Abstract
Background: Takotsubo syndrome is, by definition, a reversible form of acute heart failure. If cardiac output is severely reduced, Takotsubo syndrome can cause cardiogenic shock, and mechanical circulatory support can serve as a bridge to recovery. To date, there are no recommendations on when to use mechanical circulatory support and on which device is particularly effective in this context. Our aim was to determine the best treatment strategy. Methods: A systematic literature research and analysis of individual patient data was performed in MEDLINE/PubMed according to PRISMA guidelines. Our research considered original works published until 31 July 2023. Results: A total of 93 publications that met the inclusion criteria were identified, providing individual data from 124 patients. Of these, 62 (50%) were treated with veno-arterial extracorporeal life support (va-ECLS), and 44 (35.5%) received a microaxial left ventricular assist device (Impella). Eighteen patients received an Impella CP and twenty-one an Impella 2.5. An intra-aortic balloon pump (IABP) without other devices was used in only 13 patients (10.5%), while other devices (BiVAD or Tandem Heart) were used in 5 patients (4%). The median initial left ventricular ejection fraction was 20%, with no difference between the four device groups except for the IABP group, which was less affected by cardiac output failure (p = 0.015). The overall survival was 86.3%. Compared to the other groups, the time to cardiac recovery was shorter with Impella (p < 0.001). Conclusions: Though the Impella treatment is new, our analysis may show a significant benefit of Impella compared to other MCS strategies for cardiogenic shock in Takotsubo syndrome.
1. Introduction
Takotsubo syndrome (TTS) is an acute and reversible manifestation of heart failure, commonly found in patients presenting with symptoms leading to acute coronary syndrome (ACS), especially women [,]. The 2019 coronavirus pandemic, as a global event with major psychosocial impacts, resulted in a dramatic increase in the number of TTS cases and further demonstrated the importance of TTS as a differential diagnosis of ACS. During the COVID-19 pandemic, the incidence of patients presenting with ACS with subsequently confirmed TTS increased to 7.8%, from 3% previously [,]. However, TTS remains underdiagnosed, largely due to the interplay of acute coronary syndrome.
The incidence of cardiogenic shock (CS) in patients with TTS is estimated as ranging between 6% and 20% [,]. Patients with TTS in CS have a high in-hospital mortality of 15% []. Both the Mayo Clinic Criteria [] and the InterTAK Diagnostic Criteria [] are used as instruments for diagnosing TTS. They include echocardiographic evidence of hypo-, a- or dyskinesia extending beyond a single coronary vascular territory, as well as exclusion by coronary angiography of coronary atherosclerosis as a causal agent of regional wall motion abnormalities. Physical or emotional triggers should additionally be detectable. The main difference between the two criteria is that, for the Mayo Clinic Criteria, the absence of pheochromocytoma must be confirmed, whereas the InterTAK Diagnostic Criteria postulate that certain neurological disorders (e.g., subarachnoid haemorrhage, stroke/transient ischaemic attack or seizures) as well as pheochromocytoma may serve as triggers for TTS [,]. Even though research has paid increasing attention to TTS in recent years, many aspects remain unclear. Besides its aetiology, which is not completely understood, the management of patients with TTS, especially when a severe course of stress cardiomyopathy leads to cardiogenic shock, is unclear and there is no evidence-based therapy concept available. As mechanical circulatory support (MCS) devices are increasingly used in patients with CS and are recommended for SCAI shock stages C, D and E [], they are also used in patients with TTS with good results [,,].
A so-called broken heart can be repaired mechanically. However, there are no recommendations for when MCS should be used and which device should be preferred. The literature increasingly recommends considering MCS as a bridge-to-recovery strategy in TTS-CS [,], which by definition is reversible, in order to minimise the use of inotropes, as these may further worsen the clinical picture, which is potentially triggered by catecholamines [].
Data on the use of MCS in TTS-CS are rare and only retrospective. The use of IABP was evaluated in a European registry of 2248 TTS patients, of whom 43 were patients with CS treated with IABP. The data show no significant difference in 30-day mortality, length of hospitalisation, need for invasive ventilation and 42-month follow-up []. A review and meta-analysis of MCS for TTS-CS by Mariani et al., including data until 2019, also confirms that the use of IABP is decreasing, while the use of va-ECLS and Impella in TTS patients with CS is increasing []. Since the microaxial LVAD Impella is a relatively new device, its frequent use is reflected only in the most recent literature; therefore, the review from Mariani et al. from 2020 included only 10 patients supported by Impella. The Impella is a non-durable microaxial left ventricular assist device (LVAD). Different surgical Impella procedures exist: the implantation of an Impella CP in combination with veno-arterial ECLS via the femoral artery is called ECMELLA 1.0 [], while the combination of a single axillary artery approach for an Impella CP with veno-arterial ECLS is referred to as ECMELLA 2.0 []. The various procedures are associated with different complications (e.g., wound infection rates) and possibilities (e.g., early patient mobilisation) []. In recent years, it has been increasingly suggested that microaxial LVADs may be of particular value in the treatment of CS caused by TTS, as they enable venting of the left ventricle [,,]. There is thus a growing interest to evaluate patients with TTS-CS who have been treated with Impella [,,,].
A retrospective multicentre analysis conducted in 2022 evaluated 16 patients with TTS-CS treated with Impella; the data indicated favourable outcomes, with good survival and full recovery of LV function [].
In general, the literature contains reports on the use of intra-aortic balloon pumps (IABP), TandemHeart, extracorporeal membrane oxygenation (ECLS) and microaxial pumps (i.e., Impella) for the treatment of TTS-CS. Since interest in this clinical cardiac entity is increasing, there is also a rapidly growing number of published case reports and series relating to the use of MCS devices in TTS-CS. To recognise the trends and outcomes of this currently rapidly growing population of patients with TTS-CS treated with MCS and to compare the different support systems that can be applied, we performed a systematic review and analysis of the existing literature.
2. Materials and Methods
This review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines []. Prior to the literature review, the concept, inclusion criteria, research question and hypothesis were defined. The aim was to compare the different devices used so far as MSC strategies in the context of TTS-CS in the existing literature. The underlying hypothesis was that left ventricular venting and physiological antegrade support provided by the microaxial Impella device allows the overloaded ventricle to recover faster and that MCS is therefore needed for a shorter period of time.
2.1. Search Strategies
A systematic search in PubMed of articles published until 31 July 2023 was carried out using the following mesh terms: (‘takotsubo’ or ‘tako-tsubo’ or ‘stress cardiomyopathy’ or ‘apical ballooning’) AND (‘ECLS’ or ‘ECMO’ or ‘Impella’ or ‘IABP’ or ‘mechanical support’ or ‘mechanical circulatory support’ or ‘MCS’ or ‘assist device’). The titles of the initial results were screened and all publications matching the inclusion criteria of confirmed TTS and use of MCS were imported into the reference management software EndNote 20® (Clarivate Analytics, London, UK). Duplicates were removed and the remaining publications were assessed for inclusion and screened for additional matching references. Full texts of all relevant articles were obtained and evaluated by the first author (Figure 1).
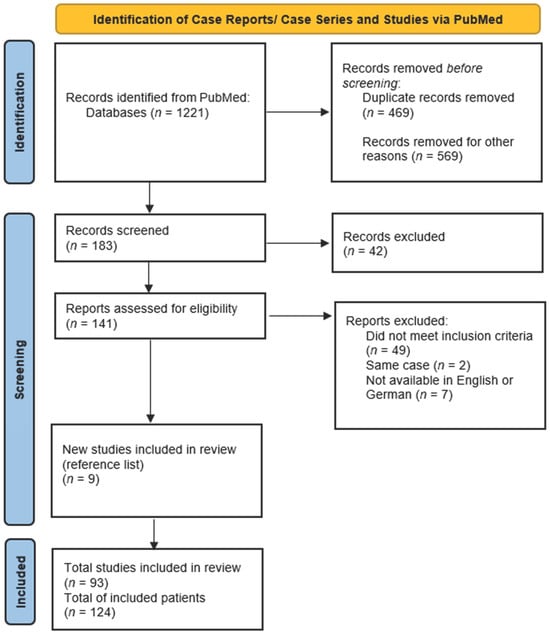
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) flowchart of the systematic literature review [].
2.2. Inclusion Criteria
Published case reports and case series as well as retrospective, observational or randomised studies among patients diagnosed with TTS were considered. Inclusion required documentation of the following criteria:
- TTS diagnosis consistent with the InterTAK Diagnostic Criteria and designated as such by the authors;
- Use of temporary MCS: IABP, ECLS, Impella, TandemHeart;
- Individually reported patient data in terms of pre-intervention status, survival and time on support.
Studies not written in English or German, poorly described cases and review articles were excluded. Case series or studies not providing primary data or where the analysis was pooled without a description of individual patient data were excluded.
2.3. Data Extraction
Data were extracted by the first author and independently checked for accuracy by the last author. Data extracted included authors, title, year of publication, patient demographics (age, sex), MCS strategy, vital signs before circulatory support (systolic/diastolic blood pressure or MAP, heart rate), respiratory failure, use of inotropes before MCS, performed angiography (LVEDP), cardiac arrest before MCS, new ECG abnormalities (ST elevation, ST depression and T wave inversion), new arrhythmias, LVEF (before MCS, on MCS and in follow-up), NTproBNP/BNP (baseline and follow-up), baseline lactate, presence of left ventricular outflow tract obstruction (LVOTO) (gradient) or mitral regurgitation (MR) (systolic anterior motion), trigger, TTS type (inverted or normal), RV involvement, time to weaning of inotropes under MCS, time on MCS and follow-up (last documented date after implantation, LV recovery and survival). Patients treated with more than one device were assigned to a device group for statistical analysis (see Figure 2). The assignment was based on the device to which the authors attributed therapy success. Thus, if MCS therapy was escalated to another device under which therapy success was achieved, the patient was assigned to the device group to which it was escalated.
