Mechanical Circulatory Support Strategies in Takotsubo Syndrome with Cardiogenic Shock: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategies
2.2. Inclusion Criteria
- TTS diagnosis consistent with the InterTAK Diagnostic Criteria and designated as such by the authors;
- Use of temporary MCS: IABP, ECLS, Impella, TandemHeart;
- Individually reported patient data in terms of pre-intervention status, survival and time on support.
2.3. Data Extraction
2.4. Statistics
3. Results
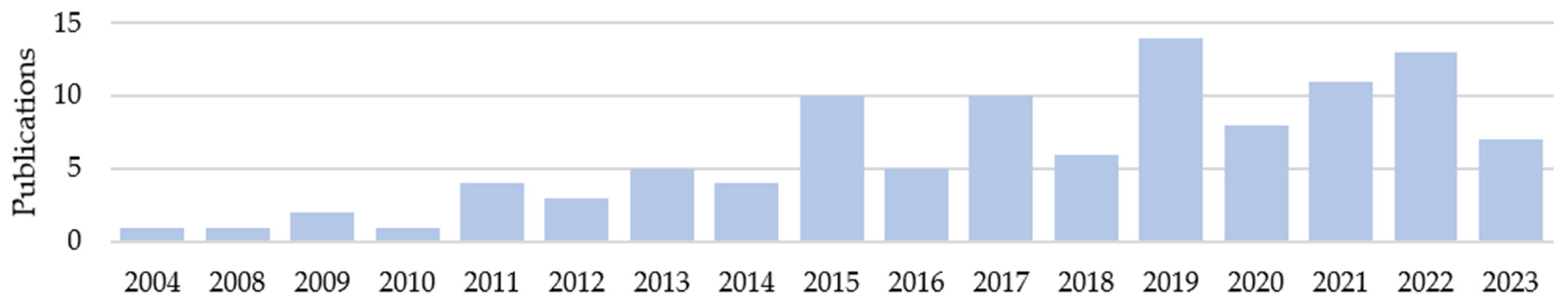
3.1. Literature Research
3.2. Demographics and Device Groups
3.3. Pre-MCS Status of the Patients
3.4. Outcome and Follow-Up
3.5. Comparison of the Device Groups
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
| J.K.R.v.M. | None. |
| V.I.T.Z. | None. |
| A.E.S. | None. |
| K.M.V.P. | None. |
| R.H. | None. |
| F.S. | Institutional grants from Novartis and Abbott; non-financial support from Medtronic and institutional fees (speaker honoraria) from Orion Pharma outside of the submitted work. |
| J.K. | Grants or contracts from any entity: Edwards and LivaNova. Payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events: Edwards, Medtronic, Abbott, LivaNova and CryoLife. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: TC EACTS, ECSC Board, and ISMICS Board. |
| C.T.S. | Payment to his institution in the form of speaker fees, honoraria, consultancy, advisory board fees, investigator and committee membership: AngioDynamics, Abiomed, Medtronic, Spectranetics, Biotronik, LivaNova (Sorin) and Cook Medical. Departmental or institutional research funding: Cook Medical. |
| E.V.P. | Consulting fees: Abbott (institutional grants), Medtronic (institutional grants) and Abiomed (institutional grants). Payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events: Abbott (institutional grants), Medtronic (institutional grants) and Abiomed (institutional grants). Support for attending meetings and/or travel: Abbott (institutional grants), Medtronic (institutional grants) and Abiomed (institutional grants). Participation in Data Safety Monitoring Board or Advisory Board: Abbott and Medtronic. |
| V.F. | Grants or contracts from any entity: Medtronic GmbH, Biotronik SE & Co., Abbott GmbH & Co. KG, Boston Scientific, Edwards Lifesciences, Berlin Heart, Novartis Pharma GmbH, JOTEC/CryoLife GmbH, LivaNova and Zurich Heart. I hereby declare that I have relevant (institutional) financial activities outside the submitted work with the mentioned commercial entities in relation to educational grants (including travel support), fees for lectures and speeches, fees for professional consultation, research and study funds. |
| L.W. | None. |
Glossary of Abbreviations
| LV | left ventricle/left ventricular |
| LVAD | left ventricular assist device |
| BiVAD | biventricular assist device |
| ECMO | extracorporeal membrane oxygenation |
| ECLS | extracorporeal life support |
| IABP | intra-aortic balloon pump |
| LVEF | left ventricular ejection fraction |
| NYHA | New York Heart Association |
| TTS | Takotsubo syndrome |
| CS | cardiogenic shock |
| ACS | acute coronary syndrome |
| MCS | mechanical circulatory support |
| MR | mitral regurgitation |
| SAM | systolic anterior motion |
| MAP | mean arterial pressure |
| CO | cardiac output |
| AKI | acute kidney injury |
| HB | haemoglobin |
Appendix A. Included Publications
| Author | Journal | Year | Title | Number of Patients |
| A. A. Oredegbe and M. Awad | Cureus | 2023 | Catecholamine Mega Storm Triggered by Cocaine Use and Thyrotoxicosis Crisis | 1 |
| A. Badouin and O. Bastien | Anaesth Crit Care Pain Med | 2015 | ECLS indication for a case of stress myocardiopathy associated with severe asthma | 1 |
| A. Benak, M. Sramko, B. Janek et al. | Cureus | 2022 | Successful Treatment of Cardiogenic Shock Due to Takotsubo Cardiomyopathy With Left Ventricular Outflow Tract Obstruction and Acute Mitral Regurgitation by Impella CP | 1 |
| A. Beneduce, L. Fausta Bertoldi, F. Melillo et al. | JACC Cardiovasc Interv | 2019 | Mechanical Circulatory Support With Impella Percutaneous Ventricular Assist Device as a Bridge to Recovery in Takotsubo Syndrome Complicated by Cardiogenic Shock and Left Ventricular Outflow Tract Obstruction | 1 |
| A. H. Koop, R. E. Bailey and P. E. Lowman | BMJ Case Rep | 2018 | Acute pancreatitis-induced Takotsubo cardiomyopathy and cardiogenic shock treated with a percutaneous left ventricular assist device | 1 |
| A. K. Tiwari and N. D’Attellis | J Cardiothorac Vasc Anesth | 2008 | Intraoperative left ventricular apical ballooning: transient Takotsubo cardiomyopathy during orthotopic liver transplantation | 1 |
| A. Lauterio, M. Bottiroli, A. Cannata et al. | Minerva Anestesiol | 2022 | Successful recovery from severe inverted Takotsubo cardiomyopathy after liver transplantation: the efficacy of extracorporeal membrane oxygenation (ECMO) | 1 |
| A. Mohammedzein, A. Taha, A. Salwan et al. | JACC Case Rep | 2019 | Impella Use in Cardiogenic Shock Due to Takotsubo Cardiomyopathy With Left Ventricular Outflow Tract Obstruction | 1 |
| A. Omosule, M. F. Malik, L. Cisneros et al. | J Cardiothorac Vasc Anesth | 2019 | Takotsubo Cardiomyopathy After Double-Lung Transplantation: Role of Early Extracorporeal Membrane Oxygenation Support | 1 |
| A. Rashed, S. Won, M. Saad et al. | BMJ Case Rep | 2015 | Use of the Impella 2.5 left ventricular assist device in a patient with cardiogenic shock secondary to Takotsubo cardiomyopathy | 1 |
| A. Vachiat, K. McCutcheon, A. Mahomed et al. | Cardiovasc J Afr | 2016 | Takotsubo cardiomyopathy post liver transplantation | 1 |
| A. Yazicioglu, M. Subasi, S. Turkkan et al. | Turk Gogus Kalp Damar Cerrahisi Derg | 2018 | An uncommon cause for grade 3 primary graft dysfunction after lung transplantation: Takotsubo cardiomyopathy | 1 |
| B. F. Sagger Mawri, Hazem Malas, Sachin Parikh et al. | Scient Open Access | 2017 | Mechanical Hemodynamic Support as a Bridge to Recovery in Severe Takotsubo Cardiomyopathy with Marked Left Ventricular Outflow Tract Obstruction and Cardiogenic Shock | 1 |
