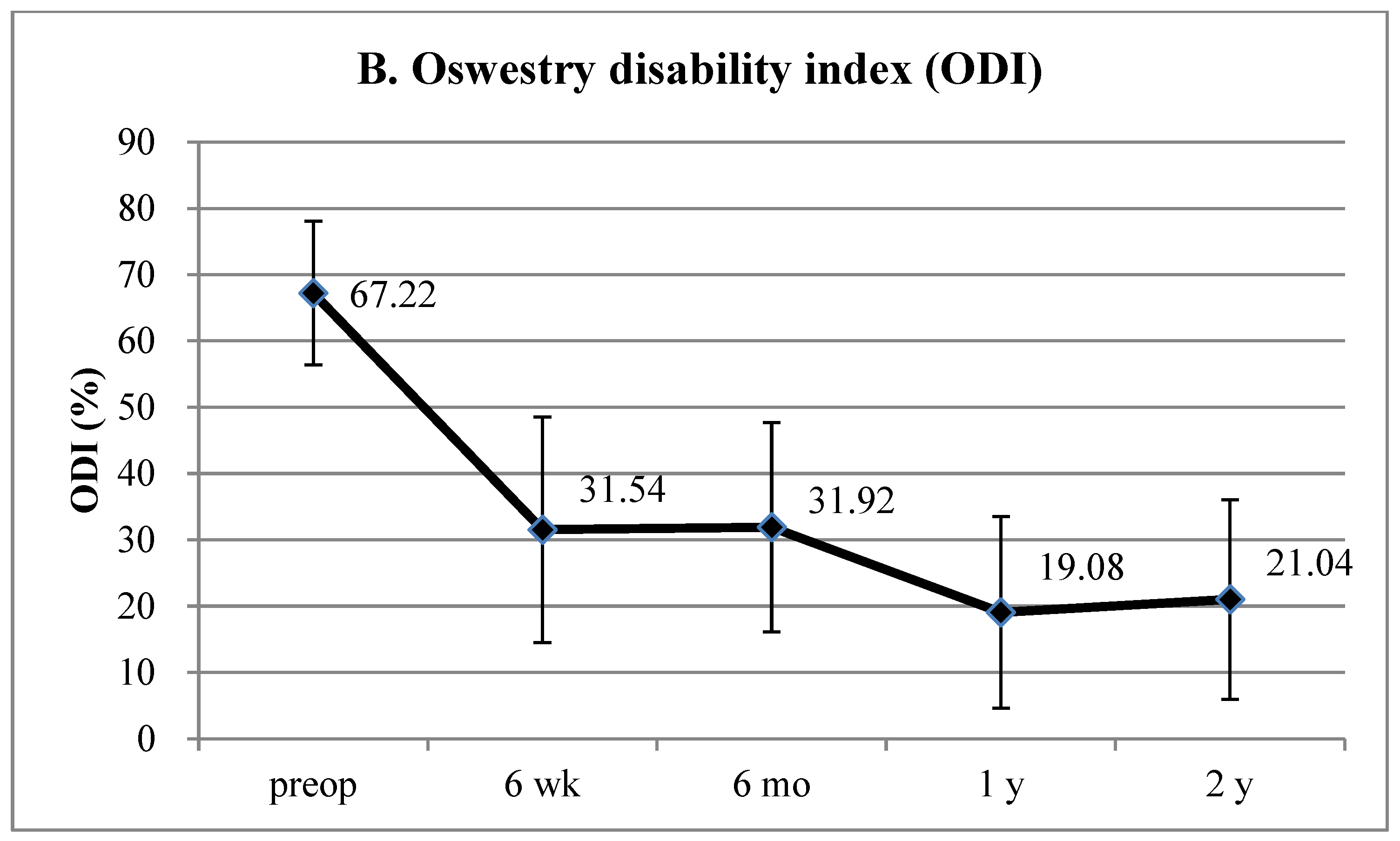
3. Results
4. Discussion
4.1. Interpretation of Clinical Results
4.2. Pros and Cons of TELLRD (Why Is Endoscopic Foraminotomy Feasible under Local Anesthesia?)
4.3. Technical Pearls to Success
4.4. Limitations of the Study
4.5. Future Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mikhael, M.A.; Ciric, I.; Tarkington, J.A.; Vick, N.A. Neuroradiological evaluation of lateral recess syndrome. Radiology 1981, 140, 97–107. [Google Scholar] [CrossRef]
- Ciric, I.; Mikhael, M.A. Lumbar spinal-lateral recess stenosis. Neurol. Clin. 1985, 3, 417–423. [Google Scholar] [CrossRef]
- Lee, C.K.; Rauschning, W.; Glenn, W. Lateral lumbar spinal canal stenosis: Classification, pathologic anatomy and surgical decompression. Spine 1988, 13, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Bartynski, W.S.; Lin, L. Lumbar root compression in the lateral recess: MR imaging, conventional myelography, and CT myelography comparison with surgical confirmation. AJNR Am. J. Neuroradiol. 2003, 24, 348–360. [Google Scholar]
- Splettstößer, A.; Khan, M.F.; Zimmermann, B.; Vogl, T.J.; Ackermann, H.; Middendorp, M.; Maataoui, A. Correlation of lumbar lateral recess stenosis in magnetic resonance imaging and clinical symptoms. World J. Radiol. 2017, 9, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Demirayak, M.; Şişman, L.; Türkmen, F.; Efe, D.; Pekince, O.; Göncü, R.G.; Sever, C. Clinical and radiological results of microsurgical posterior lumbar interbody fusion and decompression without posterior instrumentation for lateral recess stenosis. Asian Spine J. 2015, 9, 713–720. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Tosteson, T.D.; Lurie, J.D.; Tosteson, A.N.; Blood, E.; Hanscom, B.; Herkowitz, H.; Cammisa, F.; Albert, T.; Boden, S.D.; et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N. Engl. J. Med. 2008, 358, 794–810. [Google Scholar] [CrossRef] [PubMed]
- Daubs, M.D.; Lenke, L.G.; Cheh, G.; Stobbs, G.; Bridwell, K.H. Adult spinal deformity surgery: Complications and outcomes in patients over age 60. Spine 2007, 32, 2238–2244. [Google Scholar] [CrossRef]
- Cloyd, J.M.; Acosta, F.L., Jr.; Ames, C.P. Complications and outcomes of lumbar spine surgery in elderly people: A review of the literature. J. Am. Geriatr. Soc. 2008, 56, 1318–1327. [Google Scholar] [CrossRef]