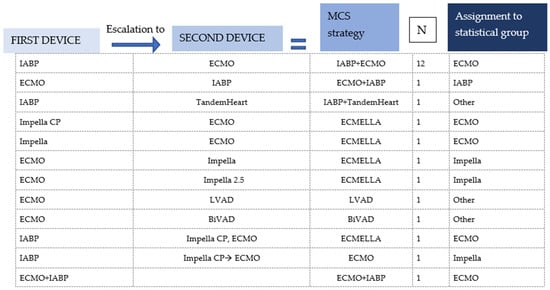
Figure 2.
Assignment to statistical group/single-device group of patients who were treated with more than one device and with a combination of two devices or where the first device was removed and replaced by another.
2.4. Statistics
Descriptive statistical analysis was performed by using the data analysis tools IBM® SPSS 29 (IBM, Armonk, New York, NY, USA) and R, Version 4.02.(R Project for Statistical Computing, the R Foundation for Statistical Computing, Vienna, Austria).
The following data were collected: median with interquartile range (IQR) or mean ± SD for the total group and for the separate device subgroups. Categorical variables are presented as N (%) and were compared using Fisher’s exact test. Metric data were analysed for >2 independent variables using the Friedman test or the Kruskal–Wallis test depending on the distribution (normal or non-normal). A two-sided p-value lower than 0.05 was considered statistically significant. To enable assignment of significant results, a pairwise comparison was performed if more than two variables (e.g., four device groups) were used.
3. Results
3.1. Literature Research
The search in PubMed yielded a total of 1221 publications. A screening of the titles identified 1038 irrelevant or double publications. A total of 183 articles were screened on the basis of the abstract; of these, 141 were classified as relevant and full-text reading was carried out. A screening of the reference list yielded a further nine publications. Forty-nine articles did not meet the inclusion criteria or provide relevant information and were therefore excluded. Two articles depicted the same case, so one was excluded [,]. Seven articles could not be included because the full text was not available in English or German [,,,,,,].
After full-text evaluation, 93 articles providing individual data on 124 cases were included (Appendix A). While the publications cover a period of almost 20 years, the majority were from the last five years (Figure 3).
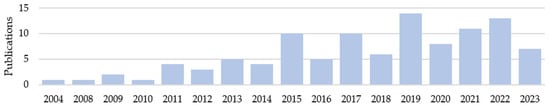
Figure 3.
Number of included publications by year.
The quality of case-related data in the publications varied widely. In particular, some articles were dedicated to specific aspects, e.g., echocardiographic control or therapy of the underlying condition, and therefore provided little general data. Overall, an almost complete data set could be collected for demographics, baseline LVEF and the duration of mechanical support. Especially information regarding follow-up and clinical status prior to the establishment of MCS, e.g., blood pressure, heart rate or catecholamines before MCS, varied greatly in terms of availability and quality. Unfortunately, important haemodynamic parameters such as right heart catheter findings or parameters of extended cardiac monitoring were not provided in the underlying literature.
3.2. Demographics and Device Groups
A data set of 124 patients was obtained, 92 of whom were female (74.2%). Five paediatric cases were included (age < 18 years). The median age was 52.2 years (Table 1).

Table 1.
Demographic characteristics (age and sex). Proportions of the Impella 2.5 and Impella CP subgroups in the Impella group. Percentage of patients in whom inverted TTS was described and percentage of patients in whom the right ventricle was also involved.
After assigning the patients with two or more devices to a device group (Figure 2), the patient collective was divided into four device groups: ECLS, Impella, IABP and Other (Figure 4). The ‘Other’ group included two cases where a TandemHeart was combined with IABP and Impella, as well as one case where central ECLS was established and two cases treated with a BiVAD [,,,,]. The Impella group was further subdivided according to the generation used: the Impella 2.5, which can increase cardiac output (CO) by about 2.5–3 L/min, was the most frequently used model, accounting for 47.7% of Impella cases. The Impella CP, which can relieve the left ventricle and support the circulation with a higher capacity of up to 4 L/min, was used in 40.9% of the cases. In 4% of the Impella cases, no exact model was named.
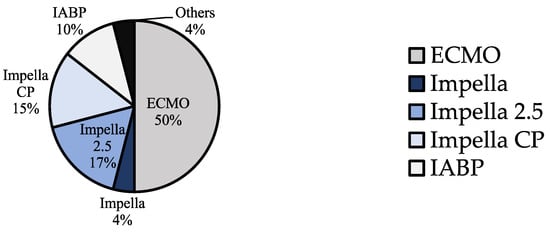
Figure 4.
Percentage of the different device groups (ECLS, Impella, Impella 2.5, Impella CP, IABP and Other) in the total collective of 124 patients with TTS and CS treated with MCS.
3.3. Pre-MCS Status of the Patients
In most cases, TTS was caused by physical triggers, but there were also cases in which a combination of physical and emotional triggers was present. Iatrogenic catecholamine administration was thought to be the cause of TTS-CS in six patients [,,,,]. Nineteen patients developed TTS-CS in the context of pheochromocytoma [,,,,,,,,,,,,], all of whom received ECLS as MCS, one in combination with IABP and one in combination with Impella. Due to pheochromocytoma, these patients exhibited a markedly higher blood pressure before MCS, with a mean systolic blood pressure of 140.9 mmHg. This is also reflected in the difference in the median systolic blood pressures between the different device groups, with the ECLS groups showing a median systolic blood pressure of 95 mmHg compared with 82 mmHg or lower in the other groups. Another difference in pre-implantation status between the groups was the frequency of cardiac catheterisation to rule out coronary obstruction. This is presumably attributable to the fact that both IABP as well as Impella 2.5 and CP are placed under angiographic support, which means that these patients inevitably visit the catheter laboratory; catheterisation is thus also mentioned in almost every case report. Another important difference is the presence of pulmonary oedema or respiratory failure. Patients who experienced a pulmonary complication or respiratory failure during CS more frequently received ECLS. There were no significant differences between the device groups with regard to cardiac arrest, ST elevations in the electrocardiogram (ECG), need for inotropes before implantation or elevated troponin levels.
Data between the groups regarding the clinical status of the patients before MCS therapy vary only for some parameters and are generally comparable (Table 2). However, for several parameters, data are only available from a fraction of the patients, making it difficult to draw conclusions as to the comparability of the groups. Regarding pre-implantation status, baseline LVEF is considered a key parameter for assessing the severity of cardiac compromise and the comparability of the severity of pump failure between the groups.

Table 2.
Clinical status before MCS implantation. Metric data are reported as mean with IQR (interquartile range) and number of available data (n). Nominal data are given in number of patients and % of the device group.
It should be emphasised that there was no significant difference in baseline LVEF across the various MCS groups. With a p value of 0.015, a significant result was found when comparing the median baseline LVEF values between the groups; however, a pairwise comparison showed that this significance existed only between IABP and the Impella as well as IABP and ECLS. The median values of baseline LVEF between the Impella and ECLS, however, did not differ significantly (p: 0.665).
3.4. Outcome and Follow-Up
In conclusion, the outcomes were good, with an overall survival rate of 86.3%. It should also be emphasised that in those cases where the patients died, they mostly suffered from very severe underlying diseases (two perioperative cases after liver transplant, one perioperative case after double-lung transplant, three cases with pheochromocytoma, two cases after polytrauma, one case with severe hypothermia and hypoglycaemia) [,,,,,]. In three cases, the authors did not attribute the patient’s death directly to the underlining condition. One suffered from advanced motor neuron disease and developed pneumonia and sepsis during MCS. One developed TTS after mitral valve reconstruction surgery. One case ‘merely’ experienced an emotional trigger but suffered from severe TTS; this patient died from the complications of MCS [,,]. Significant differences between the groups, not caused by insufficient or inconsistent data (length of follow-up), were found only with regard to median support duration. Here, the Impella device clearly showed significantly shorter support times, which were also confirmed in pairwise comparison with every other device. Especially interesting is that the analysis of the Impella subgroups regarding the duration of MCS (Figure 5) showed that the duration of support decreased with the performance of the Impella generation. In other words: the greater the degree of LV venting, the faster the heart recovers and mechanical support can be terminated.
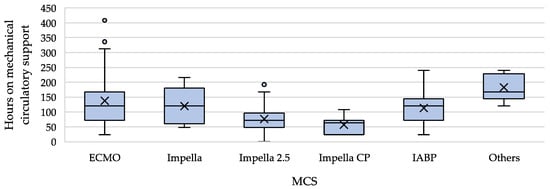
Figure 5.
Comparison of mechanical circulatory support duration with median, IQR, outliers and mean (=x). For the number of cases evaluated per group, see Table 3.
Unfortunately, it was hardly ever reported whether complications occurred during MCS therapy (Table 3). Furthermore, the recorded complications are not based on standardised definitions, but rather on what the authors of the primary literature report. In total, MCS complications were reported in 36 patients (29%). The occurrence of complications did not differ significantly between the groups (p = 0.927). Since several specific complications were mentioned in some cases and these were recorded, more individual complications are listed in some device groups in Table 4 than for the total number of patients suffering from complications in the device group. In a few publications, the persistence of cardiogenic shock under MCS was considered a complication, but because all patients in whom therapy was escalated to another device theoretically suffered from persistent CS under MCS, this category is not presented. Since the individual complication items are not based on common definitions, but merely on the fact that the authors of the case reports referred to them as such, and since complications under MCS were not mentioned in many articles, we deliberately refrained from performing a statistical evaluation of these parameters.

Table 3.
Outcome and follow-up data presented as metric data reported as means with IQRs (interquartile ranges) and number of available data (n). Nominal data given in number of patients and % of device group. Two side p-value of the statistical comparison of the different device groups.

Table 4.
Frequency of reported complications (multiple mentions per patient possible).
