Effect of Respiratory Failure on Peripheral and Organ Perfusion Markers in Severe COVID-19: A Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Fingertips Thermography
2.2. Pulse Oximetry
2.3. Capillary Refill Time
2.4. Doppler Ultrasound
2.5. High-Resolution Computed Tomography
2.6. Oxygenation Ratio—PaO2/FiO2
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.Covid19treatmentguidelines.Nih.Gov/ (accessed on 1 December 2023).
- Ince, C. The Microcirculation Is the Motor of Sepsis. Crit. Care 2005, 9 (Suppl. 4), S13–S19. [Google Scholar] [CrossRef] [PubMed]
- Edul, V.S.K.; Eguillor, J.F.C.; Ferrara, G.; Estenssoro, E.; Siles, D.S.P.; Cesio, C.E.; Dubin, A. Microcirculation Alterations in Severe COVID-19 Pneumonia. J. Crit. Care 2021, 61, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, B.; Sheng, X.; Jin, N. Accuracy of Stroke Volume Variation in Predicting Fluid Responsiveness: A Systematic Review and Meta-Analysis. J. Anesthesia 2011, 25, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; La Via, L.; Dezio, V.; Amelio, P.; Genoese, G.; Franchi, F.; Messina, A.; Robba, C.; Noto, A. Inferior Vena Cava Distensibility from Subcostal and Trans-Hepatic Imaging Using Both M-Mode or Artificial Intelligence: A Prospective Study on Mechanically Ventilated Patients. Intensive Care Med. Exp. 2023, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; La Via, L.; Dezio, V.; Santonocito, C.; Amelio, P.; Genoese, G.; Astuto, M.; Noto, A. Assessment of the Inferior vena Cava Collapsibility from Subcostal and Trans-Hepatic Imaging Using Both M-Mode or Artificial Intelligence: A Prospective Study on Healthy Volunteers. Intensive Care Med. Exp. 2023, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Moore, F.A.; Bellomo, R.; Marini, J.J. 32—Cardiovascular Monitoring. In Textbook of Critical Care, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-323-75929-8. [Google Scholar]
- Vincent, J.-L.; Moore, F.A.; Bellomo, R.; Marini, J.J. 80—Resusciation from Circulatory Shock. In Textbook of Critical Care; Elsevier: Amsterdam, The Netherlands, 2023; p. 663. ISBN 978-0-323-75929-8. [Google Scholar]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary Manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Zhao, Y.-Y.; Evans, C.E. The Stimulation of Thrombosis by Hypoxia. Thromb. Res. 2019, 181, 77–83. [Google Scholar] [CrossRef]
