A Review of Chronic Lateral Ankle Instability and Emerging Alternative Outcome Monitoring Tools in Patients following Ankle Ligament Reconstruction Surgery
Abstract
1. Introduction
2. Methods
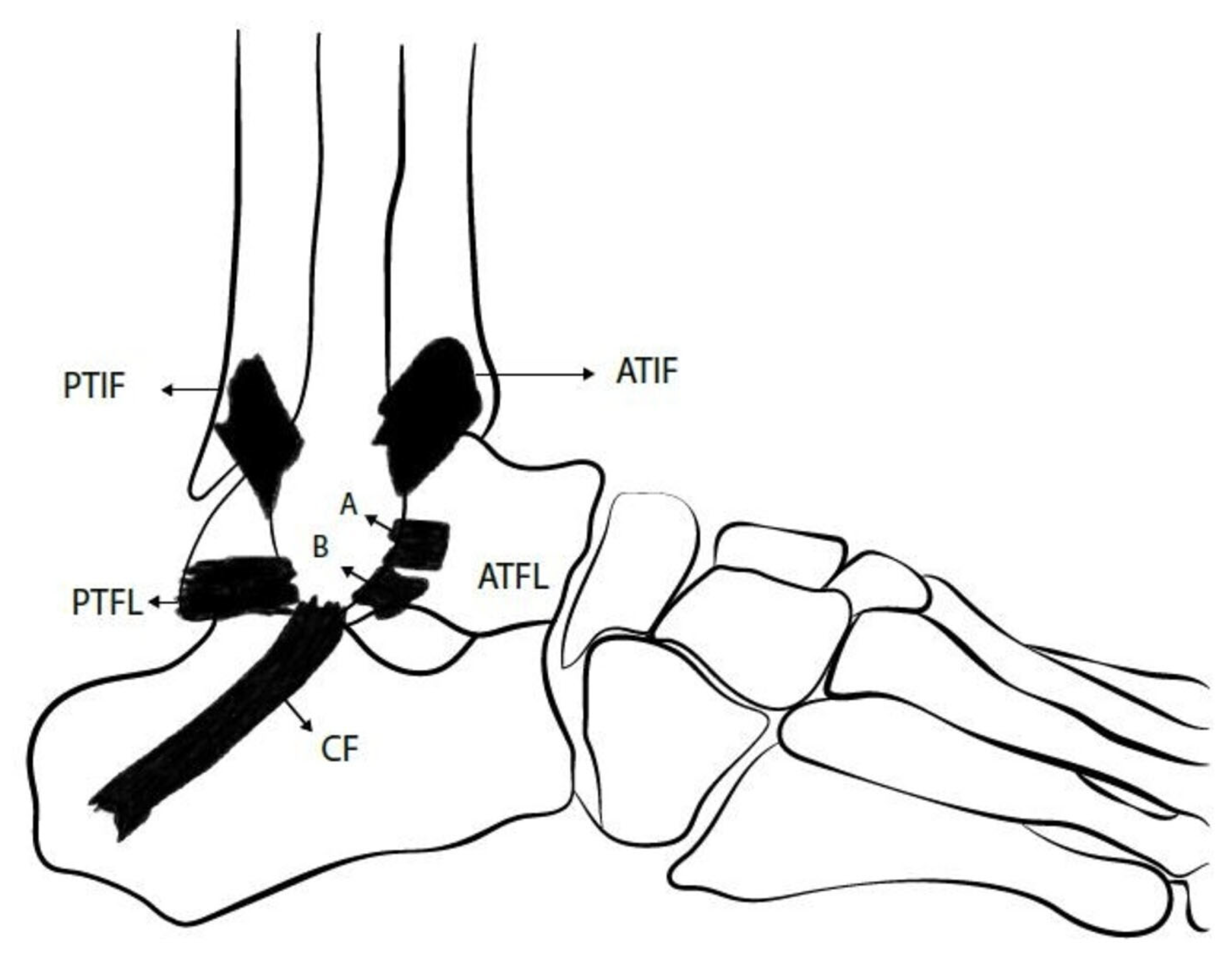
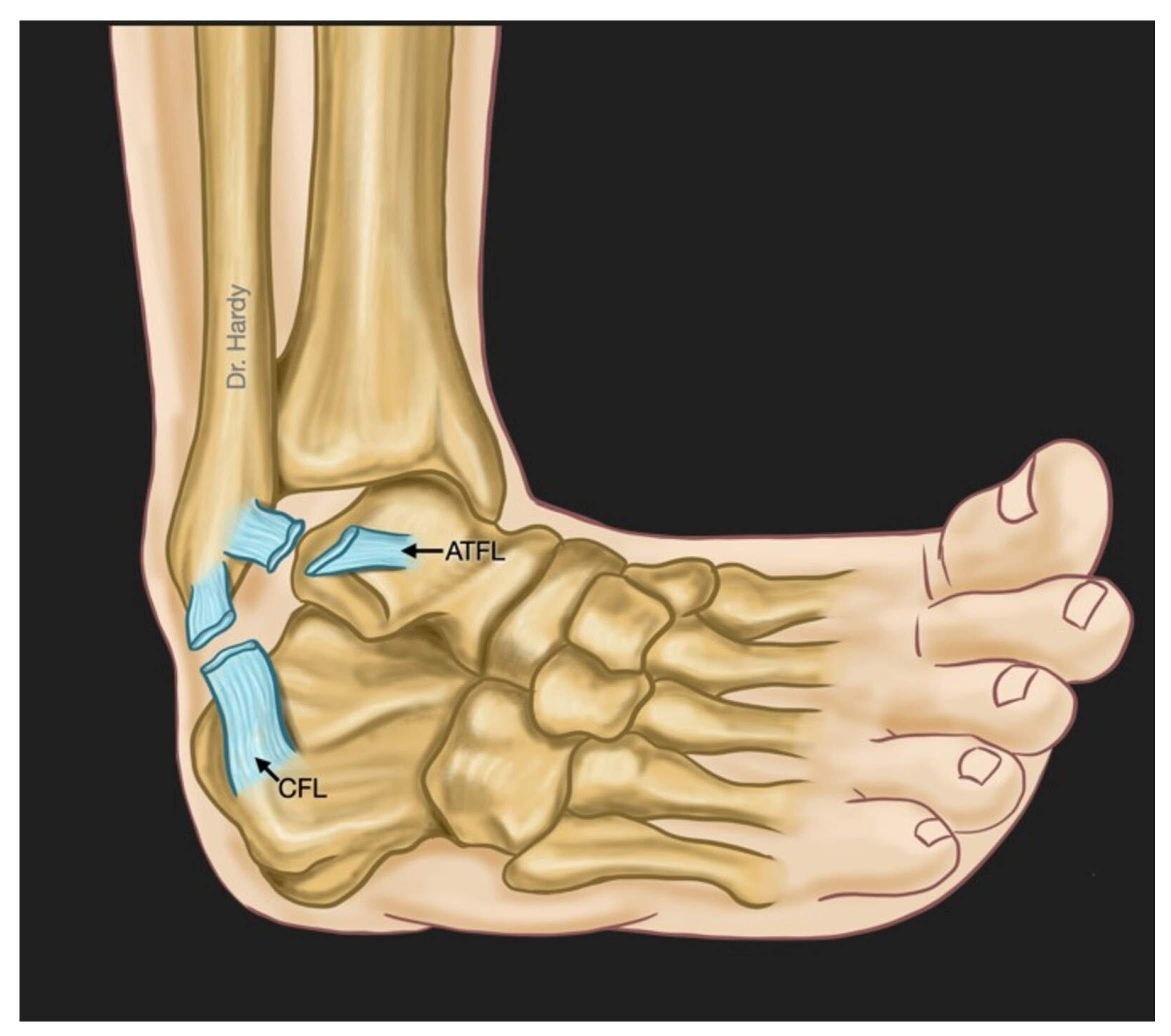