3.5. Comparison of the Device Groups
The collected data were analysed statistically with regard to the hypothesis that due to the pathophysiology of TTS-CS and the function of the different devices, the Impella is particularly suitable for circulatory support in TTS-CS. The data revealed that with comparable disease severity (especially baseline LVEF), patients in the Impella group required MCS for a significantly shorter period of time. However, there were no clear differences in the complication rates and overall survival (Figure 6).
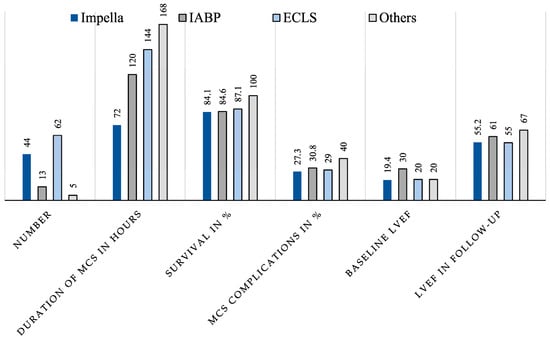
Figure 6.
Graphical representation of important parameters for comparing the four different device groups (Impella, IABP, ECMO, Other).
4. Discussion
The patient population with Takotsubo syndrome is a very heterogeneous group, both in terms of concomitant diseases and the triggers of TTS. Some patients develop TTS within the scope of a severe non-cardiac condition and therefore have a critical status simply because of their underlying disease. Other patients, however, develop TTS within the scope of an emotional stressor and are physically healthy until they develop TTS. In addition, the diagnostic criteria that can be applied differ with respect to the inclusion of patients who develop TTS in the context of a neurological disease or pheochromocytoma. These patients are included when the InterTAK criteria are applied, thus generating a much more heterogeneous patient population, even if cardiac shock develops in the context of TTS, since the usual parameters such as low systolic pressure cannot be used to assess the severity of the clinical status in patients with pheochromocytoma. In addition to the very heterogeneous patient population, this evaluation is grounded on a very heterogeneous and retrospective data base. Despite the retrospective and heterogeneous character of the data, there are indications that the data collected are representative, as they match existing data in many aspects. For example, a mortality of about 15% is described for patients with TTS-CS [,]. In the present data, the mortality was 13.7%. Other parameters such as the frequency of ECG changes or an increase in cardiac markers are also consistent with data collected in larger registries [,,,].
Concerning the question of which device might be appropriate for treating CS in TTS, the following considerations regarding the pathophysiology of TTS and the mechanisms of the devices can be made and are discussed in the literature underlying this review.
The intra-aortic balloon pump (IABP) works with counterpulsation. This therapy is only able to increase the cardiac index to a limited extent and can worsen or even cause LVOTO []. Another argument against IABP is that the rapid heartbeat that is frequently present in TTS-CS may be too fast for IABP to follow []. Furthermore, some studies suggest that IABP shows no advantage over optimal drug therapy without mechanical circulatory support [,].
Veno-arterial extracorporeal membrane oxygenation (v-a ECLS) is often used with the argument that the patient is suffering from respiratory failure on top of uncompensated acute heart failure despite inotropic and ventilatory support. Other reasons for the use of ECLS are that it also can be applied in children or patients who are too short for Impella [,].
One reason against using v-a ECLS is that, due to providing retrograde circulatory support, it increases LV afterload, which can further increase the already elevated left ventricular end-diastolic pressure (LVEDP).
The microaxial Impella pump provides physiological cardiac support which improves coronary and end-organ perfusion while simultaneously unloading the left ventricle. The main advantages of the Impella in TTS-CS are that cardiac output can be increased simultaneously with venting of the overloaded LV, thereby improving pulmonary congestion by reducing LV preload and increasing cardiac output regardless of the heart rate. Also, in several reports, the availability of the Impella was given as the reason for choosing this device [,,,,,].
The positive effect of LV unloading becomes apparent when comparing patients with CS treated with ECLS alone or with ECMELLA. LV unloading has been associated with a lower 30-day mortality [].
In order to interpret the outcome of the different device groups in this analysis, the gathered data must first be critically analysed with regard to their quality and comparability. Some parameters were only reported for a fraction of the patients in the primary literature. Other parameters, such as the categorisation of the trigger as emotional or physical, are not based on hard criteria, but on what the authors of the case reports write. The achievement of complete recovery is also a descriptive parameter, which was collected from the texts of the underlying literature and is not based on defined clinical parameters. However, some of the parameters were well reported: baseline LVEF was available for 76.6% of the patients, and the duration of mechanical circulatory support was available for 91.1% of patients. Other parameters that would have led to a more precise comparability of the groups were, unfortunately, not available at all.
It can be assumed that the patients who received the Impella for the treatment of CS in the context of TTS were equally affected by pump failure, represented by reduced LVEF, which was comparable between the groups; only the IABP group was significantly different with a better LVEF. Therefore, for the other groups, we can assume that the level of cardiac compromise was comparably severe. Additional other parameters such as NTproBNP, elevated troponin and cardiac arrest also indicate a comparable disease severity between the groups. The Impella group showed a significantly shorter duration of mechanical circulatory support. This finding, along with a comparable disease severity within the Impella group, may suggest that left ventricular venting is relevant for a rapid recovery from TTS.
Several publications about TTS-CS demonstrate high survival and recovery rates, also since TTS is, by definition, a form of temporary heart failure [,]. Therefore, when dealing with cardiogenic shock in the context of TTS, MCS can be used as part of a bridge-to-recovery concept. As the literature also clearly shows that the complications of MCS increase with time on support, MCS therapy should be administered for as short a time as possible to avoid complications []. For this reason, microaxial LVADs may be the best option for treating CS in TTS. It could therefore be assumed that in the present data, with significantly shorter support durations in the Impella group, significantly lower complication rates would also be expected. However, this was not the case. One reason for this could be that the Impella group also included data from the multicentre study of Impella use in TTS-CS by Napp et al., which also provides detailed data on the type and frequency of complications, unlike the majority of the case reports, which rarely focus on or even mention MCS complications. This demonstrates the urgent need for prospective multicentre studies investigating the use of MCS devices in TTS-CS with a critical eye on complications associated with MCS.
The available data are also insufficient to make a statement on the benefit of one device with regard to the recovery presented in the follow-up. A complicating factor here is that the underlying case reports often do not provide any information about the follow-up period or the clinical parameters that demonstrate recovery. In some cases, it was only described that complete recovery was achieved and that the patient was discharged home.
In conclusion, the data suggest that unloading the ballooned ventricle accelerates myocardial recovery, and that preference should be given to devices that increase neither the gradient across the LVOT nor the LVEDP. To provide evidence for the treatment of CS induced by TTS, prospective and multicentre studies investigating different MCS devices as well as mechanical versus conservative shock therapies in this specific setting are urgently needed.
5. Limitations
Three main points can be highlighted which limit the results of this systematic review. First, the heterogeneous data base: our systematic review represents the largest population of patients with mechanical circulatory support for CS caused by TTS to date. It is based predominantly on case reports, which are often very detailed but usually focus on a specific question and therefore do not always represent all parameters in precisely the same manner. There are also no standardized criteria for some parameters, such as a patient’s full recovery. Parameters such as the frequency of catheterization prior to MCS implantation clearly show the weakness of an analysis based on such heterogenic baseline data. In the case of an exclusion diagnosis such as TTS, a very high proportion of catheterizations would be expected to rule out ACS. However, catheterization prior to MCS is reported in only 66.9% of cases, which clearly appears too low and exemplifies the limitations of the present work.
Second, this analysis is retrospective and covers a period of 20 years. During this period, MCS technology improved and also became more widely available. This may also have changed the potential patient population in whom MCS could be applied in the setting of TTS-CS. Since the SCAI classification was established in 2019, the SCAI stage was not documented in the individual case reports, not least because the literature included in the present analysis covers a period of 20 years. Furthermore, the retrospective nature of this study poses a challenge for the inclusion of studies. The diagnosis of TTS and CS declared by the authors had to be verified on the basis of the data available in the publications (fulfilment of the InterTak diagnostic criteria and haemodynamic as well as clinical criteria of CS). If no additional information was available that would allow the assessment of the InterTak score to determine whether cardiogenic shock caused by takotsubo syndrome was a plausible diagnosis, studies were not included.
Third, when looking at the differences between the various devices, the following must be considered: there is a high cross-over rate between devices in the data set, which reduces the separation between the groups and biases this review towards finding no difference. Furthermore, the conclusion that MCS with the Impella is significantly better than other MCS strategies was based mainly on the parameters of baseline LVEF and duration of MCS. To confirm that the correlations identified here are causal and to clearly show that left ventricular ventilation leads to faster myocardial recovery, prospective multicentre studies are needed that also consider more precise parameters.
6. Conclusions
This systematic review and pooled analysis of individual data involved 124 patients with TTS-CS treated with MCS. Cardiac recovery was shorter with the Impella, with no differences in terms of survival and full recovery of left ventricular function. The superiority of microaxial LVADs over the other devices can merely be assumed on the basis of the shorter duration of mechanical support. Prospective randomized studies are needed to provide recommendations on the use of MCS in TTS-CS.
Author Contributions
J.K.R.v.M. wrote the original draft, collected the data, carried out the formal analysis and prepared all figures and tables. V.I.T.Z., A.E.S., K.M.V.P., R.H., C.T.S., F.S., E.V.P., J.K., S.J. and V.F. edited and reviewed the manuscript. L.W. conceptualised, edited, supervised and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available, see Appendix A.