- Thachil, J.; Tang, N.; Gando, S.; Falanga, A.; Cattaneo, M.; Levi, M.; Clark, C.; Iba, T. ISTH Interim Guidance on Recognition and Management of Coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 1023–1026. [Google Scholar] [CrossRef]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine Elevation in Severe and Critical COVID-19: A Rapid Systematic Review, Meta-Analysis, and Comparison with Other Inflammatory Syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Coagulation and Sepsis. Thromb. Res. 2017, 149, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.D.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the Cardiovascular Consequences. Am. J. Physiol. Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, K.Y.; Huang, Y.; Lui, K.O. Endothelial Contribution to COVID-19: An Update on Mechanisms and Therapeutic Implications. J. Mol. Cell. Cardiol. 2022, 164, 69–82. [Google Scholar] [CrossRef]
- Beigee, F.S.; Toutkaboni, M.P.; Khalili, N.; Nadji, S.A.; Dorudinia, A.; Rezaei, M.; Askari, E.; Farzanegan, B.; Marjani, M.; Rafiezadeh, A. Diffuse Alveolar Damage and Thrombotic Microangiopathy Are the Main Histopathological Findings in Lung Tissue biopsy Samples of COVID-19 Patients. Pathol. Res. Pract. 2020, 216, 153228. [Google Scholar] [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A.B.; Nie, X.; et al. Renal Histopathological Analysis of 26 Postmortem Findings of Patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Copin, M.-C.; Parmentier, E.; Duburcq, T.; Poissy, J.; Mathieu, D.; The Lille COVID-19 ICU and Anatomopathology Group, A. Time to Consider Histologic Pattern of Lung Injury to Treat Critically Ill Patients with COVID-19 Infection. Intensive Care Med. 2020, 46, 1124–1126. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients with COVID-19: A Prospective Cohort Study. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Gill, S.E.; Lawson COVID19 Study Team; dos Santos, C.C.; O’gorman, D.B.; Carter, D.E.; Patterson, E.K.; Slessarev, M.; Martin, C.; Daley, M.; Miller, M.R.; et al. Transcriptional Profiling of Leukocytes in Critically Ill COVID19 Patients: Implications for Interferon Response and Coagulation. Intensive Care Med. Exp. 2020, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Hariri, G.; Joffre, J.; Leblanc, G.; Bonsey, M.; Lavillegrand, J.-R.; Urbina, T.; Guidet, B.; Maury, E.; Bakker, J.; Ait-Oufella, H. Narrative Review: Clinical Assessment of Peripheral Tissue Perfusion in Septic Shock. Ann. Intensive Care 2019, 9, 37. [Google Scholar] [CrossRef]