- Deyo, R.A.; Mirza, S.K.; Martin, B.I. Error in trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 2011, 306, 1088. [Google Scholar] [CrossRef]
- Ong, K.L.; Auerbach, J.D.; Lau, E.; Schmier, J.; Ochoa, J.A. Perioperative outcomes, complications, and costs associated with lumbar spinal fusion in older patients with spinal stenosis and spondylolisthesis. Neurosurg. Focus 2014, 36, E5. [Google Scholar] [CrossRef]
- Ding, J.Z.; Kong, C.; Sun, X.Y.; Lu, S.B. Perioperative complications and risk factors in degenerative lumbar scoliosis surgery for patients older than 70 years of age. Clin. Interv. Aging 2019, 14, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Choi, M.K.; Kim, S.B. Perioperative results and complications after posterior lumbar interbody fusion for spinal stenosis in geriatric patients over than 70 years old. J. Korean Neurosurg. Soc. 2017, 60, 684–690. [Google Scholar] [CrossRef]
- Fritsch, E.W.; Heisel, J.; Rupp, S. The failed back surgery syndrome: Reasons, intraoperative findings, and long-term results: A report of 182 operative treatments. Spine 1996, 21, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Berger, M.; Eckenhoff, R.G.; Seitz, D.P. General anesthetic and the risk of dementia in elderly patients: Current insights. Clin. Interv. Aging 2014, 9, 1619–1628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Finsterwald, M.; Muster, M.; Farshad, M.; Saporito, A.; Brada, M.; Aguirre, J.A. Spinal versus general anesthesia for lumbar spine surgery in high risk patients: Perioperative hemodynamic stability, complications and costs. J. Clin. Anesth. 2018, 46, 3–7. [Google Scholar] [CrossRef]
- Gu, G.; Wang, C.; Gu, X.; Zhang, H.; Zhao, Y.; He, S. Percutaneous transforaminal endoscopic discectomy for adjacent segment disease after lumbar fusion in elderly patients over 65 years old. World Neurosurg. 2018, 112, e830–e836. [Google Scholar] [CrossRef]
- Kim, C.T.; Myung, W.; Lewis, M.; Lee, H.; Kim, S.E.; Lee, K.; Lee, C.; Choi, J.; Kim, H.; Carroll, B.J.; et al. Exposure to general anesthesia and risk of dementia: A nationwide population-based cohort study. J. Alzheimer’s Dis. 2018, 63, 395–405. [Google Scholar] [CrossRef]
- Li, G.; Patil, C.G.; Lad, S.P.; Ho, C.; Tian, W.; Boakye, M. Effects of age and comorbidities on complication rates and adverse outcomes after lumbar laminectomy in elderly patients. Spine 2008, 33, 1250–1255. [Google Scholar] [CrossRef]