Conflicts of Interest
The work presented received no financial support, the following table contains the conflict of interest statement of each author.
| J.K.R.v.M. | None. |
| V.I.T.Z. | None. |
| A.E.S. | None. |
| K.M.V.P. | None. |
| R.H. | None. |
| F.S. | Institutional grants from Novartis and Abbott; non-financial support from Medtronic and institutional fees (speaker honoraria) from Orion Pharma outside of the submitted work. |
| J.K. | Grants or contracts from any entity: Edwards and LivaNova. Payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events: Edwards, Medtronic, Abbott, LivaNova and CryoLife. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: TC EACTS, ECSC Board, and ISMICS Board. |
| C.T.S. | Payment to his institution in the form of speaker fees, honoraria, consultancy, advisory board fees, investigator and committee membership: AngioDynamics, Abiomed, Medtronic, Spectranetics, Biotronik, LivaNova (Sorin) and Cook Medical. Departmental or institutional research funding: Cook Medical. |
| E.V.P. | Consulting fees: Abbott (institutional grants), Medtronic (institutional grants) and Abiomed (institutional grants). Payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing or educational events: Abbott (institutional grants), Medtronic (institutional grants) and Abiomed (institutional grants). Support for attending meetings and/or travel: Abbott (institutional grants), Medtronic (institutional grants) and Abiomed (institutional grants). Participation in Data Safety Monitoring Board or Advisory Board: Abbott and Medtronic. |
| V.F. | Grants or contracts from any entity: Medtronic GmbH, Biotronik SE & Co., Abbott GmbH & Co. KG, Boston Scientific, Edwards Lifesciences, Berlin Heart, Novartis Pharma GmbH, JOTEC/CryoLife GmbH, LivaNova and Zurich Heart. I hereby declare that I have relevant (institutional) financial activities outside the submitted work with the mentioned commercial entities in relation to educational grants (including travel support), fees for lectures and speeches, fees for professional consultation, research and study funds. |
| L.W. | None. |
Glossary of Abbreviations
| LV | left ventricle/left ventricular |
| LVAD | left ventricular assist device |
| BiVAD | biventricular assist device |
| ECMO | extracorporeal membrane oxygenation |
| ECLS | extracorporeal life support |
| IABP | intra-aortic balloon pump |
| LVEF | left ventricular ejection fraction |
| NYHA | New York Heart Association |
| TTS | Takotsubo syndrome |
| CS | cardiogenic shock |
| ACS | acute coronary syndrome |
| MCS | mechanical circulatory support |
| MR | mitral regurgitation |
| SAM | systolic anterior motion |
| MAP | mean arterial pressure |
| CO | cardiac output |
| AKI | acute kidney injury |
| HB | haemoglobin |
Appendix A. Included Publications
| Author | Journal | Year | Title | Number of Patients |
| A. A. Oredegbe and M. Awad | Cureus | 2023 | Catecholamine Mega Storm Triggered by Cocaine Use and Thyrotoxicosis Crisis | 1 |
| A. Badouin and O. Bastien | Anaesth Crit Care Pain Med | 2015 | ECLS indication for a case of stress myocardiopathy associated with severe asthma | 1 |
| A. Benak, M. Sramko, B. Janek et al. | Cureus | 2022 | Successful Treatment of Cardiogenic Shock Due to Takotsubo Cardiomyopathy With Left Ventricular Outflow Tract Obstruction and Acute Mitral Regurgitation by Impella CP | 1 |
| A. Beneduce, L. Fausta Bertoldi, F. Melillo et al. | JACC Cardiovasc Interv | 2019 | Mechanical Circulatory Support With Impella Percutaneous Ventricular Assist Device as a Bridge to Recovery in Takotsubo Syndrome Complicated by Cardiogenic Shock and Left Ventricular Outflow Tract Obstruction | 1 |
| A. H. Koop, R. E. Bailey and P. E. Lowman | BMJ Case Rep | 2018 | Acute pancreatitis-induced Takotsubo cardiomyopathy and cardiogenic shock treated with a percutaneous left ventricular assist device | 1 |
| A. K. Tiwari and N. D’Attellis | J Cardiothorac Vasc Anesth | 2008 | Intraoperative left ventricular apical ballooning: transient Takotsubo cardiomyopathy during orthotopic liver transplantation | 1 |
| A. Lauterio, M. Bottiroli, A. Cannata et al. | Minerva Anestesiol | 2022 | Successful recovery from severe inverted Takotsubo cardiomyopathy after liver transplantation: the efficacy of extracorporeal membrane oxygenation (ECMO) | 1 |
| A. Mohammedzein, A. Taha, A. Salwan et al. | JACC Case Rep | 2019 | Impella Use in Cardiogenic Shock Due to Takotsubo Cardiomyopathy With Left Ventricular Outflow Tract Obstruction | 1 |
| A. Omosule, M. F. Malik, L. Cisneros et al. | J Cardiothorac Vasc Anesth | 2019 | Takotsubo Cardiomyopathy After Double-Lung Transplantation: Role of Early Extracorporeal Membrane Oxygenation Support | 1 |
| A. Rashed, S. Won, M. Saad et al. | BMJ Case Rep | 2015 | Use of the Impella 2.5 left ventricular assist device in a patient with cardiogenic shock secondary to Takotsubo cardiomyopathy | 1 |
| A. Vachiat, K. McCutcheon, A. Mahomed et al. | Cardiovasc J Afr | 2016 | Takotsubo cardiomyopathy post liver transplantation | 1 |
| A. Yazicioglu, M. Subasi, S. Turkkan et al. | Turk Gogus Kalp Damar Cerrahisi Derg | 2018 | An uncommon cause for grade 3 primary graft dysfunction after lung transplantation: Takotsubo cardiomyopathy | 1 |
| B. F. Sagger Mawri, Hazem Malas, Sachin Parikh et al. | Scient Open Access | 2017 | Mechanical Hemodynamic Support as a Bridge to Recovery in Severe Takotsubo Cardiomyopathy with Marked Left Ventricular Outflow Tract Obstruction and Cardiogenic Shock | 1 |
| B. Flam, M. Broome, B. Frenckner et al. | J Intensive Care Med | 2015 | Pheochromocytoma-Induced Inverted Takotsubo-Like Cardiomyopathy Leading to Cardiogenic Shock Successfully Treated With Extracorporeal Membrane Oxygenation | 1 |
| B. Hassid, S. Azmoon, W. S. Aronow et al. | Arch Med Sci | 2010 | Hemodynamic support with TandemHeart in tako-tsubo cardiomyopathy—a case report | 1 |
| B. Laliberte and B. N. Reed | Am J Health Syst Pharm | 2017 | Use of an argatroban-based purge solution in a percutaneous ventricular assist device | 1 |
| B. M. M. Faria, J. Portugues, R. Roncon-Albuquerque et al. | Eur Heart J Case Rep | 2020 | Inverted Takotsubo syndrome complicated with cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation in a patient with bilateral pheochromocytoma: a case report | 1 |
| C. Dominedo, E. D’Avino, A. Martinotti et al. | Eur Heart J Case Rep | 2021 | A rare pheochromocytoma complicated by cardiogenic shock and posterior reversible encephalopathy syndrome: case report | 1 |
| C. J. van Zwet, A. Rist, A. Haeussler et al. | A A Case Rep | 2016 | Extracorporeal Membrane Oxygenation for Treatment of Acute Inverted Takotsubo-Like Cardiomyopathy From Hemorrhagic Pheochromocytoma in Late Pregnancy | 1 |
| C. Zeballos, R. J. Moraca, S. H. Bailey et al. | J Card Surg | 2012 | Temporary mechanical circulatory support for Takotsubo cardiomyopathy secondary to primary mediastinal B-cell lymphoma | 1 |
| D. Basic, G. Klug, B. Haubner et al. | Eur Heart J | 2019 | Left ventricular unloading by percutaneous mechanical circulatory support in Takotsubo syndrome with severe cardiogenic shock | 1 |
| D. Ghanim, Z. Adler, D. Qarawani et al. | J Med Case Rep | 2015 | Takotsubo cardiomyopathy caused by epinephrine-treated bee sting anaphylaxis: a case report | 1 |
| D. Laghlam, O. Touboul, M. Herry et al. | Front Cardiovasc Med | 2022 | Takotsubo cardiomyopathy after cardiac surgery: A case-series and systematic review of literature | 2 |
| D. W. Donker, E. Pragt, P. W. Weerwind et al. | Int J Cardiol | 2012 | Rescue extracorporeal life support as a bridge to reflection in fulminant stress-induced cardiomyopathy | 1 |
| E. Barsoum, S. Elhosseiny, B. Patel et al. | Heart Lung | 2021 | Successful use of the impella ventricular assist device for management of reverse Takotsubo Cardiomyopathy in the setting of acute intracranial hemorrhage | 1 |
| E. C. Busse and J. M. Wiater | JBJS Case Connect | 2015 | Perioperative Takotsubo Cardiomyopathy: A Rare Cardiac Complication Following Orthopaedic Surgery: A Case Report | 1 |
| E. D Foley, Ricardo Diaz and Manuel R Castresana | SAGE Open Med Case Rep | 2017 | Prolonged circulatory support with an Impella assist device in the management of cardiogenic shock associated with Takotsubo syndrome, severe sepsis and acute respiratory distress syndrome | 1 |