3. Overview of Ankle Lateral Ligamentous Anatomy
4. Mechanism of Injury
5. Diagnosis
6. Imaging
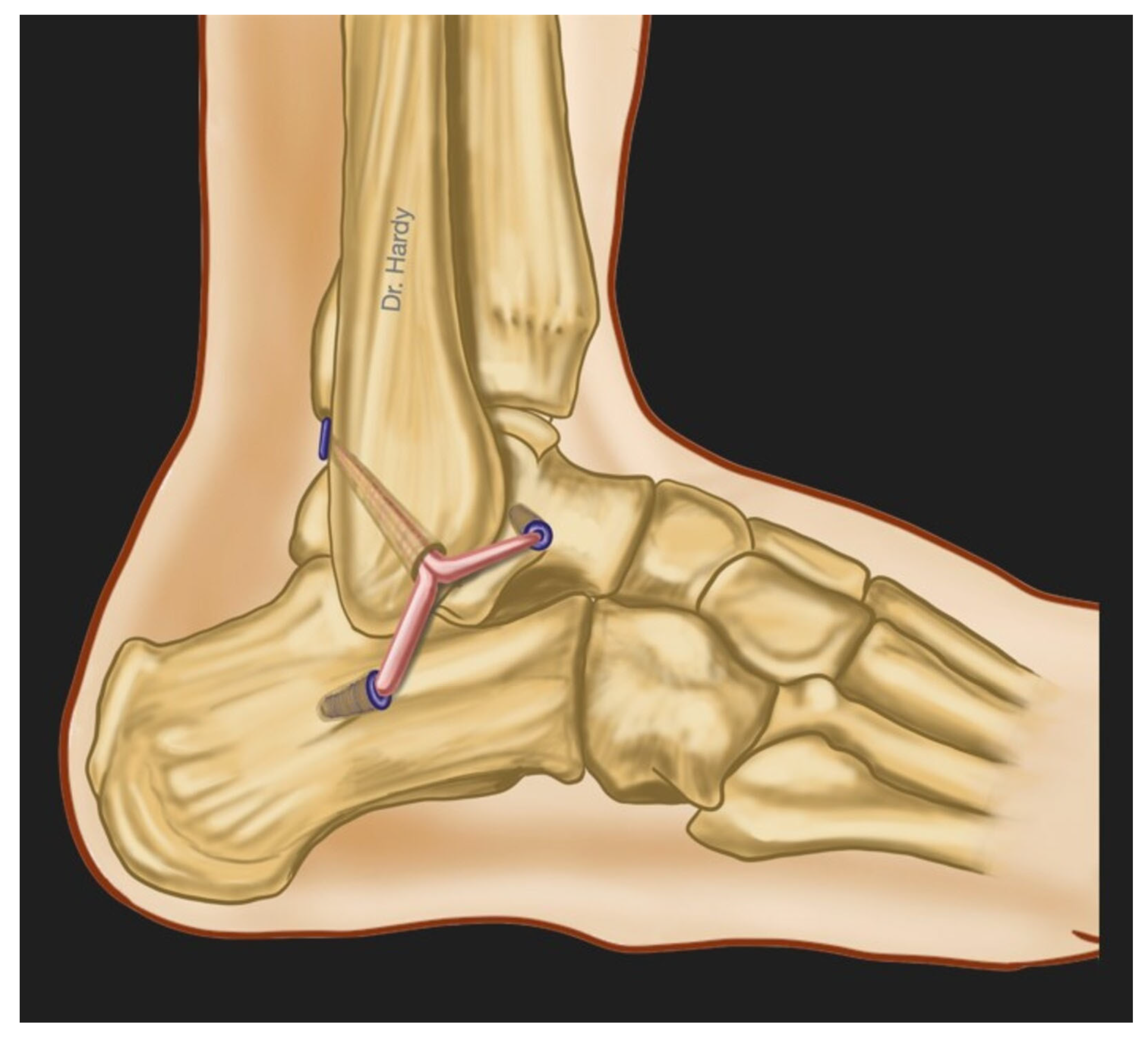
7. Surgical Treatment