- Hernández, G.; Castro, R.; Bakker, J. Capillary Refill Time: The Missing Link between Macrocirculation and Microcirculation in Septic Shock? J. Thorac. Dis. 2020, 12, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Dosal, A.; Kolosovas-Machuca, E.S.; Rivera-Vega, R.; Simón, J.; González, F.J. Use of Infrared Thermography in Children with Shock: A Case Series. SAGE Open Med. Case Rep. 2014, 2, 2050313X14561779. [Google Scholar] [CrossRef]
- Ammer, K. The Glamorgan Protocol for Recording and Evaluation of Thermal Images of the Human Body. Thermol. Int. 2008, 18, 125–144. [Google Scholar]
- Kaplan, L.J.; McPartland, K.; Santora, T.A.; Trooskin, S.Z. Start with a Subjective Assessment of Skin Temperature to Identify Hypoperfusion in Intensive Care Unit Patients. J. Trauma Inj. Infect. Crit. Care 2001, 50, 620–627. [Google Scholar] [CrossRef]
- Thompson, M.J.; Ninis, N.; Perera, R.; Mayon-White, R.; Phillips, C.; Bailey, L.; Harnden, A.; Mant, D.; Levin, M. Clinical Recognition of Meningococcal Disease in Children and Adolescents. Lancet 2006, 367, 397–403. [Google Scholar] [CrossRef]
- Evans, J.A.; May, J.; Ansong, D.; Antwi, S.; Asafo-Adjei, E.; Nguah, S.B.; Osei-Kwakye, K.; Akoto, A.O.Y.; Ofori, A.O.; Sambian, D.; et al. Capillary Refill Time as an Independent Prognostic Indicator in Severe and Complicated Malaria. J. Pediatr. 2006, 149, 676–681. [Google Scholar] [CrossRef]
- Lima, A.; Jansen, T.C.; van Bommel, J.; Ince, C.; Bakker, J. The Prognostic Value of the Subjective Assessment of Peripheral Perfusion in Critically Ill Patients. Crit. Care Med. 2009, 37, 934–938. [Google Scholar] [CrossRef]
- Magnin, M.; Junot, S.; Cardinali, M.; Ayoub, J.Y.; Paquet, C.; Louzier, V.; Garin, J.M.B.; Allaouchiche, B. Use of Infrared Thermography to Detect Early Alterations of Peripheral Perfusion: Evaluation in a Porcine Model. Biomed. Opt. Express 2020, 11, 2431–2446. [Google Scholar] [CrossRef]
- Sood, M.; Mandelzweig, K.; Rigatto, C.; Tangri, N.; Komenda, P.; Martinka, G.; Arabi, Y.; Keenan, S.; Kumar, A.; Kumar, A. Non-Pulmonary Infections but Not Specific Pathogens Are Associated with Increased Risk of AKI in Septic Shock. Intensive Care Med. 2014, 40, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Nisula, S.; Kaukonen, K.-M.; Vaara, S.T.; Korhonen, A.