| E. f. J. Hamid T, Fraser D, Fath-Ordoubadi F | J Clin Exp Cardiolog | 2013 | Use of the Impella Left Ventricular Assist Device as a Bridge to Recovery in a Patient with Cardiogenic Shock Related to Takotsubo Cardiomyopathy. | 1 |
| E. T. Wu, T. H. Lin, C. H. Lin et al. | Taiwan J Obstet Gynecol | 2014 | Left ventricular assist device for stress-induced cardiomyopathy after postpartum hemorrhage | 1 |
| F. F. Zhou, J. S. Ding, M. Zhang et al. | Open Med (Wars) | 2022 | Paraganglioma-induced inverted Takotsubo-like cardiomyopathy leading to cardiogenic shock successfully treated with extracorporeal membrane oxygenation | 1 |
| F. Yazdi, M. Blackmon, A. Kattubadi et al. | Cureus | 2023 | Seizure-Induced Cardiomyopathy: A Case of Takotsubo Cardiomyopathy Following an Epileptic Event | 1 |
| F. Zilio, S. Muraglia and R. Bonmassari | European Heart Journal—Case Reports | 2021 | Cardiac arrest complicating cardiogenic shock: from pathophysiological insights to Impella-assisted cardiopulmonary resuscitation in a pheochromocytoma-induced Takotsubo cardiomyopathy-a case report | 1 |
| G. Caturegli, M. A. Crane, E. Etchill et al. | ASAIO J | 2022 | Stress-Induced (Takotsubo) Cardiomyopathy After Liver Transplant Rescued with Venoarterial Extracorporeal Membrane Oxygenation | 1 |
| G. Hekimian, F. Kharcha, N. Brechot et al. | Ann Intensive Care | 2016 | Extracorporeal membrane oxygenation for pheochromocytoma-induced cardiogenic shock | 9 |
| G. Rojas-Marte, J. John, A. Sadiq et al. | Cardiovasc Revasc Med | 2015 | Medication-induced Takotsubo Cardiomyopathy presenting with cardiogenic shock-utility of extracorporeal membrane oxygenation (ECMO): case report and review of the literature | 1 |
| H. Sumida, K. Morihisa, K. Katahira et al. | Intern Med | 2017 | Isolated Right Ventricular Stress (Takotsubo) Cardiomyopathy | 1 |
| H. Zhang and X. Liao | J Card Surg | 2021 | Takotsubo cardiomyopathy following pericardiectomy: A case report | 1 |
| I. Schroeder, M. Zoller, M. Angstwurm et al. | nt J Artif Organs | 2017 | Venlafaxine intoxication with development of Takotsubo cardiomyopathy: successful use of extracorporeal life support, intravenous lipid emulsion and CytoSorb® | 1 |
| J. Feghaly, Z. Oman, D. Das et al. | Cureus | 2021 | Recurrent Stress-Induced Cardiomyopathy With Cardiogenic Shock Requiring Impella Left Ventricular Assist Device | 1 |
| J. H. Choi, I. D. Oh, E. Shin et al. | Acute Crit Care | 2020 | Extracorporeal membrane oxygenation for Takotsubo cardiomyopathy that developed after mitral valve replacement | 1 |
| J. J. J. Aalberts, T. J. Klinkenberg, M. A. Mariani et al. | Eur Heart J | 2017 | Mechanical circulatory support for refractory cardiogenic shock in Takotsubo syndrome: a case report and review of the literature | 1 |
| J. Kirigaya, N. Iwahashi, R. Tanaka et al. | Cureus | 2022 | A Fatal Case of Takotsubo Cardiomyopathy Secondary to Refractory Hypoglycemia in Severe Starvation: An Autopsy Case Report | 1 |
| J. Mierke, T. Loehn, A. Linke et al. | Eur Heart J Case Rep | 2019 | Reverse Takotsubo cardiomyopathy- life-threatening symptom of an incidental pheochromocytoma: a case report | 1 |
| J. O’Brien, S. Mahony, R. J. Byrne et al. | Eur Heart J Case Rep | 2021 | Dynamic left ventricular outflow tract gradient resulting from Takotsubo cardiomyopathy ameliorated by intra-aortic balloon pump counterpulsation: a case report | 1 |
| J. Wei, L. Zhang, X. Ruan et al. | Front Cardiovasc Med | 2022 | Case Report: Takotsubo Syndrome Induced by Severe Anaphylactic Reaction During Anesthesia Induction and Subsequent High-Dose Epinephrine Resuscitation | 1 |
| K. T. Webster, T. Apridonidze, P. R. Mopala et al. | Can J Cardiol 2019 | 2019 | Stress-Induced Cardiomyopathy Complicated by Dynamic Left Ventricular Outflow Obstruction, Cardiogenic Shock, and Ventricular Septal Rupture | 1 |
| K. X. Fu, B. H. Z. Ng and M. H. X. Chua | BMC Pediatr | 2019 | A unique case of acute brain haemorrhage with left ventricular systolic failure requiring ECMO | 1 |
| K. Yamane, H. Hirose, G. R. Reeves et al. | J Heart Valve Dis | 2011 | Left ventricular dysfunction mimicking Takotsubo cardiomyopathy following cardiac surgery | 1 |
| L. C. Napp, R. Westenfeld, J. E. Møller et al. | Cardiovasc Revasc Med | 2022 | Impella Mechanical Circulatory Support for Takotsubo Syndrome With Shock: A Retrospective Multicenter Analysis | 16 |
| L. Paton and I. Quasim | Br J Anaesth | 2013 | Takotsubo cardiomyopathy: issues for the intensivist | 1 |
| L. Wert, J. Kempfert, V. Falk et al. | Interdiscip Cardiovasc Thorac Surg | 2023 | Transaxillary implantation of a temporary microaxial left ventricular assist device in a patient with a rectangular kinked subclavian artery | 1 |
| M. Bonacchi, A. Vannini, G. Harmelin et al. | Interact Cardiovasc Thorac Surg | 2015 | Inverted-Takotsubo cardiomyopathy: severe refractory heart failure in poly-trauma patients saved by emergency extracorporeal life support | 4 |
| M. Bonacchi, S. Valente, G. Harmelin et al. | Artif Organs | 2009 | extracorporeal life support as ultimate strategy for refractory severe cardiogenic shock induced by Tako-tsubo cardiomyopathy: a new effective therapeutic option | 1 |
| M. Hanif, M. A. Haider, Q. Xi et al. | Cureus | 2020 | Takotsubo Cardiomyopathy Triggered by the Death of Pets (Cats): Two Case Reports | 1 |
| M. Husaini, J. N. Baker, S. Cresci et al. | JACC Case Rep | 2022 | Recurrent Takotsubo Cardiomyopathy in a Patient With Hypertrophic Cardiomyopathy Leading to Cardiogenic Shock Requiring VA-ECMO | 1 |
| M. Moguilevitch, M. Rufino, J. Leff et al. | Liver Transpl | 2015 | Novel approach for heart failure treatment after liver transplantation | 1 |
| M. Nakamura, M. Nakagaito, M. Hori et al. | J Artif Organs | 2019 | A case of Takotsubo cardiomyopathy with cardiogenic shock after influenza infection successfully recovered by IMPELLA support | 1 |
| M. P. Silva, E. M. Vilela, R. L. Lopes et al. | Rev Port Cardiol | 2015 | Cardiogenic shock induced by Takotsubo cardiomyopathy: A new therapeutic option | 1 |
| O. Kiamanesh, E. N. Vu, D. L. Webber et al. | JACC Case Rep | 2019 | Pheochromocytoma-Induced Takotsubo Syndrome Treated With Extracorporeal Membrane Oxygenation: Beware of the Apical Sparing Pattern | 1 |
| P. A. Cotinet, P. Bizouarn, F. Roux et al. | Heart Lung | 2021 | Management of cardiogenic shock by circulatory support during reverse Tako-Tsubo following amphetamine exposure: A report of two cases | 2 |
| R. Dalla Pozza, A. Lehner, S. Ulrich, M. Nabauer et al. | World J Pediatr Congenit Heart Surg | 2020 | Takotsubo Cardiomyopathy Complicating Percutaneous Pulmonary Valve Implantation in a Child | 1 |
| R. Gurreri, P. Poommipanit and A. Alghamdi | Oxf Med Case Reports | 2023 | Cardiogenic shock secondary to stress-induced cardiomyopathy precipitated by severe diabetic ketoacidosis | 1 |
| R. Hamdan, M. E. Nassef, J. Khan et al. | Ann Cardiol Angeiol | 2022 | Reverse Takotsubo ou myocardite fulminante ? Succes de VA ECMO chez une patiente ayant une atteinte cardiaque liee COVID 19 | 1 |
| R. Kakizaki, N. Bunya, S. Uemura et al. | Acute Med Surg | 2019 | Takotsubo cardiomyopathy developed during rewarming of accidental hypothermia with extracorporeal membrane oxygenation | 1 |
| R. Korabathina, W. Abel and A. Labovitz | Case Rep Cardiol | 2016 | Cardiogenic Shock due to Psychosis-Induced Inverted Takotsubo Cardiomyopathy Bridged-to-Recovery with a Percutaneous Left Ventricular Assist Device | 1 |
| R. Nishikawa, N. Nagano, N. Kokubu et al. | Int Heart J | 2021 | Favorable Effects of Impella on Takotsubo Syndrome Complicated with Cardiogenic Shock | 4 |
| R. S. Biondi, V. S. Barzilai, A. L. C. Watanabe et al. | Rev Bras Ter Intensiva | 2018 | Use of extracorporeal membrane oxygenation for treating acute cardiomyopathy after liver transplantation: a case report | 1 |
| R. V. Reddy, S. Agarwal, V. Choudhary et al. | Indian J Anaesth | 2018 | Reverse stress cardiomyopathy post-liver transplant needing mechanical circulatory support | 1 |
| S. An, H. I. Ma, J. Song et al. | BMC Neurol | 2020 | Stress cardiomyopathy associated with area postrema syndrome as a presentation of neuromyelitis optica: case report | 1 |