| B. Flam, M. Broome, B. Frenckner et al. | J Intensive Care Med | 2015 | Pheochromocytoma-Induced Inverted Takotsubo-Like Cardiomyopathy Leading to Cardiogenic Shock Successfully Treated With Extracorporeal Membrane Oxygenation | 1 |
| B. Hassid, S. Azmoon, W. S. Aronow et al. | Arch Med Sci | 2010 | Hemodynamic support with TandemHeart in tako-tsubo cardiomyopathy—a case report | 1 |
| B. Laliberte and B. N. Reed | Am J Health Syst Pharm | 2017 | Use of an argatroban-based purge solution in a percutaneous ventricular assist device | 1 |
| B. M. M. Faria, J. Portugues, R. Roncon-Albuquerque et al. | Eur Heart J Case Rep | 2020 | Inverted Takotsubo syndrome complicated with cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation in a patient with bilateral pheochromocytoma: a case report | 1 |
| C. Dominedo, E. D’Avino, A. Martinotti et al. | Eur Heart J Case Rep | 2021 | A rare pheochromocytoma complicated by cardiogenic shock and posterior reversible encephalopathy syndrome: case report | 1 |
| C. J. van Zwet, A. Rist, A. Haeussler et al. | A A Case Rep | 2016 | Extracorporeal Membrane Oxygenation for Treatment of Acute Inverted Takotsubo-Like Cardiomyopathy From Hemorrhagic Pheochromocytoma in Late Pregnancy | 1 |
| C. Zeballos, R. J. Moraca, S. H. Bailey et al. | J Card Surg | 2012 | Temporary mechanical circulatory support for Takotsubo cardiomyopathy secondary to primary mediastinal B-cell lymphoma | 1 |
| D. Basic, G. Klug, B. Haubner et al. | Eur Heart J | 2019 | Left ventricular unloading by percutaneous mechanical circulatory support in Takotsubo syndrome with severe cardiogenic shock | 1 |
| D. Ghanim, Z. Adler, D. Qarawani et al. | J Med Case Rep | 2015 | Takotsubo cardiomyopathy caused by epinephrine-treated bee sting anaphylaxis: a case report | 1 |
| D. Laghlam, O. Touboul, M. Herry et al. | Front Cardiovasc Med | 2022 | Takotsubo cardiomyopathy after cardiac surgery: A case-series and systematic review of literature | 2 |
| D. W. Donker, E. Pragt, P. W. Weerwind et al. | Int J Cardiol | 2012 | Rescue extracorporeal life support as a bridge to reflection in fulminant stress-induced cardiomyopathy | 1 |
| E. Barsoum, S. Elhosseiny, B. Patel et al. | Heart Lung | 2021 | Successful use of the impella ventricular assist device for management of reverse Takotsubo Cardiomyopathy in the setting of acute intracranial hemorrhage | 1 |
| E. C. Busse and J. M. Wiater | JBJS Case Connect | 2015 | Perioperative Takotsubo Cardiomyopathy: A Rare Cardiac Complication Following Orthopaedic Surgery: A Case Report | 1 |
| E. D Foley, Ricardo Diaz and Manuel R Castresana | SAGE Open Med Case Rep | 2017 | Prolonged circulatory support with an Impella assist device in the management of cardiogenic shock associated with Takotsubo syndrome, severe sepsis and acute respiratory distress syndrome | 1 |
| E. f. J. Hamid T, Fraser D, Fath-Ordoubadi F | J Clin Exp Cardiolog | 2013 | Use of the Impella Left Ventricular Assist Device as a Bridge to Recovery in a Patient with Cardiogenic Shock Related to Takotsubo Cardiomyopathy. | 1 |
| E. T. Wu, T. H. Lin, C. H. Lin et al. | Taiwan J Obstet Gynecol | 2014 | Left ventricular assist device for stress-induced cardiomyopathy after postpartum hemorrhage | 1 |
| F. F. Zhou, J. S. Ding, M. Zhang et al. | Open Med (Wars) | 2022 | Paraganglioma-induced inverted Takotsubo-like cardiomyopathy leading to cardiogenic shock successfully treated with extracorporeal membrane oxygenation | 1 |
| F. Yazdi, M. Blackmon, A. Kattubadi et al. | Cureus | 2023 | Seizure-Induced Cardiomyopathy: A Case of Takotsubo Cardiomyopathy Following an Epileptic Event | 1 |
| F. Zilio, S. Muraglia and R. Bonmassari | European Heart Journal—Case Reports | 2021 | Cardiac arrest complicating cardiogenic shock: from pathophysiological insights to Impella-assisted cardiopulmonary resuscitation in a pheochromocytoma-induced Takotsubo cardiomyopathy-a case report | 1 |
| G. Caturegli, M. A. Crane, E. Etchill et al. | ASAIO J | 2022 | Stress-Induced (Takotsubo) Cardiomyopathy After Liver Transplant Rescued with Venoarterial Extracorporeal Membrane Oxygenation | 1 |
| G. Hekimian, F. Kharcha, N. Brechot et al. | Ann Intensive Care | 2016 | Extracorporeal membrane oxygenation for pheochromocytoma-induced cardiogenic shock | 9 |
| G. Rojas-Marte, J. John, A. Sadiq et al. | Cardiovasc Revasc Med | 2015 | Medication-induced Takotsubo Cardiomyopathy presenting with cardiogenic shock-utility of extracorporeal membrane oxygenation (ECMO): case report and review of the literature | 1 |
| H. Sumida, K. Morihisa, K. Katahira et al. | Intern Med | 2017 | Isolated Right Ventricular Stress (Takotsubo) Cardiomyopathy | 1 |
| H. Zhang and X. Liao | J Card Surg | 2021 | Takotsubo cardiomyopathy following pericardiectomy: A case report | 1 |
| I. Schroeder, M. Zoller, M. Angstwurm et al. | nt J Artif Organs | 2017 | Venlafaxine intoxication with development of Takotsubo cardiomyopathy: successful use of extracorporeal life support, intravenous lipid emulsion and CytoSorb® | 1 |
| J. Feghaly, Z. Oman, D. Das et al. | Cureus | 2021 | Recurrent Stress-Induced Cardiomyopathy With Cardiogenic Shock Requiring Impella Left Ventricular Assist Device | 1 |
| J. H. Choi, I. D. Oh, E. Shin et al. | Acute Crit Care | 2020 | Extracorporeal membrane oxygenation for Takotsubo cardiomyopathy that developed after mitral valve replacement | 1 |
| J. J. J. Aalberts, T. J. Klinkenberg, M. A. Mariani et al. | Eur Heart J | 2017 | Mechanical circulatory support for refractory cardiogenic shock in Takotsubo syndrome: a case report and review of the literature | 1 |
| J. Kirigaya, N. Iwahashi, R. Tanaka et al. | Cureus | 2022 | A Fatal Case of Takotsubo Cardiomyopathy Secondary to Refractory Hypoglycemia in Severe Starvation: An Autopsy Case Report | 1 |