8. Postoperative Rehabilitation and Management
9. Alternative Outcome Monitoring Methods in Patients after CLAI Surgery
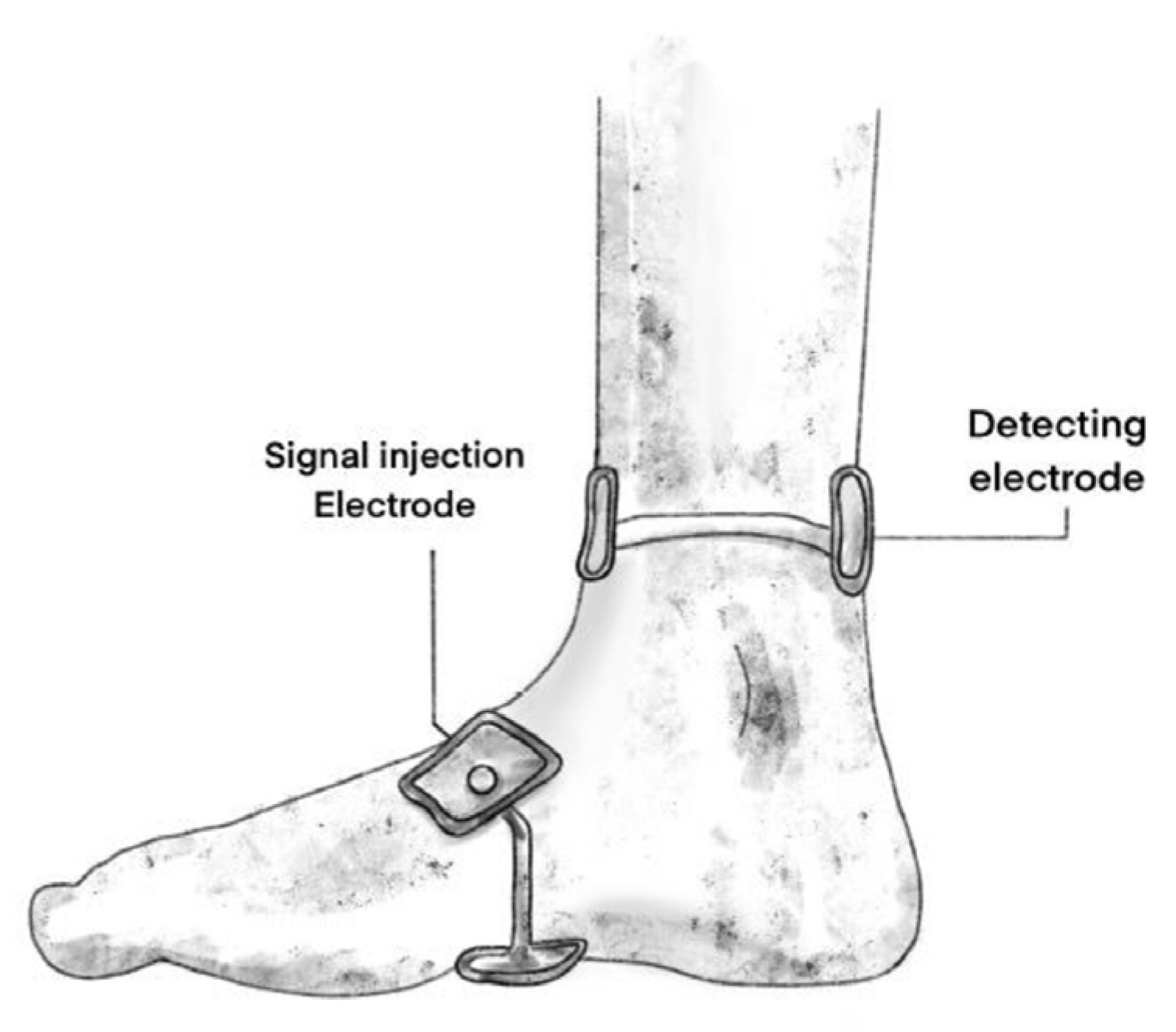