- Acosta, F.L., Jr.; McClendon, J., Jr.; O’Shaughnessy, B.A.; Koller, H.; Neal, C.J.; Meier, O.; Ames, C.P.; Koski, T.R.; Ondra, S.L. Morbidity and mortality after spinal deformity surgery in patients 75 years and older: Complications and predictive factors. J. Neurosurg. Spine 2011, 15, 667–674. [Google Scholar] [CrossRef]
- Mao, G.; Gigliotti, M.J.; Tomycz, N.; Altman, D.T.; Philp, F.H. Clinical outcomes after spine surgery for traumatic injury in the octogenarian population. World Neurosurg. 2019, 129, e97–e103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.D.; Dietz, N.; Sharma, M.; Cruz, A.; Counts, C.E.; Wang, D.; Ugiliweneza, B.; Boakye, M.; Drazin, D. Spine surgery in the octogenarian population: A comparison of demographics, surgical approach, and healthcare utilization with the PearlDiver Database. Cureus 2021, 13, e14561. [Google Scholar] [CrossRef] [PubMed]
- Galiano, K.; Obwegeser, A.A.; Gabl, M.V.; Bauer, R.; Twerdy, K. Long-term outcome of laminectomy for spinal stenosis in octogenarians. Spine 2005, 30, 332–335. [Google Scholar] [CrossRef]
- Shabat, S.; Arinzon, Z.; Folman, Y.; Leitner, J.; David, R.; Pevzner, E.; Gepstein, R.; Pekarsky, I.; Shuval, I. Long-term outcome of decompressive surgery for lumbar spinal stenosis in octogenarians. Eur. Spine J. 2008, 17, 193–198. [Google Scholar] [CrossRef]
- Nie, H.; Hao, J.; Peng, C.; Ou, Y.; Quan, Z.; An, H. Clinical outcomes of discectomy in octogenarian patients with lumbar disc herniation. Clin. Spine Surg. 2013, 26, 74–78. [Google Scholar] [CrossRef]
- Mihailidis, H.G.; Manners, S.; Churilov, L.; Quan, G.M.Y. Is spinal surgery safe in octogenarians? ANZ J. Surg. 2017, 87, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Kambin, P.; Casey, K.; O’Brien, E.; Zhou, L. Transforaminal arthroscopic decompression of lateral recess stenosis. J. Neurosurg. 1996, 84, 462–467. [Google Scholar] [CrossRef]
- Schubert, M.; Hoogland, T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper. Orthop. Traumatol. 2005, 17, 641–661. [Google Scholar] [CrossRef]
- Kitahama, Y.; Sairyo, K.; Dezawa, A. Percutaneous endoscopic transforaminal approach to decompress the lateral recess in an elderly patient with spinal canal stenosis, herniated nucleus pulposus and pulmonary comorbidities. Asian J. Endosc. Surg. 2013, 6, 130–133. [Google Scholar] [CrossRef]