| J. Mierke, T. Loehn, A. Linke et al. | Eur Heart J Case Rep | 2019 | Reverse Takotsubo cardiomyopathy- life-threatening symptom of an incidental pheochromocytoma: a case report | 1 |
| J. O’Brien, S. Mahony, R. J. Byrne et al. | Eur Heart J Case Rep | 2021 | Dynamic left ventricular outflow tract gradient resulting from Takotsubo cardiomyopathy ameliorated by intra-aortic balloon pump counterpulsation: a case report | 1 |
| J. Wei, L. Zhang, X. Ruan et al. | Front Cardiovasc Med | 2022 | Case Report: Takotsubo Syndrome Induced by Severe Anaphylactic Reaction During Anesthesia Induction and Subsequent High-Dose Epinephrine Resuscitation | 1 |
| K. T. Webster, T. Apridonidze, P. R. Mopala et al. | Can J Cardiol 2019 | 2019 | Stress-Induced Cardiomyopathy Complicated by Dynamic Left Ventricular Outflow Obstruction, Cardiogenic Shock, and Ventricular Septal Rupture | 1 |
| K. X. Fu, B. H. Z. Ng and M. H. X. Chua | BMC Pediatr | 2019 | A unique case of acute brain haemorrhage with left ventricular systolic failure requiring ECMO | 1 |
| K. Yamane, H. Hirose, G. R. Reeves et al. | J Heart Valve Dis | 2011 | Left ventricular dysfunction mimicking Takotsubo cardiomyopathy following cardiac surgery | 1 |
| L. C. Napp, R. Westenfeld, J. E. Møller et al. | Cardiovasc Revasc Med | 2022 | Impella Mechanical Circulatory Support for Takotsubo Syndrome With Shock: A Retrospective Multicenter Analysis | 16 |
| L. Paton and I. Quasim | Br J Anaesth | 2013 | Takotsubo cardiomyopathy: issues for the intensivist | 1 |
| L. Wert, J. Kempfert, V. Falk et al. | Interdiscip Cardiovasc Thorac Surg | 2023 | Transaxillary implantation of a temporary microaxial left ventricular assist device in a patient with a rectangular kinked subclavian artery | 1 |
| M. Bonacchi, A. Vannini, G. Harmelin et al. | Interact Cardiovasc Thorac Surg | 2015 | Inverted-Takotsubo cardiomyopathy: severe refractory heart failure in poly-trauma patients saved by emergency extracorporeal life support | 4 |
| M. Bonacchi, S. Valente, G. Harmelin et al. | Artif Organs | 2009 | extracorporeal life support as ultimate strategy for refractory severe cardiogenic shock induced by Tako-tsubo cardiomyopathy: a new effective therapeutic option | 1 |
| M. Hanif, M. A. Haider, Q. Xi et al. | Cureus | 2020 | Takotsubo Cardiomyopathy Triggered by the Death of Pets (Cats): Two Case Reports | 1 |
| M. Husaini, J. N. Baker, S. Cresci et al. | JACC Case Rep | 2022 | Recurrent Takotsubo Cardiomyopathy in a Patient With Hypertrophic Cardiomyopathy Leading to Cardiogenic Shock Requiring VA-ECMO | 1 |
| M. Moguilevitch, M. Rufino, J. Leff et al. | Liver Transpl | 2015 | Novel approach for heart failure treatment after liver transplantation | 1 |
| M. Nakamura, M. Nakagaito, M. Hori et al. | J Artif Organs | 2019 | A case of Takotsubo cardiomyopathy with cardiogenic shock after influenza infection successfully recovered by IMPELLA support | 1 |
| M. P. Silva, E. M. Vilela, R. L. Lopes et al. | Rev Port Cardiol | 2015 | Cardiogenic shock induced by Takotsubo cardiomyopathy: A new therapeutic option | 1 |
| O. Kiamanesh, E. N. Vu, D. L. Webber et al. | JACC Case Rep | 2019 | Pheochromocytoma-Induced Takotsubo Syndrome Treated With Extracorporeal Membrane Oxygenation: Beware of the Apical Sparing Pattern | 1 |
| P. A. Cotinet, P. Bizouarn, F. Roux et al. | Heart Lung | 2021 | Management of cardiogenic shock by circulatory support during reverse Tako-Tsubo following amphetamine exposure: A report of two cases | 2 |
| R. Dalla Pozza, A. Lehner, S. Ulrich, M. Nabauer et al. | World J Pediatr Congenit Heart Surg | 2020 | Takotsubo Cardiomyopathy Complicating Percutaneous Pulmonary Valve Implantation in a Child | 1 |
| R. Gurreri, P. Poommipanit and A. Alghamdi | Oxf Med Case Reports | 2023 | Cardiogenic shock secondary to stress-induced cardiomyopathy precipitated by severe diabetic ketoacidosis | 1 |
| R. Hamdan, M. E. Nassef, J. Khan et al. | Ann Cardiol Angeiol | 2022 | Reverse Takotsubo ou myocardite fulminante ? Succes de VA ECMO chez une patiente ayant une atteinte cardiaque liee COVID 19 | 1 |
| R. Kakizaki, N. Bunya, S. Uemura et al. | Acute Med Surg | 2019 | Takotsubo cardiomyopathy developed during rewarming of accidental hypothermia with extracorporeal membrane oxygenation | 1 |
| R. Korabathina, W. Abel and A. Labovitz | Case Rep Cardiol | 2016 | Cardiogenic Shock due to Psychosis-Induced Inverted Takotsubo Cardiomyopathy Bridged-to-Recovery with a Percutaneous Left Ventricular Assist Device | 1 |
| R. Nishikawa, N. Nagano, N. Kokubu et al. | Int Heart J | 2021 | Favorable Effects of Impella on Takotsubo Syndrome Complicated with Cardiogenic Shock | 4 |
| R. S. Biondi, V. S. Barzilai, A. L. C. Watanabe et al. | Rev Bras Ter Intensiva | 2018 | Use of extracorporeal membrane oxygenation for treating acute cardiomyopathy after liver transplantation: a case report | 1 |
| R. V. Reddy, S. Agarwal, V. Choudhary et al. | Indian J Anaesth | 2018 | Reverse stress cardiomyopathy post-liver transplant needing mechanical circulatory support | 1 |
| S. An, H. I. Ma, J. Song et al. | BMC Neurol | 2020 | Stress cardiomyopathy associated with area postrema syndrome as a presentation of neuromyelitis optica: case report | 1 |
| S. Elapavaluru, A. Gologorsky, N. Thai et al. | J Cardiothorac Vasc Anesth | 2017 | Perioperative Stress Cardiomyopathy in Simultaneous Liver and Kidney Transplantation: A Call for Early Consideration of Mechanical Circulatory Support | 1 |
| S. Fang, Y. Wang, P. K. He et al. | Medicine (Baltimore) | 2021 | Cardiogenic shock caused by Takotsubo syndrome complicated with severe anxiety: A case report and literature review | 1 |
| S. Forsberg, L. Abazi and P. Forsman | J Med Case Rep | 2021 | Successful use of extended cardiopulmonary resuscitation followed by extracorporeal oxygenation after venlafaxine-induced Takotsubo cardiomyopathy and cardiac arrest: a case report | 1 |
| S. H. Park, M. K. Song, G. B. Kim et al. | Chonnam Med J | 2019 | Stress Induced Cardiomyopathy Requiring Ventricular Assist Device Support in an 8-Year-Old Girl with Acute Leukemia | 1 |