| S. Elapavaluru, A. Gologorsky, N. Thai et al. | J Cardiothorac Vasc Anesth | 2017 | Perioperative Stress Cardiomyopathy in Simultaneous Liver and Kidney Transplantation: A Call for Early Consideration of Mechanical Circulatory Support | 1 |
| S. Fang, Y. Wang, P. K. He et al. | Medicine (Baltimore) | 2021 | Cardiogenic shock caused by Takotsubo syndrome complicated with severe anxiety: A case report and literature review | 1 |
| S. Forsberg, L. Abazi and P. Forsman | J Med Case Rep | 2021 | Successful use of extended cardiopulmonary resuscitation followed by extracorporeal oxygenation after venlafaxine-induced Takotsubo cardiomyopathy and cardiac arrest: a case report | 1 |
| S. H. Park, M. K. Song, G. B. Kim et al. | Chonnam Med J | 2019 | Stress Induced Cardiomyopathy Requiring Ventricular Assist Device Support in an 8-Year-Old Girl with Acute Leukemia | 1 |
| S. Kaese, C. Schulke, D. Fischer et al. | Intensive Care Med | 2013 | Pheochromocytoma-induced Takotsubo-like cardiomyopathy and global heart failure with need for extracorporal life support | 1 |
| S. Kurisu, K. Ishibashi, Y. Kato et al. | Intern Med | 2012 | Tako-tsubo cardiomyopathy complicated by QRS prolongation | 1 |
| S. Lee, S. P. Lim, J. H. Yu et al. | Korean J Thorac Cardiovasc Surg | 2011 | Stress-induced Cardiomyopathy during Pulmonary Resection (Takotsubo Syndrome)—A case report | 1 |
| S. Li, M. M. Koerner, A. El-Banayosy et al. | Ann Thorac Surg | 2014 | Takotsubo’s syndrome after mitral valve repair and rescue with extracorporeal membrane oxygenation | 1 |
| S. Modi and D. Ramsdale | Int J Cardiol | 2011 | Tako-tsubo, hypertrophic obstructive cardiomyopathy & muscle bridging--separate disease entities or a single condition? | 1 |
| S. Park, M. Kim, D. I. Lee et al. | Acute Crit Care | 2022 | Successful extracorporeal membrane oxygenation treatment of catecholamine-induced cardiomyopathy-associated pheochromocytoma | 1 |
| S. Sossalla, C. Meindl, M. Fischer et al. | Circ Cardiovasc Interv | 2019 | Bail-Out Alcohol Septal Ablation for Hypertrophic Obstructive Cardiomyopathy in a Patient With Takotsubo Cardiomyopathy-Induced Cardiogenic Shock | 1 |
| S. Sundaravel, A. Alrifai, M. Kabach et al. | Case Rep Cardiol | 2017 | FOLFOX Induced Takotsubo Cardiomyopathy Treated with Impella Assist Device | 1 |
| T. Attisano, A. Silverio, C. Prota et al. | ESC Heart Failure | 2020 | Impella in Takotsubo syndrome complicated by left ventricular outflow tract obstruction and severe mitral regurgitation | 1 |
| T. Bleser, C. Weth and G. Gorge | Med Klin Intensivmed Notfmed | 2013 | Reverse Takotsubo cardiomyopathy-a life-threatening disease. Successful resuscitation of a 31-year-old woman with cardiologic shock after a visit to the dentist | 1 |
| T. E. Pearson, M. A. Frizzola, M. A. Priest et al. | Air Med J | 2018 | Pediatric Extracorporeal Cardiopulmonary Resuscitation Patient With Traumatic Subarachnoid Hemorrhage and Takotsubo Syndrome | 1 |
| T. K. Yoo, J. Y. Lee, K. C. Sung et al. | J Cardiovasc Ultrasound | 2016 | Stress-Induced Cardiomyopathy Presenting as Shock | 1 |
| T. Lyu, J. Niu, Z. Liu et al. | Front Cardiovasc Med | 2022 | Case Report: Early Resection of Pheochromocytoma in a Patient With Cardiogenic Shock Due to Pheochromocytoma-Induced Cardiomyopathy With Extracorporeal Life Support | 1 |
| V. V. Garla, S. Gosi, S. Kanduri et al. | BMJ Case Rep | 2019 | A case of catecholamine-induced cardiomyopathy treated with extracorporeal membrane oxygenation | 1 |
| X. Fan, P. Liu and L. Bai | Eur Heart J Case Rep | 2022 | Cardiogenic shock due to Takotsubo cardiomyopathy associated with thyroid crisis: a case report | 1 |
| Y. Luo, X. Ye, L. Zhang et al. | Echocardiography | 2023 | A rare case of cardiogenic shock caused by Takotsubo syndrome associated with SARS-CoV-2 infection: The value of echocardiography in the diagnosis and monitoring of the efficacy of extracorporeal membrane oxygenation | 1 |
| Y. Xie, A. Zhang, M. Qi et al. | BMC Endocr Disord | 2023 | Pheochromocytoma crisis with refractory Acute Respiratory Distress Syndrome (ARDS), Takotsubo syndrome, emergency adrenalectomy, and need for Extracorporeal Membrane Oxygenation (ECMO) in a previously undiagnosed and asymptomatic patient, due to the use of metoclopramide | 1 |
| Y. Y. Jo, S. Park and Y. S. Choi | Anaesth Intensive Care | 2011 | Extracorporeal membrane oxygenation in a patient with stress-induced cardiomyopathy after caesarean section | 1 |
| Z. Su, Y. Wang and H. Fei | CASE (Phila) | 2019 | Takotsubo-Like Cardiomyopathy in Pheochromocytoma | 1 |
| Z. Y. Zhang, J. J. Sun, J. H. Wang et al. | BMC Cardiovasc Disord | 2023 | Successful treatment of a severe Takotsubo syndrome case complicated by liver abscess | 1 |
References
- Lyon, A.R.; Bossone, E.; Schneider, B.; Sechtem, U.; Citro, R.; Underwood, S.R.; Sheppard, M.N.; Figtree, G.A.; Parodi, G.; Akashi, Y.J.; et al. Current state of knowledge on Takotsubo syndrome: A Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 8–27. [Google Scholar] [CrossRef]
- Stiermaier, T.; Eitel, C.; Desch, S.; Fuernau, G.; Schuler, G.; Thiele, H.; Eitel, I. Incidence, determinants and prognostic relevance of cardiogenic shock in patients with Takotsubo cardiomyopathy. Eur. Heart J. Acute Cardiovasc. Care 2016, 5, 489–496. [Google Scholar] [CrossRef]
- Matta, A.G.; Carrie, D. Epidemiology, Pathophysiology, Diagnosis, and Principles of Management of Takotsubo Cardiomyopathy: A Review. Med. Sci. Monit. 2023, 29, e939020. [Google Scholar] [CrossRef]
- Jabri, A.; Kalra, A.; Kumar, A.; Alameh, A.; Adroja, S.; Bashir, H.; Nowacki, A.S.; Shah, R.; Khubber, S.; Kanaa, N.A.; et al. Incidence of Stress Cardiomyopathy During the Coronavirus Disease 2019 Pandemic. JAMA Netw Open. 2020, 3, e2014780. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Athanasiadis, A.; Schwab, J.; Pistner, W.; Gottwald, U.; Schoeller, R.; Toepel, W.; Winter, K.D.; Stellbrink, C.; Müller-Honold, T.; et al. Complications in the clinical course of tako-tsubo cardiomyopathy. Int. J. Cardiol. 2014, 176, 199–205. [Google Scholar] [CrossRef]
- Vallabhajosyula, S.; Dunlay, S.M.; Murphree, D.H.; Barsness, G.W., Jr.; Sandhu, G.S.; Lerman, A.; Prasad, A. Cardiogenic Shock in Takotsubo Cardiomyopathy Versus Acute Myocardial Infarction: An 8-Year National Perspective on Clinical Characteristics, Management, and Outcomes. JACC Heart Fail. 2019, 7, 469–476. [Google Scholar] [CrossRef]
- Madhavan, M.; Rihal, C.S.; Lerman, A.; Prasad, A. Acute heart failure in apical ballooning syndrome (TakoTsubo/stress cardiomyopathy): Clinical correlates and Mayo Clinic risk score. J. Am. Coll. Cardiol. 2011, 57, 1400–1401. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Cammann, V.L.; Jurisic, S.; Seifert, B.; Napp, L.C.; Diekmann, J.; Bataiosu, D.R.; D’Ascenzo, F.; Ding, K.J.; Sarcon, A.; et al. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: Results from the International Takotsubo Registry. Eur. J. Heart Fail. 2017, 19, 1036–1042. [Google Scholar] [CrossRef]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [PubMed]
- Napierkowski, S.; Banerjee, U.; Anderson, H.V.; Charitakis, K.; Madjid, M.; Smalling, R.W.; Dhoble, A. Trends and Impact of the Use of Mechanical Circulatory Support for Cardiogenic Shock Secondary to Takotsubo Cardiomyopathy. Am. J. Cardiol. 2021, 139, 28–33. [Google Scholar] [CrossRef]
- Terasaki, S.; Kanaoka, K.; Nakai, M.; Sumita, Y.; Onoue, K.; Soeda, T.; Watanabe, M.; Miyamoto, Y.; Saito, Y. Outcomes of catecholamine and/or mechanical support in Takotsubo syndrome. Heart 2022, 108, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Di Vece, D.; Citro, R.; Cammann, V.L.; Kato, K.; Gili, S.; Szawan, K.A.; Micek, J.; Jurisic, S.; Ding, K.J.; Bacchi, B.; et al. Outcomes Associated With Cardiogenic Shock in Takotsubo Syndrome. Circulation 2019, 139, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Elapavaluru, S.; Gologorsky, A.; Thai, N.; Uemura, T.; Khalil, R.; Mareddy, C.; Dishart, M.; Gologorsky, E. Perioperative Stress Cardiomyopathy in Simultaneous Liver and Kidney Transplantation: A Call for Early Consideration of Mechanical Circulatory Support. J. Cardiothorac. Vasc. Anesth. 2017, 31, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Santoro, F.; Nunez Gil, I.J.; Stiermaier, T.; El-Battrawy, I.; Moeller, C.; Guerra, F.; Novo, G.; Arcari, L.; Musumeci, B.; Cacciotti, L.; et al. Impact of intra-aortic balloon counterpulsation on all-cause mortality among patients with Takotsubo syndrome complicated by cardiogenic shock: Results from the German-Italian-Spanish (GEIST) registry. Eur. Heart J. Open 2023, 3, oead003. [Google Scholar] [CrossRef] [PubMed]