-M.; Poukkanen, M.; Karlsson, S.; Haapio, M.; Inkinen, O.; Parviainen, I.; Suojaranta-Ylinen, R.; et al. Incidence, Risk Factors and 90-Day Mortality of Patients with Acute Kidney Injury in Finnish Intensive Care Units: The FINNAKI Study. Intensive Care Med. 2013, 39, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Matejovic, M.; Ince, C.; Chawla, L.S.; Blantz, R.; Molitoris, B.A.; Rosner, M.H.; Okusa, M.D.; Kellum, J.A.; Ronco, C.; Group, A.X.W. Renal Hemodynamics in AKI: In Search of New Treatment Targets. J. Am. Soc. Nephrol. 2016, 27, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gomez, H.; Ince, C.; De Backer, D.; Pickkers, P.; Payen, D.; Hotchkiss, J.; Kellum, J.A. A Unified Theory of Sepsis-Induced Acute Kidney Injury: Inflammation, Microcirculatory Dysfunction, Bioenergetics, and the Tubular Cell Adaptation to Injury. Shock 2014, 41, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Libert, N.; Harrois, A.; Duranteau, J. Haemodynamic Coherence in Haemorrhagic Shock. Best Pract. Res. Clin. Anaesthesiol. 2016, 30, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Scholbach, T.; Dimos, I.; Scholbach, J. A New Method of Color Doppler Perfusion Measurement via Dynamic Sonographic Signal Quantification in Renal Parenchyma. Nephron Physiol. 2004, 96, p99–p104. [Google Scholar] [CrossRef] [PubMed]
- Scholbach, T. Dynamic Tissue Perfusion Measurement—Basics and Applications. In Sonography; Thoirs, K., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-307-947-9. [Google Scholar]
- Harrois, A.; Grillot, N.; Figueiredo, S.; Duranteau, J. Acute Kidney Injury Is Associated with a Decrease in Cortical Renal Perfusion during Septic Shock. Crit. Care 2018, 22, 161. [Google Scholar] [CrossRef]
- Lubas, A.; Kade, G.; Saracyn, M.; Niemczyk, S.; Dyrla, P. Dynamic Tissue Perfusion Assessment Reflects Associations between Antihypertensive Treatment and Renal Cortical Perfusion in Patients with Chronic Kidney Disease and Hypertension. Int. Urol. Nephrol. 2018, 50, 509–516. [Google Scholar] [CrossRef]
- Scholbach, T.M.; Vogel, C.; Bergner, N. Color Doppler Sonographic Dynamic Tissue Perfusion Measurement Demonstrates Significantly Reduced Cortical Perfusion in Children with Diabetes Mellitus Type 1 without Microalbuminuria and Apparently Healthy Kidneys. Ultraschall Med. Eur. J. Ultrasound 2014, 35, 445–450. [Google Scholar] [CrossRef]
- Scholbach, T.; Sachse, C. Color-Doppler Sonographic Tissue Perfusion Measurements Reveal Significantly Diminished Renal Cortical Perfusion in Kidneys with Vesicoureteral Reflux. Indian J. Nephrol. 2016, 26, 102–106. [Google Scholar] [CrossRef]