- Li, Z.Z.; Hou, S.X.; Shang, W.L.; Cao, Z.; Zhao, H.L. Percutaneous lumbar foraminoplasty and percutaneous endoscopic lumbar decompression for lateral recess stenosis through transforaminal approach: Technique notes and 2 years follow-up. Clin. Neurol. Neurosurg. 2016, 143, 90–94. [Google Scholar] [CrossRef]
- Lewandrowski, K.U. Endoscopic transforaminal and lateral recess decompression after previous spinal surgery. Int. J. Spine Surg. 2018, 12, 98–111. [Google Scholar] [CrossRef]
- Tang, S.; Jin, S.; Liao, X.; Huang, K.; Luo, J.; Zhu, T. Transforaminal percutaneous endoscopic lumbar decompression by using rigid bendable burr for lumbar lateral recess stenosis: Technique and clinical outcome. BioMed Res. Int. 2018, 2018, 2601232. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Keum, H.J.; Lee, S.G.; Lee, S.W. Transforaminal endoscopic decompression for lumbar lateral recess stenosis: An advanced surgical technique and clinical outcomes. World Neurosurg. 2019, 125, e916–e924. [Google Scholar] [CrossRef]
- Wu, B.; Xiong, C.; Huang, B.; Zhao, D.; Yao, Z.; Yao, Y.; Xu, F.; Kang, H. Clinical outcomes of transforaminal endoscopic lateral recess decompression by using the visualized drilled foraminoplasty and visualized reamed foraminoplasty: A comparison study. BMC Musculoskelet. Disord. 2020, 21, 829. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.G.; Pan, X.K.; Hu, M.J.; Zhang, J.Q.; Sheng, J.M.; Chen, B.; Zhou, X.; Yu, Y.; Hu, X.Q. Percutaneous teransforaminal endoscopic decompression for lumbar lateral recess stenosis. Front. Surg. 2021, 8, 631419. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, S.H.; Lee, H.Y.; Lee, H.J.; Chang, S.B.; Chung, S.K.; Kim, H.J. Validation of the Korean version of the oswestry disability index. Spine 2005, 30, E123–E127. [Google Scholar] [CrossRef]
- Yeung, A.T.; Tsou, P.M. Posterolateral endoscopic excision for lumbar disc herniation: Surgical technique, outcome, and complications in 307 consecutive cases. Spine 2002, 27, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.J.; Ashton-James, C.E.; Skorpil, N.E.; Heymans, M.W.; Forouzanfar, T. What constitutes a clinically important pain reduction in patients after third molar surgery? Pain Res. Manag. 2013, 18, 319–322. [Google Scholar] [CrossRef]