| S. Kaese, C. Schulke, D. Fischer et al. | Intensive Care Med | 2013 | Pheochromocytoma-induced Takotsubo-like cardiomyopathy and global heart failure with need for extracorporal life support | 1 |
| S. Kurisu, K. Ishibashi, Y. Kato et al. | Intern Med | 2012 | Tako-tsubo cardiomyopathy complicated by QRS prolongation | 1 |
| S. Lee, S. P. Lim, J. H. Yu et al. | Korean J Thorac Cardiovasc Surg | 2011 | Stress-induced Cardiomyopathy during Pulmonary Resection (Takotsubo Syndrome)—A case report | 1 |
| S. Li, M. M. Koerner, A. El-Banayosy et al. | Ann Thorac Surg | 2014 | Takotsubo’s syndrome after mitral valve repair and rescue with extracorporeal membrane oxygenation | 1 |
| S. Modi and D. Ramsdale | Int J Cardiol | 2011 | Tako-tsubo, hypertrophic obstructive cardiomyopathy & muscle bridging--separate disease entities or a single condition? | 1 |
| S. Park, M. Kim, D. I. Lee et al. | Acute Crit Care | 2022 | Successful extracorporeal membrane oxygenation treatment of catecholamine-induced cardiomyopathy-associated pheochromocytoma | 1 |
| S. Sossalla, C. Meindl, M. Fischer et al. | Circ Cardiovasc Interv | 2019 | Bail-Out Alcohol Septal Ablation for Hypertrophic Obstructive Cardiomyopathy in a Patient With Takotsubo Cardiomyopathy-Induced Cardiogenic Shock | 1 |
| S. Sundaravel, A. Alrifai, M. Kabach et al. | Case Rep Cardiol | 2017 | FOLFOX Induced Takotsubo Cardiomyopathy Treated with Impella Assist Device | 1 |
| T. Attisano, A. Silverio, C. Prota et al. | ESC Heart Failure | 2020 | Impella in Takotsubo syndrome complicated by left ventricular outflow tract obstruction and severe mitral regurgitation | 1 |
| T. Bleser, C. Weth and G. Gorge | Med Klin Intensivmed Notfmed | 2013 | Reverse Takotsubo cardiomyopathy-a life-threatening disease. Successful resuscitation of a 31-year-old woman with cardiologic shock after a visit to the dentist | 1 |
| T. E. Pearson, M. A. Frizzola, M. A. Priest et al. | Air Med J | 2018 | Pediatric Extracorporeal Cardiopulmonary Resuscitation Patient With Traumatic Subarachnoid Hemorrhage and Takotsubo Syndrome | 1 |
| T. K. Yoo, J. Y. Lee, K. C. Sung et al. | J Cardiovasc Ultrasound | 2016 | Stress-Induced Cardiomyopathy Presenting as Shock | 1 |
| T. Lyu, J. Niu, Z. Liu et al. | Front Cardiovasc Med | 2022 | Case Report: Early Resection of Pheochromocytoma in a Patient With Cardiogenic Shock Due to Pheochromocytoma-Induced Cardiomyopathy With Extracorporeal Life Support | 1 |
| V. V. Garla, S. Gosi, S. Kanduri et al. | BMJ Case Rep | 2019 | A case of catecholamine-induced cardiomyopathy treated with extracorporeal membrane oxygenation | 1 |
| X. Fan, P. Liu and L. Bai | Eur Heart J Case Rep | 2022 | Cardiogenic shock due to Takotsubo cardiomyopathy associated with thyroid crisis: a case report | 1 |
| Y. Luo, X. Ye, L. Zhang et al. | Echocardiography | 2023 | A rare case of cardiogenic shock caused by Takotsubo syndrome associated with SARS-CoV-2 infection: The value of echocardiography in the diagnosis and monitoring of the efficacy of extracorporeal membrane oxygenation | 1 |
| Y. Xie, A. Zhang, M. Qi et al. | BMC Endocr Disord | 2023 | Pheochromocytoma crisis with refractory Acute Respiratory Distress Syndrome (ARDS), Takotsubo syndrome, emergency adrenalectomy, and need for Extracorporeal Membrane Oxygenation (ECMO) in a previously undiagnosed and asymptomatic patient, due to the use of metoclopramide | 1 |
| Y. Y. Jo, S. Park and Y. S. Choi | Anaesth Intensive Care | 2011 | Extracorporeal membrane oxygenation in a patient with stress-induced cardiomyopathy after caesarean section | 1 |
| Z. Su, Y. Wang and H. Fei | CASE (Phila) | 2019 | Takotsubo-Like Cardiomyopathy in Pheochromocytoma | 1 |
| Z. Y. Zhang, J. J. Sun, J. H. Wang et al. | BMC Cardiovasc Disord | 2023 | Successful treatment of a severe Takotsubo syndrome case complicated by liver abscess | 1 |
References
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current state of knowledge on Takotsubo syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef]
- Stiermaier, T.; Eitel, C.; Desch, S.; Fuernau, G.; Schuler, G.; Thiele, H.; Eitel, I. Incidence, determinants and prognostic relevance of cardiogenic shock in patients with Takotsubo cardiomyopathy. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 489–496. [Google Scholar] [CrossRef]
- Matta, A.G.; Carrie, D. Epidemiology, Pathophysiology, Diagnosis, and Principles of Management of Takotsubo Cardiomyopathy: A Review. Med. Sci. Monit. 2023, 29, e939020. [Google Scholar] [CrossRef]
- Jabri, A.; Kalra, A.; Kumar, A.; Alameh, A.; Adroja, S.; Bashir, H.; Nowacki, A.S.; Shah, R.; Khubber, S.; Kanaa, N.A.; et al. Incidence of Stress Cardiomyopathy During the Coronavirus Disease 2019 Pandemic. JAMA Netw Open. 2020, 3, e2014780. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Athanasiadis, A.; Schwab, J.; Pistner, W.; Gottwald, U.; Schoeller, R.; Toepel, W.; Winter, K.D.; Stellbrink, C.; Müller-Honold, T.; et al. Complications in the clinical course of tako-tsubo cardiomyopathy. Int. J. Cardiol. 2014, 176, 199–205. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Dunlay, S.M.; Murphree, D.H.; Barsness, G.W., Jr.; Sandhu, G.S.; Lerman, A.; Prasad, A. Cardiogenic Shock in Takotsubo Cardiomyopathy Versus Acute Myocardial Infarction: An 8-Year National Perspective on Clinical Characteristics, Management, and Outcomes. JACC Heart Fail. 2019, 7, 469–476. [Google Scholar] [CrossRef]
- Madhavan, M.; Rihal, C.S.; Lerman, A.; Prasad, A. Acute heart failure in apical ballooning syndrome (TakoTsubo/stress cardiomyopathy): Clinical correlates and Mayo Clinic risk score. J. Am. Coll. Cardiol. 2011, 57, 1400–1401. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Cammann, V.L.; Jurisic, S.; Seifert, B.; Napp, L.C.; Diekmann, J.; Bataiosu, D.R.; D’Ascenzo, F.; Ding, K.J.; Sarcon, A.; et al. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: Results from the International Takotsubo Registry. Eur. J. Heart Fail. 2017, 19, 1036–1042. [Google Scholar] [CrossRef]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [PubMed]