- Lubas, A.; Kade, G.; Ryczek, R.; Banasiak, P.; Dyrla, P.; Szamotulska, K.; Schneditz, D.; Niemczyk, S. Ultrasonic Evaluation of Renal Cortex Arterial Area Enables Differentiation between Hypertensive and Glomerulonephritis-Related Chronic Kidney Disease. Int. Urol. Nephrol. 2017, 49, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Lubas, A.; Zegadło, A.; Frankowska, E.; Klimkiewicz, J.; Jędrych, E.; Niemczyk, S. Ultrasound Doppler Flow Parameters Are Independently Associated with Renal Cortex Contrast-Enhanced Multidetector Computed Tomography Perfusion and Kidney Function. J. Clin. Med. 2023, 12, 2111. [Google Scholar] [CrossRef]
- Rustecki, B.; Rustecka, A.; Kalicki, B.; Ring, F.; Kwasiborski, P.; Truszczyński, A.; Jung, A. A Study of Heat Loss in Patients Undergoing General Anesthesia Warmed with a Heated Mattress with Esophageal Temperature Monitoring Compared to Facial Infrared Thermography. J. Med. Imaging Health Inform. 2016, 6, 141–145. [Google Scholar] [CrossRef]
- Hernández, G.; Ospina-Tascón, G.A.; Damiani, L.P.; Estenssoro, E.; Dubin, A.; Hurtado, J.; Friedman, G.; Castro, R.; Alegría, L.; Teboul, J.-L.; et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients with Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA 2019, 321, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Francone, M.; Iafrate, F.; Masci, G.M.; Coco, S.; Cilia, F.; Manganaro, L.; Panebianco, V.; Andreoli, C.; Colaiacomo, M.C.; Zingaropoli, M.A.; et al. Chest CT Score in COVID-19 Patients: Correlation with Disease Severity and Short-Term Prognosis. Eur. Radiol. 2020, 30, 6808–6817. [Google Scholar] [CrossRef]
- Grasselli, G.; Calfee, C.S.; Camporota, L.; Poole, D.; Amato, M.B.P.; Antonelli, M.; Arabi, Y.M.; Baroncelli, F.; Beitler, J.R.; Bellani, G.; et al. ESICM Guidelines on Acute Respiratory Distress Syndrome: Definition, Phenotyping and Respiratory Support Strategies. Intensive Care Med. 2023, 49, 727–759. [Google Scholar] [CrossRef] [PubMed]
- Segal, S.S. Regulation of Blood Flow in the Microcirculation. Microcirculation 2005, 12, 33–45. [Google Scholar] [CrossRef]
- De Backer, D.; Donadello, K.; Favory, R. Link between Coagulation Abnormalities and Microcirculatory Dysfunction in Critically Ill Patients. Curr. Opin. Anaesthesiol. 2009, 22, 150–154. [Google Scholar] [CrossRef]
- De Backer, D.; Ortiz, J.A.; Salgado, D. Coupling Microcirculation to Systemic Hemodynamics. Curr. Opin. Crit. Care 2010, 16, 250–254. [Google Scholar] [CrossRef]