- Ng, L.C.; Tafazal, S.; Sell, P. The effect of duration of symptoms on standard outcome measures in the surgical treatment of spinal stenosis. Eur. Spine J. 2007, 16, 199–206. [Google Scholar] [CrossRef][Green Version]
- Ostelo, R.W.; Deyo, R.A.; Stratford, P.; Waddell, G.; Croft, P.; Von Korff, M.; Bouter, L.M.; de Vet, H.C. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine 2008, 33, 90–94. [Google Scholar] [CrossRef]
- Ahn, Y.; Lee, S.; Son, S.; Kim, H.; Kim, J.E. Learning curve for transforaminal percutaneous endoscopic lumbar discectomy: A systematic review. World Neurosurg. 2020, 143, 471–479. [Google Scholar] [CrossRef] [PubMed]
| Octogenarian (n = 21) | Younger (n = 95) | p Values | |
|---|---|---|---|
| Age (mean, y) | 82.86 (81–88) | 66.28 (42–79) | <0.0001 |
| Male:Female | 9:12 | 46:49 | NS |
| Operative level | NS | ||
| L2–3 | 0 | 2 | |
| L3–4 | 6 | 23 | |
| L4–5 | 10 | 48 | |
| L5–S1 | 5 | 22 | |
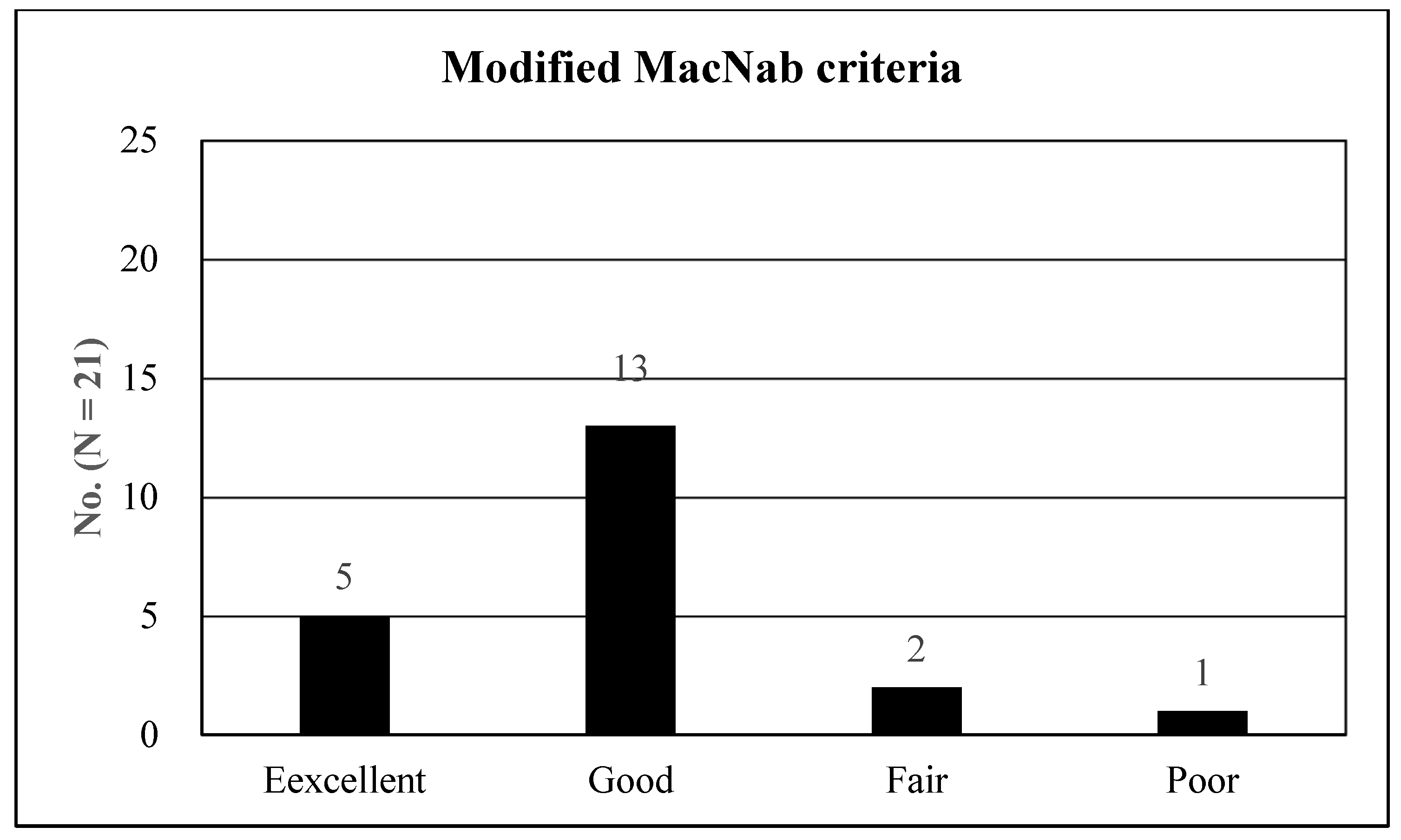
| MacNab criteria | NS | ||
| Excellent | 5 (23.81%) | 24 (254.12%) | |
| Good | 13 (61.90%) | 60 (63.16%) | |
| Fair | 2 (9.52%) | 7 (7.37%) | |
| Poor | 1 (4.76%) | 4 (4.21%) | |
| Reoperation (%) | 1 (open laminotomy) | 3 (open laminotomy) | NS |
| Complication (%) | NS | ||
| Infection | 0 | 0 | |
| hematoma | 0 | 1 (minor, epidural) | |
| dural tear | 1 (minor, intraoperative) | 1 (required revision) | |
| dysesthesia | 2 (1 transient) | 5 (2 transient) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, Y.; Jung, J.-H. Transforaminal Endoscopic Lumbar Lateral Recess Decompression for Octogenarian Patients. J. Clin. Med. 2024, 13, 515. https://doi.org/10.3390/jcm13020515
Ahn Y, Jung J-H. Transforaminal Endoscopic Lumbar Lateral Recess Decompression for Octogenarian Patients. Journal of Clinical Medicine. 2024; 13(2):515. https://doi.org/10.3390/jcm13020515
Chicago/Turabian StyleAhn, Yong, and Jun-Hyeok Jung. 2024. "Transforaminal Endoscopic Lumbar Lateral Recess Decompression for Octogenarian Patients" Journal of Clinical Medicine 13, no. 2: 515. https://doi.org/10.3390/jcm13020515
APA StyleAhn, Y., & Jung, J.-H. (2024). Transforaminal Endoscopic Lumbar Lateral Recess Decompression for Octogenarian Patients. Journal of Clinical Medicine, 13(2), 515. https://doi.org/10.3390/jcm13020515