- Napierkowski, S.; Banerjee, U.; Anderson, H.V.; Charitakis, K.; Madjid, M.; Smalling, R.W.; Dhoble, A. Trends and Impact of the Use of Mechanical Circulatory Support for Cardiogenic Shock Secondary to Takotsubo Cardiomyopathy. Am. J. Cardiol. 2021, 139, 28–33. [Google Scholar] [CrossRef]
- Terasaki, S.; Kanaoka, K.; Nakai, M.; Sumita, Y.; Onoue, K.; Soeda, T.; Watanabe, M.; Miyamoto, Y.; Saito, Y. Outcomes of catecholamine and/or mechanical support in Takotsubo syndrome. Heart 2022, 108, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Di Vece, D.; Citro, R.; Cammann, V.L.; Kato, K.; Gili, S.; Szawan, K.A.; Micek, J.; Jurisic, S.; Ding, K.J.; Bacchi, B.; et al. Outcomes Associated With Cardiogenic Shock in Takotsubo Syndrome. Circulation 2019, 139, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Elapavaluru, S.; Gologorsky, A.; Thai, N.; Uemura, T.; Khalil, R.; Mareddy, C.; Dishart, M.; Gologorsky, E. Perioperative Stress Cardiomyopathy in Simultaneous Liver and Kidney Transplantation: A Call for Early Consideration of Mechanical Circulatory Support. J. Cardiothorac. Vasc. Anesth. 2017, 31, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Nunez Gil, I.J.; Stiermaier, T.; El-Battrawy, I.; Moeller, C.; Guerra, F.; Novo, G.; Arcari, L.; Musumeci, B.; Cacciotti, L.; et al. Impact of intra-aortic balloon counterpulsation on all-cause mortality among patients with Takotsubo syndrome complicated by cardiogenic shock: Results from the German-Italian-Spanish (GEIST) registry. Eur. Heart J. Open 2023, 3, oead003. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Richter, J.; Pappalardo, F.; Bělohlávek, J.; Lorusso, R.; Schmitto, J.D.; Bauersachs, J.; Napp, L.C. Mechanical circulatory support for Takotsubo syndrome: A systematic review and meta-analysis. Int. J. Cardiol. 2020, 316, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nersesian, G.; Hennig, F.; Muller, M.; Mulzer, J.; Tsyganenko, D.; Starck, C.; Gromann, T.; Falk, V.; Potapov, E.; Schoenrath, F. Temporary mechanical circulatory support for refractory heart failure: The German Heart Center Berlin experience. Ann. Cardiothorac. Surg. 2019, 8, 76–83. [Google Scholar] [CrossRef]
- Eulert-Grehn, J.J.; Starck, C.; Kempfert, J.; Falk, V.; Potapov, E. ECMELLA 2.0: Single Arterial Access Technique for a Staged Approach in Cardiogenic Shock. Ann. Thorac. Surg. 2021, 111, e135–e137. [Google Scholar] [CrossRef]
- Wert, L.; Kempfert, J.; Falk, V.; Potapov, E.V. Transaxillary implantation of a temporary microaxial left ventricular assist device in a patient with a rectangular kinked subclavian artery. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad088. [Google Scholar] [CrossRef]
- Schrage, B.; Becher, P.M.; Bernhardt, A.; Bezerra, H.; Blankenberg, S.; Brunner, S.; Colson, P.; Cudemus Deseda, G.; Dabboura, S.; Eckner, D.; et al. Left Ventricular Unloading Is Associated With Lower Mortality in Patients With Cardiogenic Shock Treated With Venoarterial Extracorporeal Membrane Oxygenation: Results From an International, Multicenter Cohort Study. Circulation 2020, 142, 2095–2106. [Google Scholar] [CrossRef]
- Bairashevskaia, A.V.; Belogubova, S.Y.; Kondratiuk, M.R.; Rudnova, D.S.; Sologova, S.S.; Tereshkina, O.I.; Avakyan, E.I. Update of Takotsubo cardiomyopathy: Present experience and outlook for the future. Int. J. Cardiol. Heart Vasc. 2022, 39, 100990. [Google Scholar] [CrossRef]
- Nishikawa, R.; Nagano, N.; Kokubu, N.; Hashimoto, K.; Nakata, J.; Kishiue, N.; Takahashi, R.; Otomo, S.; Tsuchihashi, K.; Yano, T. Favorable Effects of Impella on Takotsubo Syndrome Complicated with Cardiogenic Shock. Int. Heart J. 2021, 62, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Napp, L.C.; Westenfeld, R.; Møller, J.E.; Pappalardo, F.; Ibrahim, K.; Bonello, L.; Wilkins, C.; Pershad, A.; Mannino, S.F.; Schreiber, T.L.; et al. Impella Mechanical Circulatory Support for Takotsubo Syndrome With Shock: A Retrospective Multicenter Analysis. Cardiovasc. Revasc. Med. 2022, 40, 113–119. [Google Scholar] [CrossRef]
- Barsoum, E.; Elhosseiny, S.; Patel, B.; Pathak, S.; Patel, A.; Vaidya, P. Successful use of the impella ventricular assist device for management of reverse Takotsubo Cardiomyopathy in the setting of acute intracranial hemorrhage. Heart Lung 2021, 50, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, B.; Reed, B.N. Use of an argatroban-based purge solution in a percutaneous ventricular assist device. Am. J. Health Syst. Pharm. 2017, 74, e163–e169. [Google Scholar] [CrossRef]
- Attisano, T.; Silverio, A.; Prota, C.; Briguori, C.; Galasso, G.; Citro, R. Impella in Takotsubo syndrome complicated by left ventricular outflow tract obstruction and severe mitral regurgitation. ESC Heart Fail. 2020, 7, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bonacchi, M.; Maiani, M.; Harmelin, G.; Sani, G. Intractable cardiogenic shock in stress cardiomyopathy with left ventricular outflow tract obstruction: Is extra-corporeal life support the best treatment? Eur. J. Heart Fail. 2009, 11, 721–727. [Google Scholar] [CrossRef]
- Bonacchi, M.; Valente, S.; Harmelin, G.; Gensini, G.F.; Sani, G. Extracorporeal life support as ultimate strategy for refractory severe cardiogenic shock induced by Tako-tsubo cardiomyopathy: A new effective therapeutic option. Artif. Organs 2009, 33, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Lazcano-Diaz, E.A.; Gonzalez-Ruiz, F.J.; Sarre-Alvarez, D.; Alvarez-Alvarez, R.J.; Bucio-Reta, E.; Garcia-Cruz, E.; Rojas-Velazco, G.; Ramos-Enriquez, A.; Melano-Carranza, E.; Santos-Martinez, L.E.; et al. Circulatory support with venoarterial ECMO in a patient with biventricular Takotsubo cardiomyopathy. Arch. Cardiol. Mex. 2020, 91, 100–104. [Google Scholar] [CrossRef]
- Redfors, B.; Shao, Y.; Omerovic, E. “Primum nil nocere” first choice in Takotsubo cardiomyopathy. Mechanical support as a bridge to recovery should be tested in severe cases. Lakartidningen 2014, 111, 1366–1369. [Google Scholar] [PubMed]