- Lamprea, S.; Fernández-Sarmiento, J.; Barrera, S.; Mora, A.; Fernández-Sarta, J.P.; Acevedo, L. Capillary Refill Time in Sepsis: A Useful and Easily Accessible Tool for Evaluating Perfusion in Children. Front. Pediatr. 2022, 10, 1035567. [Google Scholar] [CrossRef]
- Kazune, S.; Vasiljevs, E.; Caica-Rinca, A.; Marcinkevics, Z.; Grabovskis, A. Infrared Thermography Imaging for Assessment of Peripheral Perfusion in Patients with Septic Shock. Bioengineering 2023, 10, 729. [Google Scholar] [CrossRef]
- Amson, H.; Vacheron, C.-H.; Thiolliere, F.; Piriou, V.; Magnin, M.; Allaouchiche, B. Core-to-Skin Temperature Gradient Measured by Thermography Predicts Day-8 Mortality in Septic Shock: A Prospective Observational Study. J. Crit. Care 2020, 60, 294–299. [Google Scholar] [CrossRef] [PubMed]
- De Backer, D.; Cortes, D.O.; Donadello, K.; Vincent, J.-L. Pathophysiology of Microcirculatory Dysfunction and the Pathogenesis of Septic Shock. Virulence 2014, 5, 73–79. [Google Scholar] [CrossRef]
- Post, E.H.; Kellum, J.A.; Bellomo, R.; Vincent, J.-L. Renal Perfusion in Sepsis: From Macro- to Microcirculation. Kidney Int. 2017, 91, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Jakob, S.M.; Takala, J. ARDS: Monitoring Tissue Perfusion. Crit. Care Clin. 2002, 18, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Mok, G.; Hendin, A.; Reardon, P.; Hickey, M.; Gray, S.; Yadav, K. Macrocirculatory and Microcirculatory Endpoints in Sepsis Resuscitation. J. Intensive Care Med. 2021, 36, 1385–1391. [Google Scholar] [CrossRef]
- Luther, T.; Eckerbom, P.; Cox, E.; Lipcsey, M.; Bülow, S.; Hultström, M.; Torrente, F.M.; Weis, J.; Palm, F.; Francis, S.; et al. Decreased Renal Perfusion during Acute Kidney Injury in Critical COVID-19 Assessed by Magnetic Resonance Imaging: A Prospective Case Control Study. Crit. Care 2022, 26, 262. [Google Scholar] [CrossRef]
- He, H.-W.; Liu, D.-W.; Long, Y.; Wang, X.-T. The Peripheral Perfusion Index and Transcutaneous Oxygen Challenge Test Are Predictive of Mortality in Septic Patients after Resuscitation. Crit. Care 2013, 17, R116. [Google Scholar] [CrossRef]
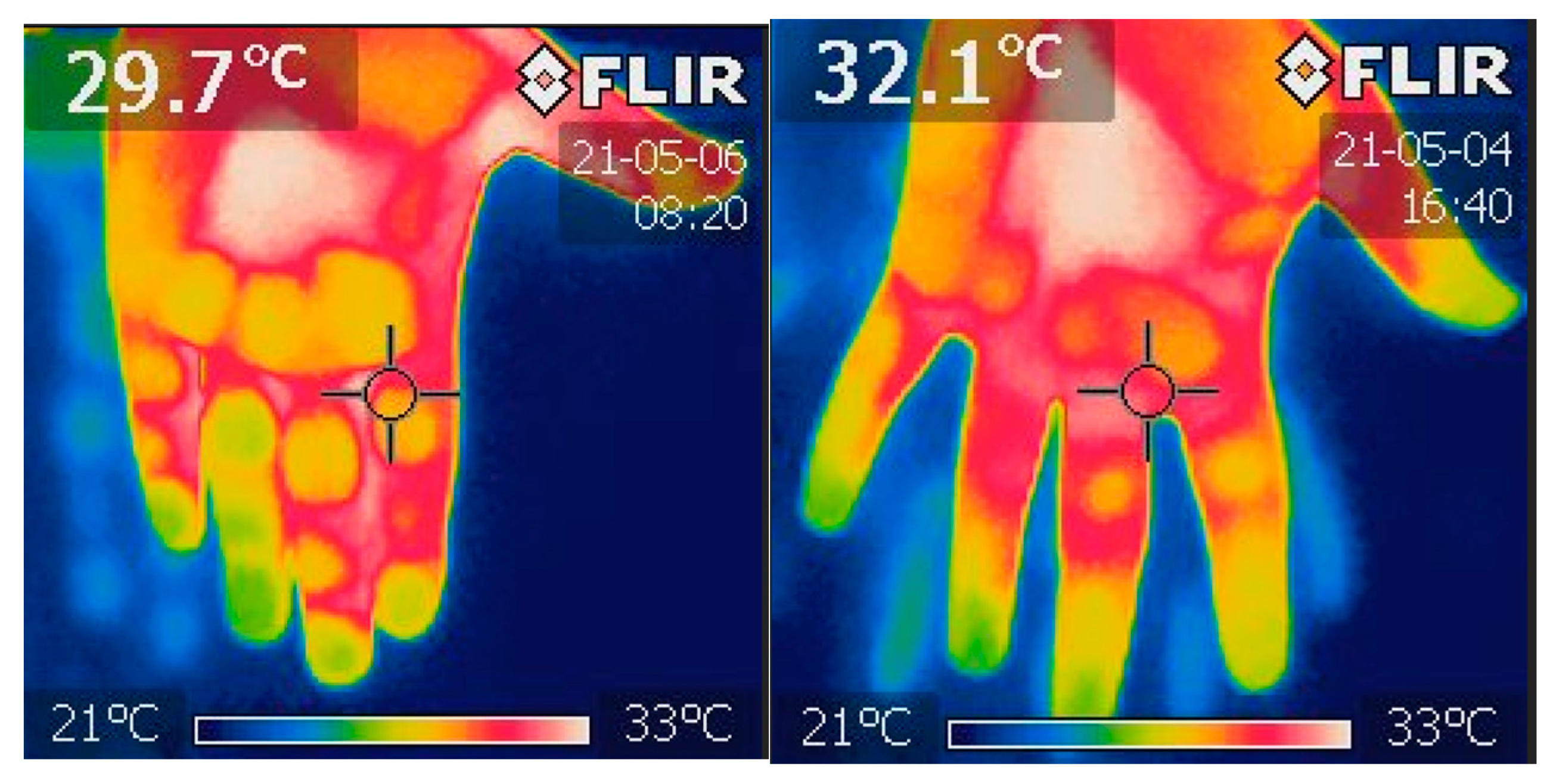


| Variable | N | % | ||
|---|---|---|---|---|
| Gender | Male | 70 | 60.3 | |
| Female | 46 | 39.7 | ||
| Ward Type | Intensive Care Unit | 38 | 32.8 | |
| High-Dependency Unit | 78 | 67.2 | ||
| Comorbidity | Malignancy | 11 | 9.5 | |
| Obesity | 19 | 16.4 | ||