- Mariani, S.; Richter, J.; Pappalardo, F.; Bělohlávek, J.; Lorusso, R.; Schmitto, J.D.; Bauersachs, J.; Napp, L.C. Mechanical circulatory support for Takotsubo syndrome: A systematic review and meta-analysis. Int. J. Cardiol. 2020, 316, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nersesian, G.; Hennig, F.; Muller, M.; Mulzer, J.; Tsyganenko, D.; Starck, C.; Gromann, T.; Falk, V.; Potapov, E.; Schoenrath, F. Temporary mechanical circulatory support for refractory heart failure: The German Heart Center Berlin experience. Ann. Cardiothorac. Surg. 2019, 8, 76–83. [Google Scholar] [CrossRef]
- Eulert-Grehn, J.J.; Starck, C.; Kempfert, J.; Falk, V.; Potapov, E. ECMELLA 2.0: Single Arterial Access Technique for a Staged Approach in Cardiogenic Shock. Ann. Thorac. Surg. 2021, 111, e135–e137. [Google Scholar] [CrossRef]
- Wert, L.; Kempfert, J.; Falk, V.; Potapov, E.V. Transaxillary implantation of a temporary microaxial left ventricular assist device in a patient with a rectangular kinked subclavian artery. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad088. [Google Scholar] [CrossRef]
- Schrage, B.; Becher, P.M.; Bernhardt, A.; Bezerra, H.; Blankenberg, S.; Brunner, S.; Colson, P.; Cudemus Deseda, G.; Dabboura, S.; Eckner, D.; et al. Left Ventricular Unloading Is Associated With Lower Mortality in Patients With Cardiogenic Shock Treated With Venoarterial Extracorporeal Membrane Oxygenation: Results From an International, Multicenter Cohort Study. Circulation 2020, 142, 2095–2106. [Google Scholar] [CrossRef]
- Bairashevskaia, A.V.; Belogubova, S.Y.; Kondratiuk, M.R.; Rudnova, D.S.; Sologova, S.S.; Tereshkina, O.I.; Avakyan, E.I. Update of Takotsubo cardiomyopathy: Present experience and outlook for the future. Int. J. Cardiol. Heart Vasc. 2022, 39, 100990. [Google Scholar] [CrossRef]
- Nishikawa, R.; Nagano, N.; Kokubu, N.; Hashimoto, K.; Nakata, J.; Kishiue, N.; Takahashi, R.; Otomo, S.; Tsuchihashi, K.; Yano, T. Favorable Effects of Impella on Takotsubo Syndrome Complicated with Cardiogenic Shock. Int. Heart J. 2021, 62, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Napp, L.C.; Westenfeld, R.; Møller, J.E.; Pappalardo, F.; Ibrahim, K.; Bonello, L.; Wilkins, C.; Pershad, A.; Mannino, S.F.; Schreiber, T.L.; et al. Impella Mechanical Circulatory Support for Takotsubo Syndrome With Shock: A Retrospective Multicenter Analysis. Cardiovasc. Revasc. Med. 2022, 40, 113–119. [Google Scholar] [CrossRef]
- Barsoum, E.; Elhosseiny, S.; Patel, B.; Pathak, S.; Patel, A.; Vaidya, P. Successful use of the impella ventricular assist device for management of reverse Takotsubo Cardiomyopathy in the setting of acute intracranial hemorrhage. Heart Lung 2021, 50, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, B.; Reed, B.N. Use of an argatroban-based purge solution in a percutaneous ventricular assist device. Am. J. Health Syst. Pharm. 2017, 74, e163–e169. [Google Scholar] [CrossRef]
- Attisano, T.; Silverio, A.; Prota, C.; Briguori, C.; Galasso, G.; Citro, R. Impella in Takotsubo syndrome complicated by left ventricular outflow tract obstruction and severe mitral regurgitation. ESC Heart Fail. 2020, 7, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bonacchi, M.; Maiani, M.; Harmelin, G.; Sani, G. Intractable cardiogenic shock in stress cardiomyopathy with left ventricular outflow tract obstruction: Is extra-corporeal life support the best treatment? Eur. J. Heart Fail. 2009, 11, 721–727. [Google Scholar] [CrossRef]
- Bonacchi, M.; Valente, S.; Harmelin, G.; Gensini, G.F.; Sani, G. Extracorporeal life support as ultimate strategy for refractory severe cardiogenic shock induced by Tako-tsubo cardiomyopathy: A new effective therapeutic option. Artif. Organs 2009, 33, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Lazcano-Diaz, E.A.; Gonzalez-Ruiz, F.J.; Sarre-Alvarez, D.; Alvarez-Alvarez, R.J.; Bucio-Reta, E.; Garcia-Cruz, E.; Rojas-Velazco, G.; Ramos-Enriquez, A.; Melano-Carranza, E.; Santos-Martinez, L.E.; et al. Circulatory support with venoarterial ECMO in a patient with biventricular Takotsubo cardiomyopathy. Arch. Cardiol. Mex. 2020, 91, 100–104. [Google Scholar] [CrossRef]
- Redfors, B.; Shao, Y.; Omerovic, E. “Primum nil nocere” first choice in Takotsubo cardiomyopathy. Mechanical support as a bridge to recovery should be tested in severe cases. Lakartidningen 2014, 111, 1366–1369. [Google Scholar] [PubMed]
- Besnier, E.; Hubscher, C.; Doguet, F.; Bessou, J.P.; Dureuil, B. Extracorporeal membrane oxygenation support for tako-tsubo syndrome after urgent caesarean section. Ann. Fr. Anesth. Reanim. 2013, 32, 704–706. [Google Scholar] [CrossRef]
- Martin-Villen, L.; Corcia-Palomo, Y.; Escalona-Rodriguez, S.; Roldan-Reina, A.; Acosta-Delgado, D.; Martin-Bermudez, R. Extracorporeal membrane oxygenation support in a patient with pheochromocytoma stress myocardyopathy. Med. Intensiv. (Engl. Ed.) 2018, 42, 566–568. [Google Scholar] [CrossRef]
- Garcia-Delgado, M.; Garcia-Huertas, D.; Navarrete-Sanchez, I.; Olivencia-Pena, L.; Garrido, J.M. Extracorporeal membrane oxygenation support for Takotsubo syndrome and long QT after cardiac surgery. Med. Intensiv. 2017, 41, 441–443. [Google Scholar] [CrossRef]
- Esnault, P.; Nee, L.; Signouret, T.; Jaussaud, N.; Kerbaul, F. Reverse Takotsubo cardiomyopathy after iatrogenic epinephrine injection requiring percutaneous extracorporeal membrane oxygenation. Can. J. Anaesth. 2014, 61, 1093–1097. [Google Scholar] [CrossRef]
- Nagao, T.; Ohwada, T.; Hashimoto, M.; Aoki, K.; Shimizu, H.; Katayama, M. Intra-aortic balloon pumping is effective for hemodynamic management of catecholamine resistant ampulla (Takotsubo) cardiomyopathy. Masui 2004, 53, 799–802. [Google Scholar]
- Vachiat, A.; McCutcheon, K.; Mahomed, A.; Schleicher, G.; Brand, L.; Botha, J.; Sussman, M.; Manga, P. Takotsubo cardiomyopathy post liver transplantation. Cardiovasc. J. Afr. 2016, 27, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Hassid, B.; Azmoon, S.; Aronow, W.S.; Palaniswamy, C.; Cohen, M.; Gass, A. Hemodynamic support with TandemHeart in tako-tsubo cardiomyopathy—A case report. Arch. Med. Sci. 2010, 6, 971–975. [Google Scholar] [CrossRef]
- Aalberts, J.J.J.; Klinkenberg, T.J.; Mariani, M.A.; van der Harst, P. Mechanical circulatory support for refractory cardiogenic shock in Takotsubo syndrome: A case report and review of the literature. Eur. Heart J. Case Rep. 2017, 1, ytx005. [Google Scholar] [CrossRef] [PubMed]
- Moguilevitch, M.; Rufino, M.; Leff, J.; Delphin, E. Novel approach for heart failure treatment after liver transplantation. Liver Transplant. 2015, 21, 1103–1104. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Lee, J.Y.; Sung, K.C.; Oh, S.S.; Song, Y.S.; Lee, S.J.; Ko, K.J. Stress-Induced Cardiomyopathy Presenting as Shock. J. Cardiovasc. Ultrasound 2016, 24, 79–83. [Google Scholar] [CrossRef][Green Version]
- Ghanim, D.; Adler, Z.; Qarawani, D.; Kusniec, F.; Amir, O.; Carasso, S. Takotsubo cardiomyopathy caused by epinephrine-treated bee sting anaphylaxis: A case report. J. Med. Case Rep. 2015, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Hamid, T.E.f.J.; Fraser, D.; Fath-Ordoubadi, F. Use of the Impella Left Ventricular Assist Device as a Bridge to Recovery in a Patient with Cardiogenic Shock Related to Takotsubo Cardiomyopathy. J. Clin. Exp. Cardiol. 2013, 4, 246. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, L.; Ruan, X.; He, K.; Yu, C.; Shen, L. Case Report: Takotsubo Syndrome Induced by Severe Anaphylactic Reaction During Anesthesia Induction and Subsequent High-Dose Epinephrine Resuscitation. Front. Cardiovasc. Med. 2022, 9, 842440. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.E.; Frizzola, M.A.; Priest, M.A.; Rochman, M.F.; Froehlich, C.D. Pediatric Extracorporeal Cardiopulmonary Resuscitation Patient With Traumatic Subarachnoid Hemorrhage and Takotsubo Syndrome. Air Med. J. 2018, 37, 64–66. [Google Scholar] [CrossRef]