- Besnier, E.; Hubscher, C.; Doguet, F.; Bessou, J.P.; Dureuil, B. Extracorporeal membrane oxygenation support for tako-tsubo syndrome after urgent caesarean section. Ann. Fr. Anesth. Reanim. 2013, 32, 704–706. [Google Scholar] [CrossRef]
- Martin-Villen, L.; Corcia-Palomo, Y.; Escalona-Rodriguez, S.; Roldan-Reina, A.; Acosta-Delgado, D.; Martin-Bermudez, R. Extracorporeal membrane oxygenation support in a patient with pheochromocytoma stress myocardyopathy. Med. Intensiv. (Engl. Ed.) 2018, 42, 566–568. [Google Scholar] [CrossRef]
- Garcia-Delgado, M.; Garcia-Huertas, D.; Navarrete-Sanchez, I.; Olivencia-Pena, L.; Garrido, J.M. Extracorporeal membrane oxygenation support for Takotsubo syndrome and long QT after cardiac surgery. Med. Intensiv. 2017, 41, 441–443. [Google Scholar] [CrossRef]
- Esnault, P.; Nee, L.; Signouret, T.; Jaussaud, N.; Kerbaul, F. Reverse Takotsubo cardiomyopathy after iatrogenic epinephrine injection requiring percutaneous extracorporeal membrane oxygenation. Can. J. Anaesth. 2014, 61, 1093–1097. [Google Scholar] [CrossRef]
- Nagao, T.; Ohwada, T.; Hashimoto, M.; Aoki, K.; Shimizu, H.; Katayama, M. Intra-aortic balloon pumping is effective for hemodynamic management of catecholamine resistant ampulla (Takotsubo) cardiomyopathy. Masui 2004, 53, 799–802. [Google Scholar]
- Vachiat, A.; McCutcheon, K.; Mahomed, A.; Schleicher, G.; Brand, L.; Botha, J.; Sussman, M.; Manga, P. Takotsubo cardiomyopathy post liver transplantation. Cardiovasc. J. Afr. 2016, 27, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Hassid, B.; Azmoon, S.; Aronow, W.S.; Palaniswamy, C.; Cohen, M.; Gass, A. Hemodynamic support with TandemHeart in tako-tsubo cardiomyopathy—A case report. Arch. Med. Sci. 2010, 6, 971–975. [Google Scholar] [CrossRef]
- Aalberts, J.J.J.; Klinkenberg, T.J.; Mariani, M.A.; van der Harst, P. Mechanical circulatory support for refractory cardiogenic shock in Takotsubo syndrome: A case report and review of the literature. Eur. Heart J. Case Rep. 2017, 1, ytx005. [Google Scholar] [CrossRef] [PubMed]
- Moguilevitch, M.; Rufino, M.; Leff, J.; Delphin, E. Novel approach for heart failure treatment after liver transplantation. Liver Transplant. 2015, 21, 1103–1104. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Lee, J.Y.; Sung, K.C.; Oh, S.S.; Song, Y.S.; Lee, S.J.; Ko, K.J. Stress-Induced Cardiomyopathy Presenting as Shock. J. Cardiovasc. Ultrasound 2016, 24, 79–83. [Google Scholar] [CrossRef][Green Version]
- Ghanim, D.; Adler, Z.; Qarawani, D.; Kusniec, F.; Amir, O.; Carasso, S. Takotsubo cardiomyopathy caused by epinephrine-treated bee sting anaphylaxis: A case report. J. Med. Case Rep. 2015, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Hamid, T.E.f.J.; Fraser, D.; Fath-Ordoubadi, F. Use of the Impella Left Ventricular Assist Device as a Bridge to Recovery in a Patient with Cardiogenic Shock Related to Takotsubo Cardiomyopathy. J. Clin. Exp. Cardiol. 2013, 4, 246. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, L.; Ruan, X.; He, K.; Yu, C.; Shen, L. Case Report: Takotsubo Syndrome Induced by Severe Anaphylactic Reaction During Anesthesia Induction and Subsequent High-Dose Epinephrine Resuscitation. Front. Cardiovasc. Med. 2022, 9, 842440. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.E.; Frizzola, M.A.; Priest, M.A.; Rochman, M.F.; Froehlich, C.D. Pediatric Extracorporeal Cardiopulmonary Resuscitation Patient With Traumatic Subarachnoid Hemorrhage and Takotsubo Syndrome. Air Med. J. 2018, 37, 64–66. [Google Scholar] [CrossRef]
- Flam, B.; Broome, M.; Frenckner, B.; Branstrom, R.; Bell, M. Pheochromocytoma-Induced Inverted Takotsubo-Like Cardiomyopathy Leading to Cardiogenic Shock Successfully Treated With Extracorporeal Membrane Oxygenation. J. Intensive Care Med. 2015, 30, 365–372. [Google Scholar] [CrossRef]
- Faria, B.M.M.; Portugues, J.; Roncon-Albuquerque, R.; Pimentel, R., Jr. Inverted takotsubo syndrome complicated with cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation in a patient with bilateral pheochromocytoma: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- Dominedo, C.; D’Avino, E.; Martinotti, A.; Cingolani, E. A rare pheochromocytoma complicated by cardiogenic shock and posterior reversible encephalopathy syndrome: Case report. Eur. Heart J. Case Rep. 2021, 5, ytaa513. [Google Scholar] [CrossRef]
- van Zwet, C.J.; Rist, A.; Haeussler, A.; Graves, K.; Zollinger, A.; Blumenthal, S. Extracorporeal Membrane Oxygenation for Treatment of Acute Inverted Takotsubo-Like Cardiomyopathy From Hemorrhagic Pheochromocytoma in Late Pregnancy. A A Case Rep. 2016, 7, 196–199. [Google Scholar] [CrossRef]
- Zilio, F.; Muraglia, S.; Bonmassari, R. Cardiac arrest complicating cardiogenic shock: From pathophysiological insights to Impella-assisted cardiopulmonary resuscitation in a pheochromocytoma-induced Takotsubo cardiomyopathy-a case report. Eur. Heart J. Case Rep. 2021, 5, ytab092. [Google Scholar] [CrossRef]
- Mierke, J.; Loehn, T.; Linke, A.; Ibrahim, K. Reverse takotsubo cardiomyopathy- life-threatening symptom of an incidental pheochromocytoma: A case report. Eur. Heart J. Case Rep. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Kiamanesh, O.; Vu, E.N.; Webber, D.L.; Lau, E.; Kapeluto, J.E.; Stuart, H.; Wood, D.A.; Wong, G.C. Pheochromocytoma-Induced Takotsubo Syndrome Treated With Extracorporeal Membrane Oxygenation: Beware of the Apical Sparing Pattern. JACC Case Rep. 2019, 1, 85–90. [Google Scholar] [CrossRef]
- Kaese, S.; Schulke, C.; Fischer, D.; Lebiedz, P. Pheochromocytoma-induced takotsubo-like cardiomyopathy and global heart failure with need for extracorporal life support. Intensive Care Med. 2013, 39, 1473–1474. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.; Lee, D.I.; Lee, J.H.; Kim, S.; Lee, S.Y.; Bae, J.W.; Hwang, K.K.; Kim, D.W.; Cho, M.C.; et al. Successful extracorporeal membrane oxygenation treatment of catecholamine-induced cardiomyopathy-associated pheochromocytoma. Acute Crit. Care 2022. ahead-of-print. [Google Scholar]