| Chronic Kidney Disease | Non-dialyzed | 4 | 3.4 | |
| Dialyzed | 1 | 0.9 | ||
| Coronary Artery Disease | 7 | 6.0 | ||
| Heart Failure | 6 | 5.2 | ||
| Myocardial infarction | 2 | 1.7 | ||
| CABG/Stent | 7 | 6.0 | ||
| Atrial fibrillation | Chronic | 7 | 6.0 | |
| De novo | 4 | 3.4 | ||
| Hypertension | 33 | 28.4 | ||
| Diabetes | Non-insulin | 14 | 12.1 | |
| Insulin dependent | 8 | 6.9 | ||
| Asthma | 7 | 6.0 | ||
| Chronic Obstructive Pulmonary Disease | 6 | 5.2 | ||
| Smoker | 4 | 3.4 | ||
| ARDS | PaO2/FiO2 > 300 | No ARDS | 34 | 29.3 |
| PaO2/FiO2 300 to 200 | MILD | 24 | 20.7 | |
| PaO2/FiO2 200 to 100 | MODERATE | 27 | 23.3 | |
| PaO2/FiO2 < 100 | SEVERE | 31 | 26.7 | |
| Median (Mean) | IQR (±SD) | |
|---|---|---|
| CT score [0/25] | 16.0 | 10.0 |
| FiO2 [%] | 0.40 | 0.59 |
| PaO2 [mmHg] | 75.0 | 24.0 |
| Oxygenation ratio | 205.0 | 215.5 |
| Saturation [%] * | (93.7) | (±2.7) |
| FIT [°C] | 32.6 | 4.0 |
| CRT [s] | 3.0 | 1.8 |
| RCP [cm/s] | 0.149 | 0.224 |
| MAP [mmHg] | 94.3 | 15.0 |
| SBP [mmHg] | 130.0 | 21.0 |
| DBP [mmHg] * | (78.4) | (±12.6) |
| HR [1/min] | 84.0 | 21.5 |
| Albumin [g/dL] * | (3.1) | (±0.4) |
| Creatinine [mg/dL] | 0.90 | 0.40 |
| Urea [mg/dL] | 35.0 | 24.0 |
| LDH [U/L] | 408.5 | 244.0 |
| Variable | Correlation Coefficient—r | p-Value |
|---|---|---|
| Saturation and Creatinine | −0.194 | 0.038 |
| Saturation and LDH | −0.314 | 0.002 |
| CRT and Oxygenation Ratio | −0.202 | 0.030 |
| CRT and HR | 0.196 | 0.042 |
| CRT and Albumin | −0.403 | 0.005 |
| CRT and Urea | 0.235 | 0.011 |
| RCP and CT score | −0.275 | 0.010 |
| RCP and Oxygenation Ratio | 0.396 | <0.0001 |
| RCP and LDH | −0.221 | 0.049 |
| RCP and Urea | −0.213 | 0.031 |
| Variable | Correlation Coefficient—r | p-Value |
|---|---|---|
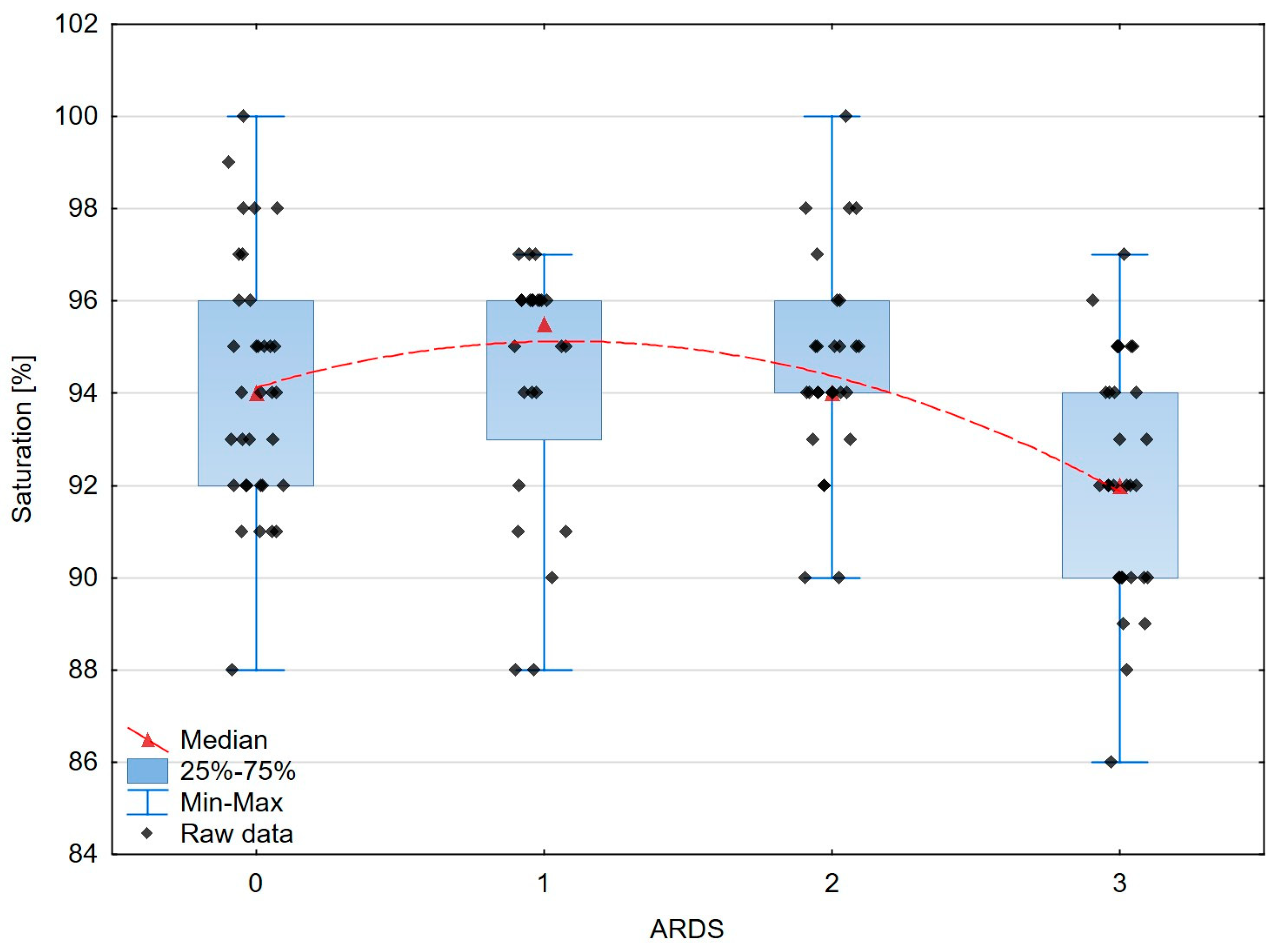
| Saturation and ARDS severity | −0.232 | 0.012 |
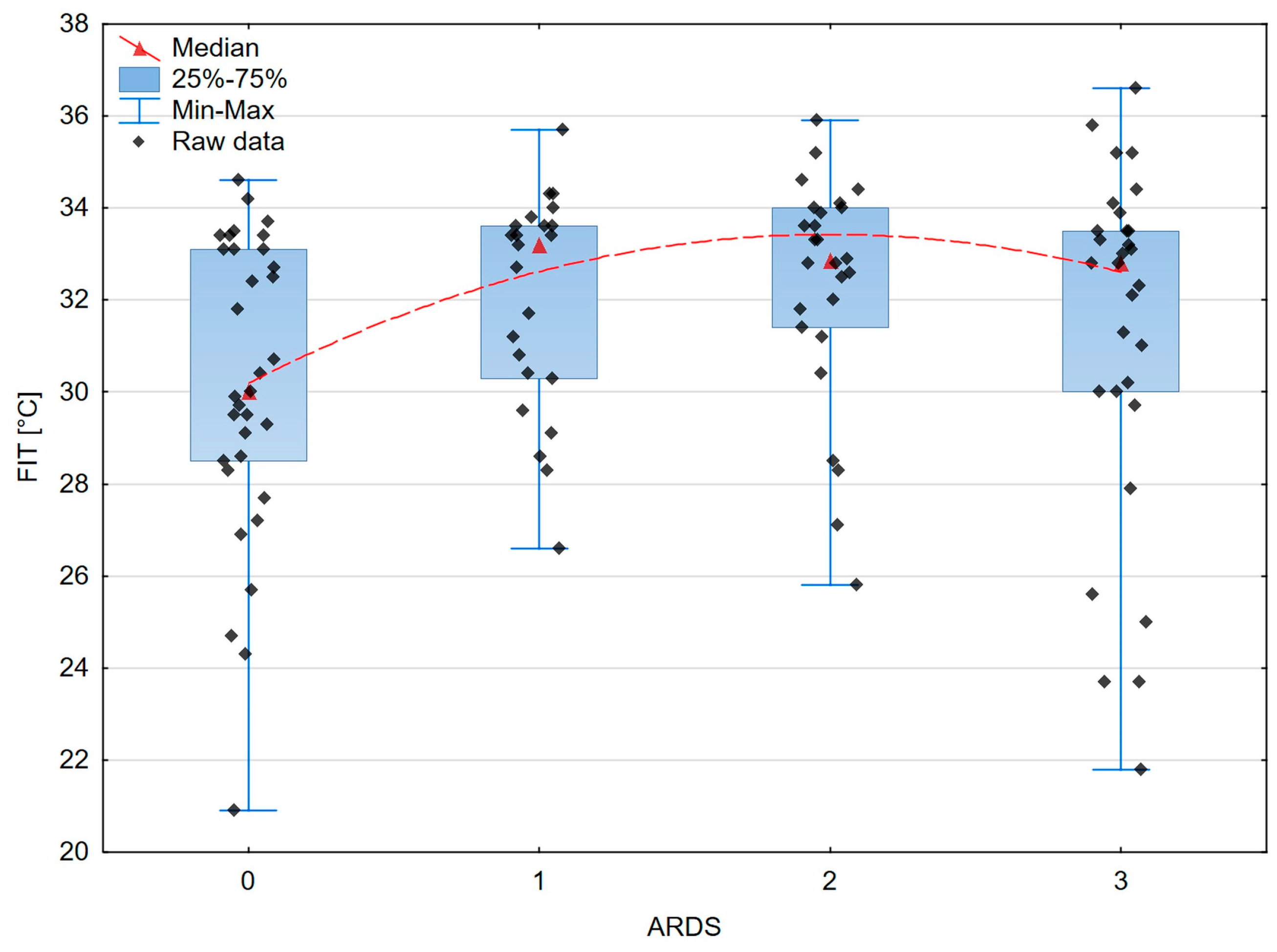
| FIT and ARDS severity | 0.183 | 0.054 |
| CRT and ARDS severity | 0.173 | 0.064 |
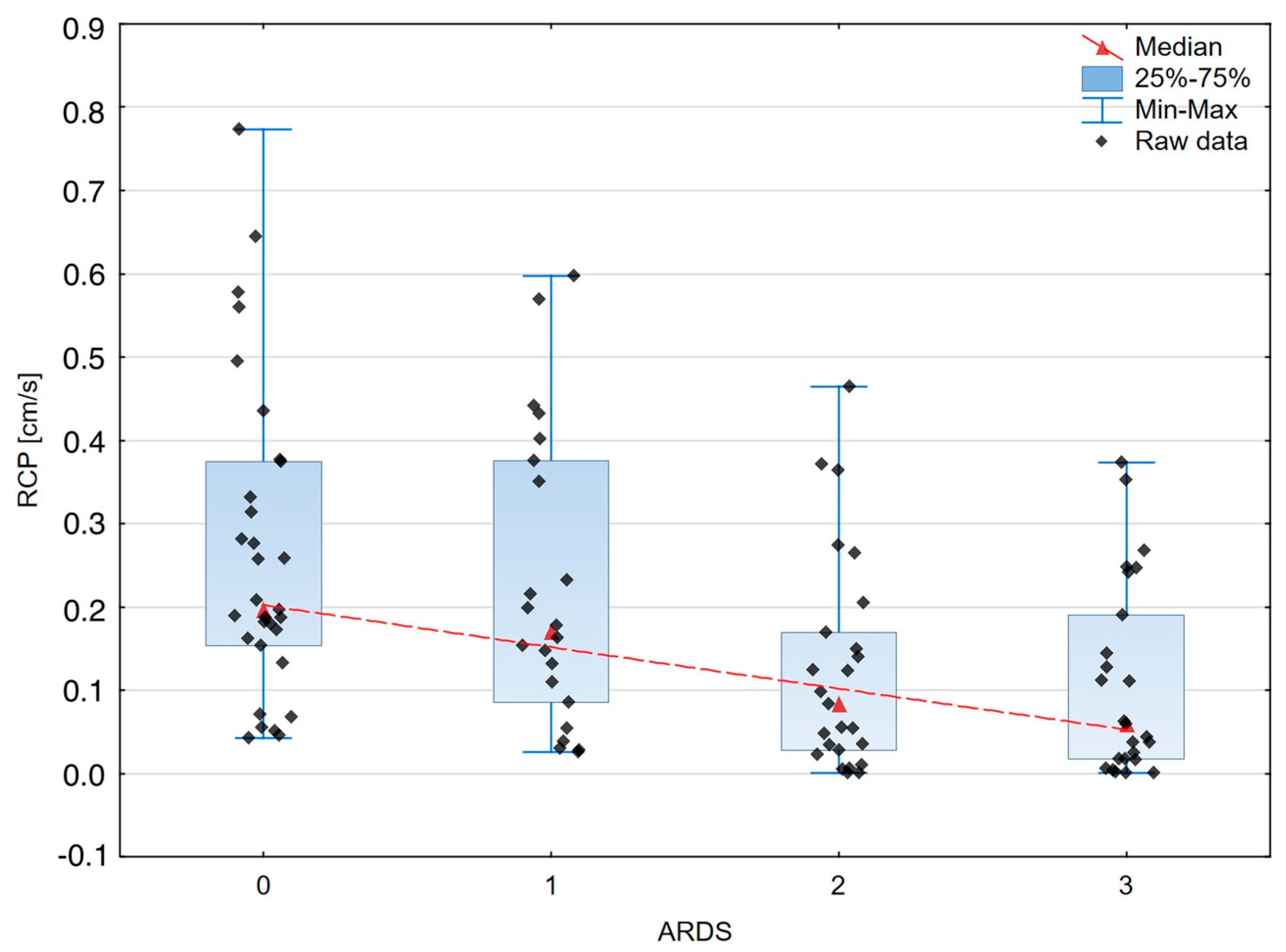
| RCP and ARDS severity | −0.426 | <0.0001 |
| ARDS | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No ARDS PaO2/FiO2 > 300 | 1 MILD PaO2/FiO2 300 to 200 | 2 MODERATE PaO2/FiO2 300 to 200 | 3 SEVERE PaO2/FiO2 300 to 200 | ||||||
| Median (Mean) | IQR (±SD) | Median (Mean) | IQR (±SD) | Median (Mean) | IQR (±SD) | Median (Mean) | IQR (±SD) | ||
| Saturation [%] | 94 | 4 | 96 | 3 | 94 | 2 | 92 | 4.0 | 0.0021 |