- Flam, B.; Broome, M.; Frenckner, B.; Branstrom, R.; Bell, M. Pheochromocytoma-Induced Inverted Takotsubo-Like Cardiomyopathy Leading to Cardiogenic Shock Successfully Treated With Extracorporeal Membrane Oxygenation. J. Intensive Care Med. 2015, 30, 365–372. [Google Scholar] [CrossRef]
- Faria, B.M.M.; Portugues, J.; Roncon-Albuquerque, R.; Pimentel, R., Jr. Inverted takotsubo syndrome complicated with cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation in a patient with bilateral pheochromocytoma: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- Dominedo, C.; D’Avino, E.; Martinotti, A.; Cingolani, E. A rare pheochromocytoma complicated by cardiogenic shock and posterior reversible encephalopathy syndrome: Case report. Eur. Heart J. Case Rep. 2021, 5, ytaa513. [Google Scholar] [CrossRef]
- van Zwet, C.J.; Rist, A.; Haeussler, A.; Graves, K.; Zollinger, A.; Blumenthal, S. Extracorporeal Membrane Oxygenation for Treatment of Acute Inverted Takotsubo-Like Cardiomyopathy From Hemorrhagic Pheochromocytoma in Late Pregnancy. A A Case Rep. 2016, 7, 196–199. [Google Scholar] [CrossRef]
- Zilio, F.; Muraglia, S.; Bonmassari, R. Cardiac arrest complicating cardiogenic shock: From pathophysiological insights to Impella-assisted cardiopulmonary resuscitation in a pheochromocytoma-induced Takotsubo cardiomyopathy-a case report. Eur. Heart J. Case Rep. 2021, 5, ytab092. [Google Scholar] [CrossRef]
- Mierke, J.; Loehn, T.; Linke, A.; Ibrahim, K. Reverse takotsubo cardiomyopathy- life-threatening symptom of an incidental pheochromocytoma: A case report. Eur. Heart J. Case Rep. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Kiamanesh, O.; Vu, E.N.; Webber, D.L.; Lau, E.; Kapeluto, J.E.; Stuart, H.; Wood, D.A.; Wong, G.C. Pheochromocytoma-Induced Takotsubo Syndrome Treated With Extracorporeal Membrane Oxygenation: Beware of the Apical Sparing Pattern. JACC Case Rep. 2019, 1, 85–90. [Google Scholar] [CrossRef]
- Kaese, S.; Schulke, C.; Fischer, D.; Lebiedz, P. Pheochromocytoma-induced takotsubo-like cardiomyopathy and global heart failure with need for extracorporal life support. Intensive Care Med. 2013, 39, 1473–1474. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.; Lee, D.I.; Lee, J.H.; Kim, S.; Lee, S.Y.; Bae, J.W.; Hwang, K.K.; Kim, D.W.; Cho, M.C.; et al. Successful extracorporeal membrane oxygenation treatment of catecholamine-induced cardiomyopathy-associated pheochromocytoma. Acute Crit. Care 2022. ahead-of-print. [Google Scholar]
- Garla, V.V.; Gosi, S.; Kanduri, S.; Lien, L. A case of catecholamine-induced cardiomyopathy treated with extracorporeal membrane oxygenation. BMJ Case Rep. 2019, 12, e230196. [Google Scholar] [CrossRef]
- Su, Z.; Wang, Y.; Fei, H. Takotsubo-Like Cardiomyopathy in Pheochromocytoma. CASE 2019, 3, 157–161. [Google Scholar] [CrossRef]
- Lyu, T.; Niu, J.; Liu, Z.; Li, T. Case Report: Early Resection of Pheochromocytoma in a Patient With Cardiogenic Shock Due to Pheochromocytoma-Induced Cardiomyopathy With Extracorporeal Life Support. Front. Cardiovasc. Med. 2022, 9, 788644. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, A.; Qi, M.; Xiong, B.; Zhang, S.; Zhou, J.; Cao, Y. Pheochromocytoma crisis with refractory Acute Respiratory Distress Syndrome (ARDS), Takotsubo syndrome, emergency adrenalectomy, and need for Extracorporeal Membrane Oxygenation (ECMO) in a previously undiagnosed and asymptomatic patient, due to the use of metoclopramide. BMC Endocr. Disord. 2023, 23, 145. [Google Scholar]
- Tiwari, A.K.; D’Attellis, N. Intraoperative left ventricular apical ballooning: Transient Takotsubo cardiomyopathy during orthotopic liver transplantation. J. Cardiothorac. Vasc. Anesth. 2008, 22, 442–445. [Google Scholar] [CrossRef]
- Caturegli, G.; Crane, M.A.; Etchill, E.; Giuliano, K.; Nguyen, M.; Philosophe, B.; Cho, S.M.; Wittstein, I.S.; Whitman, G.J.R. Stress-Induced (Takotsubo) Cardiomyopathy After Liver Transplant Rescued with Venoarterial Extracorporeal Membrane Oxygenation. ASAIO J. 2022, 68, e66–e68. [Google Scholar] [CrossRef] [PubMed]
- Omosule, A.; Malik, M.F.; Cisneros, L.; Guruswamy, J. Takotsubo Cardiomyopathy After Double-Lung Transplantation: Role of Early Extracorporeal Membrane Oxygenation Support. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2503–2507. [Google Scholar] [CrossRef] [PubMed]
- Kirigaya, J.; Iwahashi, N.; Tanaka, R.; Inayama, Y.; Takeuchi, I. A Fatal Case of Takotsubo Cardiomyopathy Secondary to Refractory Hypoglycemia in Severe Starvation: An Autopsy Case Report. Cureus 2022, 14, e23287. [Google Scholar] [CrossRef] [PubMed]
- Hekimian, G.; Kharcha, F.; Brechot, N.; Schmidt, M.; Ghander, C.; Lebreton, G.; Girerd, X.; Tresallet, C.; Trouillet, J.L.; Leprince, P.; et al. Extracorporeal membrane oxygenation for pheochromocytoma-induced cardiogenic shock. Ann. Intensive Care. 2016, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Bonacchi, M.; Vannini, A.; Harmelin, G.; Batacchi, S.; Bugetti, M.; Sani, G.; Peris, A. Inverted-Takotsubo cardiomyopathy: Severe refractory heart failure in poly-trauma patients saved by emergency extracorporeal life support. Interact. Cardiovasc. Thorac. Surg. 2015, 20, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.T.; Apridonidze, T.; Mopala, P.R.; Sherman, A.E.; Potakamuri, L.N.; Storms, D.R.; Kono, A.T.; Mohanty, B.D. Stress-Induced Cardiomyopathy Complicated by Dynamic Left Ventricular Outflow Obstruction, Cardiogenic Shock, and Ventricular Septal Rupture. Can. J. Cardiol. 2019, 35, 229.e7–229.e9. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Ramsdale, D. Tako-tsubo, hypertrophic obstructive cardiomyopathy & muscle bridging--separate disease entities or a single condition? Int. J. Cardiol. 2011, 147, 133–134. [Google Scholar] [PubMed]
- Laghlam, D.; Touboul, O.; Herry, M.; Estagnasie, P.; Dib, J.C.; Baccouche, M.; Brusset, A.; Nguyen, L.S.; Squara, P. Takotsubo cardiomyopathy after cardiac surgery: A case-series and systematic review of literature. Front. Cardiovasc. Med. 2022, 9, 1067444. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Kato, K.; Cammann, V.L.; Gili, S.; Jurisic, S.; Di Vece, D.; Candreva, A.; Ding, K.J.; Micek, J.; Szawan, K.A.; et al. Long-Term Prognosis of Patients With Takotsubo Syndrome. J. Am. Coll. Cardiol. 2018, 72, 874–882. [Google Scholar] [CrossRef]
- Almeida Junior, G.L.G.; Mansur Filho, J.; Albuquerque, D.C.; Xavier, S.S.; Pontes, A.; Gouvea, E.P.; Martins, A.B.B.; Nunes, N.S.V.; Carestiato, L.V.; Petriz, J.L.F.; et al. Takotsubo Multicenter Registry (REMUTA)—Clinical Aspects, In-Hospital Outcomes, and Long-Term Mortality. Arq. Bras. Cardiol. 2020, 115, 207–216. [Google Scholar] [CrossRef]
- Sangen, H.; Imori, Y.; Tara, S.; Yamamoto, T.; Takano, H.; Shimizu, W. Haemodynamic deterioration due to intra-aortic balloon counterpulsation in takotsubo cardiomyopathy. Eur. Heart J. 2018, 39, 2118. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Nakagaito, M.; Hori, M.; Ueno, H.; Kinugawa, K. A case of Takotsubo cardiomyopathy with cardiogenic shock after influenza infection successfully recovered by IMPELLA support. J. Artif. Organs. 2019, 22, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Oh, I.D.; Shin, E.; Lee, S.; Jeon, J.M.; Kim, H.T.; Youn, H.C. Extracorporeal membrane oxygenation for takotsubo cardiomyopathy that developed after mitral valve replacement. Acute Crit. Care 2020, 35, 51–55. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Park, S.; Choi, Y.S. Extracorporeal membrane oxygenation in a patient with stress-induced cardiomyopathy after caesarean section. Anaesth. Intensive Care 2011, 39, 954–957. [Google Scholar] [CrossRef]
- Rashed, A.; Won, S.; Saad, M.; Schreiber, T. Use of the Impella 2.5 left ventricular assist device in a patient with cardiogenic shock secondary to takotsubo cardiomyopathy. BMJ Case Rep. 2015, 2015, bcr2014208354. [Google Scholar] [CrossRef]
- Mohammedzein, A.; Taha, A.; Salwan, A.; Nambiar, R. Impella Use in Cardiogenic Shock Due to Takotsubo Cardiomyopathy With Left Ventricular Outflow Tract Obstruction. JACC Case Rep. 2019, 1, 161–165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).