- Garla, V.V.; Gosi, S.; Kanduri, S.; Lien, L. A case of catecholamine-induced cardiomyopathy treated with extracorporeal membrane oxygenation. BMJ Case Rep. 2019, 12, e230196. [Google Scholar] [CrossRef]
- Su, Z.; Wang, Y.; Fei, H. Takotsubo-Like Cardiomyopathy in Pheochromocytoma. CASE 2019, 3, 157–161. [Google Scholar] [CrossRef]
- Lyu, T.; Niu, J.; Liu, Z.; Li, T. Case Report: Early Resection of Pheochromocytoma in a Patient With Cardiogenic Shock Due to Pheochromocytoma-Induced Cardiomyopathy With Extracorporeal Life Support. Front. Cardiovasc. Med. 2022, 9, 788644. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, A.; Qi, M.; Xiong, B.; Zhang, S.; Zhou, J.; Cao, Y. Pheochromocytoma crisis with refractory Acute Respiratory Distress Syndrome (ARDS), Takotsubo syndrome, emergency adrenalectomy, and need for Extracorporeal Membrane Oxygenation (ECMO) in a previously undiagnosed and asymptomatic patient, due to the use of metoclopramide. BMC Endocr. Disord. 2023, 23, 145. [Google Scholar]
- Tiwari, A.K.; D’Attellis, N. Intraoperative left ventricular apical ballooning: Transient Takotsubo cardiomyopathy during orthotopic liver transplantation. J. Cardiothorac. Vasc. Anesth. 2008, 22, 442–445. [Google Scholar] [CrossRef]
- Caturegli, G.; Crane, M.A.; Etchill, E.; Giuliano, K.; Nguyen, M.; Philosophe, B.; Cho, S.M.; Wittstein, I.S.; Whitman, G.J.R. Stress-Induced (Takotsubo) Cardiomyopathy After Liver Transplant Rescued with Venoarterial Extracorporeal Membrane Oxygenation. ASAIO J. 2022, 68, e66–e68. [Google Scholar] [CrossRef] [PubMed]
- Omosule, A.; Malik, M.F.; Cisneros, L.; Guruswamy, J. Takotsubo Cardiomyopathy After Double-Lung Transplantation: Role of Early Extracorporeal Membrane Oxygenation Support. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2503–2507. [Google Scholar] [CrossRef] [PubMed]
- Kirigaya, J.; Iwahashi, N.; Tanaka, R.; Inayama, Y.; Takeuchi, I. A Fatal Case of Takotsubo Cardiomyopathy Secondary to Refractory Hypoglycemia in Severe Starvation: An Autopsy Case Report. Cureus 2022, 14, e23287. [Google Scholar] [CrossRef] [PubMed]
- Hekimian, G.; Kharcha, F.; Brechot, N.; Schmidt, M.; Ghander, C.; Lebreton, G.; Girerd, X.; Tresallet, C.; Trouillet, J.L.; Leprince, P.; et al. Extracorporeal membrane oxygenation for pheochromocytoma-induced cardiogenic shock. Ann. Intensive Care. 2016, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Bonacchi, M.; Vannini, A.; Harmelin, G.; Batacchi, S.; Bugetti, M.; Sani, G.; Peris, A. Inverted-Takotsubo cardiomyopathy: Severe refractory heart failure in poly-trauma patients saved by emergency extracorporeal life support. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.T.; Apridonidze, T.; Mopala, P.R.; Sherman, A.E.; Potakamuri, L.N.; Storms, D.R.; Kono, A.T.; Mohanty, B.D. Stress-Induced Cardiomyopathy Complicated by Dynamic Left Ventricular Outflow Obstruction, Cardiogenic Shock, and Ventricular Septal Rupture. Can. J. Cardiol. 2019, 35, 229.e7–229.e9. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Ramsdale, D. Tako-tsubo, hypertrophic obstructive cardiomyopathy & muscle bridging--separate disease entities or a single condition? Int. J. Cardiol. 2011, 147, 133–134. [Google Scholar] [PubMed]
- Laghlam, D.; Touboul, O.; Herry, M.; Estagnasie, P.; Dib, J.C.; Baccouche, M.; Brusset, A.; Nguyen, L.S.; Squara, P. Takotsubo cardiomyopathy after cardiac surgery: A case-series and systematic review of literature. Front. Cardiovasc. Med. 2022, 9, 1067444. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Kato, K.; Cammann, V.L.; Gili, S.; Jurisic, S.; Di Vece, D.; Candreva, A.; Ding, K.J.; Micek, J.; Szawan, K.A.; et al. Long-Term Prognosis of Patients With Takotsubo Syndrome. J. Am. Coll. Cardiol. 2018, 72, 874–882. [Google Scholar] [CrossRef]
- Almeida Junior, G.L.G.; Mansur Filho, J.; Albuquerque, D.C.; Xavier, S.S.; Pontes, A.; Gouvea, E.P.; Martins, A.B.B.; Nunes, N.S.V.; Carestiato, L.V.; Petriz, J.L.F.; et al. Takotsubo Multicenter Registry (REMUTA)—Clinical Aspects, In-Hospital Outcomes, and Long-Term Mortality. Arq. Bras. Cardiol. 2020, 115, 207–216. [Google Scholar] [CrossRef]
- Sangen, H.; Imori, Y.; Tara, S.; Yamamoto, T.; Takano, H.; Shimizu, W. Haemodynamic deterioration due to intra-aortic balloon counterpulsation in takotsubo cardiomyopathy. Eur. Heart J. 2018, 39, 2118. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Nakagaito, M.; Hori, M.; Ueno, H.; Kinugawa, K. A case of Takotsubo cardiomyopathy with cardiogenic shock after influenza infection successfully recovered by IMPELLA support. J. Artif. Organs. 2019, 22, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Oh, I.D.; Shin, E.; Lee, S.; Jeon, J.M.; Kim, H.T.; Youn, H.C. Extracorporeal membrane oxygenation for takotsubo cardiomyopathy that developed after mitral valve replacement. Acute Crit. Care 2020, 35, 51–55. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Park, S.; Choi, Y.S. Extracorporeal membrane oxygenation in a patient with stress-induced cardiomyopathy after caesarean section. Anaesth. Intensive Care 2011, 39, 954–957. [Google Scholar] [CrossRef]
- Rashed, A.; Won, S.; Saad, M.; Schreiber, T. Use of the Impella 2.5 left ventricular assist device in a patient with cardiogenic shock secondary to takotsubo cardiomyopathy. BMJ Case Rep. 2015, 2015, bcr2014208354. [Google Scholar] [CrossRef]
- Mohammedzein, A.; Taha, A.; Salwan, A.; Nambiar, R. Impella Use in Cardiogenic Shock Due to Takotsubo Cardiomyopathy With Left Ventricular Outflow Tract Obstruction. JACC Case Rep. 2019, 1, 161–165. [Google Scholar] [CrossRef]

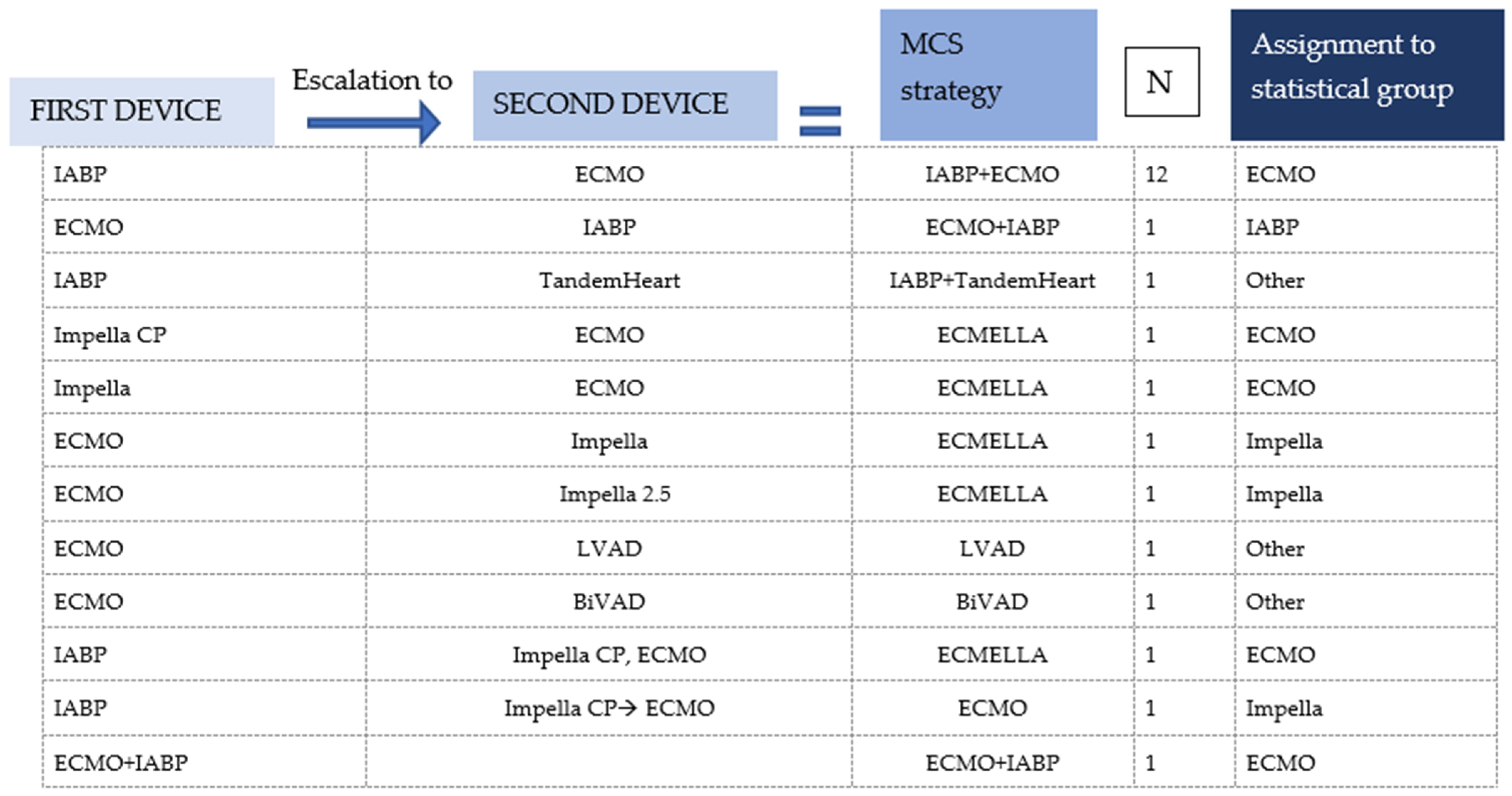
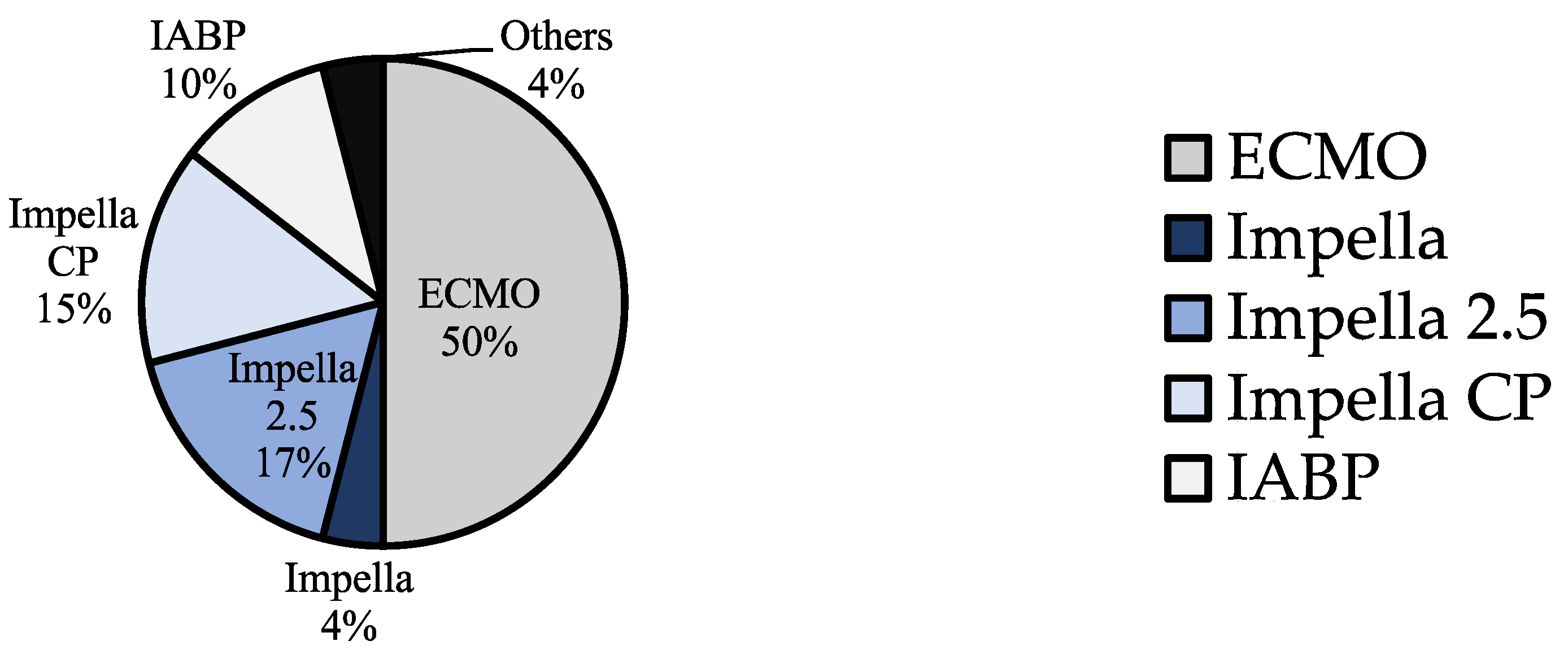


| ECMO | Impella | IABP | Others | All | p | |
|---|---|---|---|---|---|---|
| N | 62 | 44 | 13 | 5 | 124 | |
| Impella CP | 18 (40.9%) | |||||
| Impella 2.5 | 21 (47.7%) | |||||