| FIT [°C] | 30 | 4.6 | 33.2 | 3.3 | 32.9 | 2.6 | 32.8 | 3.5 | 0.038 |
| CRT [s] | 3.0 | 1.75 | 2.5 | 1 | 3 | 1.8 | 4.0 | 2.0 | <0.0006 |
| RCP [cm/s] | 0.196 | 0.22 | 0.171 | 0.29 | 0.083 | 0.141 | 0.059 | 0.173 | <0.0002 |
| MAP [mmHg] * | (93.2) | (±12.9) | (95.2) | (±16.7) | (97.6) | (±14.4) | (95.5) | (±10.0) | 0.683 |
| SBP [mmHg] | 128 | 18 | 126 | 27 | 130 | 20 | 130 | 20 | 0.718 |
| DBP [mmHg] * | (76.4) | (±12.6) | (79.7) | (±11.6) | (81.0) | (±13.8) | (77.2) | (±12.1) | 0.474 |
| HR [1/min] | 81 | 14 | 75 | 23 | 87 | 20 | 91 | 18 | 0.052 |
| Albumin [g/dL] | 3.4 | 1.05 | 3.55 | 0.6 | 3.2 | 0.3 | 3.1 | 0.4 | 0.175 |
| Creatinine [mg/dL] | 1 | 0.4 | 0.8 | 0.2 | 0.9 | 0.5 | 1 | 0.5 | 0.151 |
| Urea [mg/dL] | 34 | 24 | 27 | 17.5 | 38 | 19 | 45 | 35 | 0.016 |
| LDH [U/L] | 365.5 | 207.5 | 319 | 109 | 435 | 195 | 591.5 | 165 | <0.00001 |
| Beta | Beta Standard Error | p-Significance | |
|---|---|---|---|
| Saturation [%] | 0.225 | 0.088 | 0.013 |
| FIT [°C] | −0.293 | 0.099 | 0.004 |
| CRT [s] | −0.247 | 0.099 | 0.015 |
| RCP [cm/s] | 0.318 | 0.088 | <0.001 |
| RCP (cm/s) | Oxygenation Ratio | 95% CI |
|---|---|---|
| 0.770 | 363 | 281–444 |
| 0.185 | 219 | 196–241 |
| 0.001 | 173 | 140–206 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutowski, M.; Klimkiewicz, J.; Rustecki, B.; Michałowski, A.; Paryż, K.; Lubas, A. Effect of Respiratory Failure on Peripheral and Organ Perfusion Markers in Severe COVID-19: A Prospective Cohort Study. J. Clin. Med. 2024, 13, 469. https://doi.org/10.3390/jcm13020469
Gutowski M, Klimkiewicz J, Rustecki B, Michałowski A, Paryż K, Lubas A. Effect of Respiratory Failure on Peripheral and Organ Perfusion Markers in Severe COVID-19: A Prospective Cohort Study. Journal of Clinical Medicine. 2024; 13(2):469. https://doi.org/10.3390/jcm13020469
Chicago/Turabian StyleGutowski, Mateusz, Jakub Klimkiewicz, Bartosz Rustecki, Andrzej Michałowski, Kamil Paryż, and Arkadiusz Lubas. 2024. "Effect of Respiratory Failure on Peripheral and Organ Perfusion Markers in Severe COVID-19: A Prospective Cohort Study" Journal of Clinical Medicine 13, no. 2: 469. https://doi.org/10.3390/jcm13020469
APA StyleGutowski, M., Klimkiewicz, J., Rustecki, B., Michałowski, A., Paryż, K., & Lubas, A. (2024). Effect of Respiratory Failure on Peripheral and Organ Perfusion Markers in Severe COVID-19: A Prospective Cohort Study. Journal of Clinical Medicine, 13(2), 469. https://doi.org/10.3390/jcm13020469