| Age, median (IQR) | 45 (31–56) | 68 (55–76) | 67.5 (48–69.75) | 46 (26–53) | 52.2 | |
| Sex category | 0.275 | |||||
| Female | 42 (67.7%) | 34 (77.3%) | 12 (92.3%) | 4 (80%) | 92 (74.2%) | |
| Male | 20 (32.3%) | 10 (22.7%) | 1 (7.7%) | 1 (20%) | 32 (25.8%) | |
| Inverted TTS | 17 (27.4)% | 4 (9.1%) | 2 (15.4%) | 1 (20%) | 24 (19.4%) | 0.099 |
| RV involved | 6 (9.7%) | 1 (2.3%) | 1 (7.7%) | 1 (20%) | 9 (7.3%) | 0.205 |
| ECLS | Impella | IABP | Others | All | p | |
|---|---|---|---|---|---|---|
| Initial LVEF, median (IQR) | 20% (15–24) (n = 43) | 19.4% (19.4–21.35) (n = 38) | 30% (22.5–33) (n = 9) | 20% (9.5–28.5) (n = 5) | 20% (n = 95) | 0.015 |
| Inotropes before MCS | 47 (75.8%) | 35 (79.5%) | 11 (84.6%) | 5 (100%) | 98 (79%) | 0.876 |
| Respiratory failure | 43 (69.4%) | 13 (29.5%) | 3 (23.1%) | 2 (40%) | 61 (49.2%) | <0.001 |
| Arrhythmia | 19 (30.6%) | 14 (31.8%) | 6 (46.2%) | 3 (60%) | 42 (33.9%) | 0.002 |
| ST elevation | 24 (38.7%) | 22 (50%) | 5 (38.5%) | 2 (40%) | 53 (42.7%) | 0.88 |
| ST depression | 10 (16.1%) | 5 (11.4%) | 3 (23.1%) | 0 | 18 (14.5) | 0.002 |
| T-wave inversion | 16 (25.8%) | 6 (13.6%) | 4 (30.8%) | 0 | 26 (21%) | 0.002 |
| Troponin elevation | 44 (71%) | 24 (54.5%) | 11 (84.6%) | 2 (40%) | 81 (65.3%) | 0.067 |
| Cardiac arrest | 23 (37.1%) | 9 (20.5%) | 3 (32.1%) | 2 (40%) | 37 (29.8%) | 0.081 |
| Angiography before MCS | 31 (50%) | 41 (93.2%) | 10 (76.9%) | 1 (20%) | 83 (66.9%) | <0.001 |
| NTproBNP, mean | 8293.5 (n = 13) | 13,697.4 (n = 5) | 1832.0 (n = 3) | 22,842 (n = 1) | 9301.85 (n = 22) | 0.089 |
| BNP, mean | 6342.3 (n = 8) | 946 (n = 3) | 4900.0 (n = 1) | n = 0 | 4873 (n = 12) | |
| BP syst in mmHg, median (IQR) /MAP in mmHg, mean | 95 (79–141) (n = 35) /59.5 (n = 2) | 80 (73–100) (n = 31) /50 (n = 3) | 82 (80–115) (n = 6) /51.5 (n = 4) | 74 (n = 2) /(n = 0) | 0.134/0.399 | |
| HR median (IQR) | 129.5 (100.25–148.75) (n = 32) | 126 (106.25–136.75) (n = 28) | 127 (111–147) (n = 4) | 121 (n = 2) | 0.933 | |
| LVEDP, median (IQR) | 25 (n = 3) | 26 (23–30) (n = 11) | 12 (n = 2) | (n = 0) | 0.357 | |
| Initial lactate, median (IQR) | 69.8 (52.6–116.2) (n = 18) | 44.1 (27.5–57.6) (n = 22) | 104 (n = 2) | (n = 0) | 0.038 | |
| Pheochromocytoma | 17 | 1 (ECMELLA n = 2) | (ECLS + IABP: n = 1) | 19 | ||
| Mitral regurgitation | 7 (11.3%) | 5 (11.4%) | 2 (15.4%) | 1 (20%) | 15 (12.1%) | 0.662 |
| Systolic anterior motion | 3 (4.8%) | 5 (11.4%) | 2 (15.4%) | 0 | 10 (8.1%) | |
| LVOTO | 3 (4.8%) | 7 (15.9%) | 2 (15.4%) | 0 | 12 (9.7%) | 0.174 |
| Emotional trigger | 16 (25.8%) | 17 (38.6%) | 15.9 (53.8%) | 4 (80%) | 44 (35.5%) | 0.002 |
| Physical trigger | 58 (93.5%) | 22 (50%) | 11 (84.6%) | 4 (80%) | 95 (76.6%) | <0.001 |
| Exogenous catecholamine trigger | 4 (6.5%) | 1 (2.3%) | 1 (7.7%) | 0 | 6 (4.8%) | 0.583 |
| ECLS | Impella | IABP | Others | All | p | |
|---|---|---|---|---|---|---|
| Survival in % | 54 (87.1%) | 37 (84.1%) | 11 (84.6%) | 5 (100%) | 107 (86.3%) | 0.855 |
| Hours on MCS, median (IQR) | 144 (72–192) (n = 55) | 72 (48–96) (n = 42) | 120 (72–144) (n = 11) | 168 (144–228) | 96 (n = 113) | <0.001 |
| MCS complications | 18 (29%) | 12 (27.3%9) | 3.8 (30.8%) | 2 (40%) | 36 (24.2%) | 0.927 |
| Follow-up | 45 (72.6%) | 21 (47.7%) | 9 (69.2%) | 4 (80%) | 79 (63.7%) | 0.049 |
| Max. follow-up in weeks, median (IQR) | 8 (4–24) (n = 45) | 4 (2–8) (n = 19) | 2 (1.75–9) (n = 9) | 28 (18–44) (n = 4) | 6 (n = 77) | 0.01 |
| LVEF in follow-up, median (IQR) | 55% (55–65) (n = 22) | 55.2% (n = 22) | 61% (n = 7) | 67% (57–72) (n = 5) | 58.5% (n = 56) | 0.262 |
| Full recovery | 47 (75.8%) | 32 (72.7%) | 8 (61.5%) | 5 (100%) | 92 (74.2%) | 0.458 |
| ECLS | Impella | IABP | Others | |
|---|---|---|---|---|
| Complications under MCS | 18 (29%) | 12 (27.3%) | 4 (30.8%) | 2 (40%) |
| Stroke | 1 (1.6%) | 0 | 0 | 0 |
| Prolonged weaning of MCS | 2 (3.2%) | 0 | 0 | 0 |
| Pericardial tamponade | 2 (3.2%) | 0 | 0 | 0 |
| MR development under MCS | 0 | 1 (2.3%) | 0 | 0 |
| Ventricle rupture | 0 | 1 (2.3%) | 0 | 0 |
| Development of atrial fibrillation under MCS | 1 (1.6%) | 1 (2.3%) | 0 | 0 |
| Haemolysis | 0 | 3 (6.8%) | 0 | 0 |
| Significant HB drop/bleeding | 4 (6.5%) | 5 (11.4%) | 1 (7.7%) | 0 |
| Significant thrombocytopenia | 3 (4.8%) | 3 (6.8%) | 0 | 0 |
| AKI | 3 (4.8%) | 3 (6.8%) | 0 | 0 |
| Vascular damage in the lower limb | 5 (8.1%) | 1 (2.3%) | 1 (7.7%) | 0 |
| Vascular damage in the upper limb | 1 (1.6%) | 0 | 0 | 0 |
| Death while on support | 0 | 1 (2.3%) | 2 (15.4%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Mackensen, J.K.R.; Zwaans, V.I.T.; El Shazly, A.; Van Praet, K.M.; Heck, R.; Starck, C.T.; Schoenrath, F.; Potapov, E.V.; Kempfert, J.; Jacobs, S.; et al. Mechanical Circulatory Support Strategies in Takotsubo Syndrome with Cardiogenic Shock: A Systematic Review. J. Clin. Med. 2024, 13, 473. https://doi.org/10.3390/jcm13020473
von Mackensen JKR, Zwaans VIT, El Shazly A, Van Praet KM, Heck R, Starck CT, Schoenrath F, Potapov EV, Kempfert J, Jacobs S, et al. Mechanical Circulatory Support Strategies in Takotsubo Syndrome with Cardiogenic Shock: A Systematic Review. Journal of Clinical Medicine. 2024; 13(2):473. https://doi.org/10.3390/jcm13020473
Chicago/Turabian Stylevon Mackensen, Johanna K. R., Vanessa I. T. Zwaans, Ahmed El Shazly, Karel M. Van Praet, Roland Heck, Christoph T. Starck, Felix Schoenrath, Evgenij V. Potapov, Joerg Kempfert, Stephan Jacobs, and et al. 2024. "Mechanical Circulatory Support Strategies in Takotsubo Syndrome with Cardiogenic Shock: A Systematic Review" Journal of Clinical Medicine 13, no. 2: 473. https://doi.org/10.3390/jcm13020473
APA Stylevon Mackensen, J. K. R., Zwaans, V. I. T., El Shazly, A., Van Praet, K. M., Heck, R., Starck, C. T., Schoenrath, F., Potapov, E. V., Kempfert, J., Jacobs, S., Falk, V., & Wert, L. (2024). Mechanical Circulatory Support Strategies in Takotsubo Syndrome with Cardiogenic Shock: A Systematic Review. Journal of Clinical Medicine, 13(2), 473. https://doi.org/10.3390/jcm13020473