10. Bioelectrical Impedance Spectroscopy Measurement
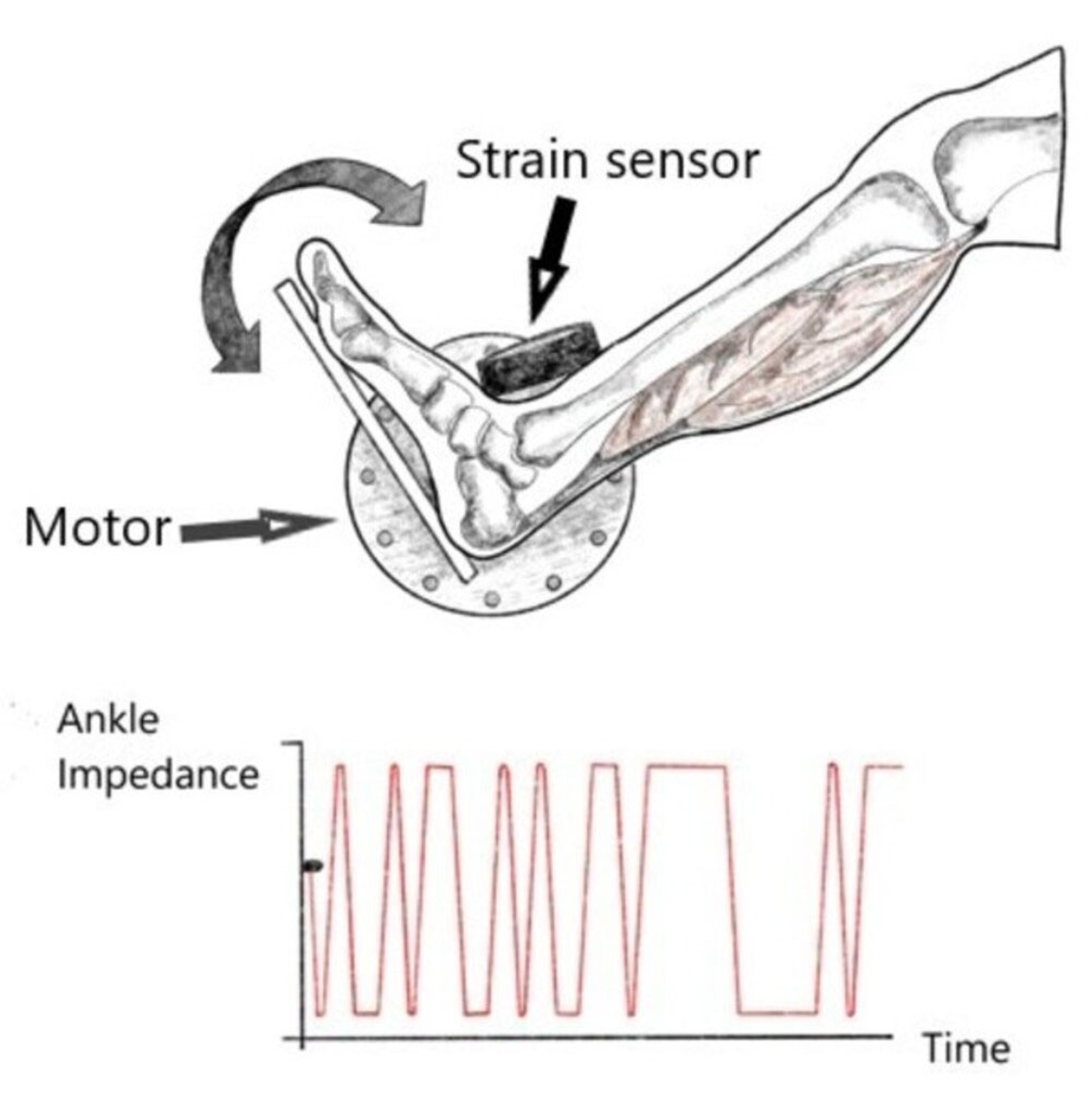
11. Mechanical Impedance Measurement
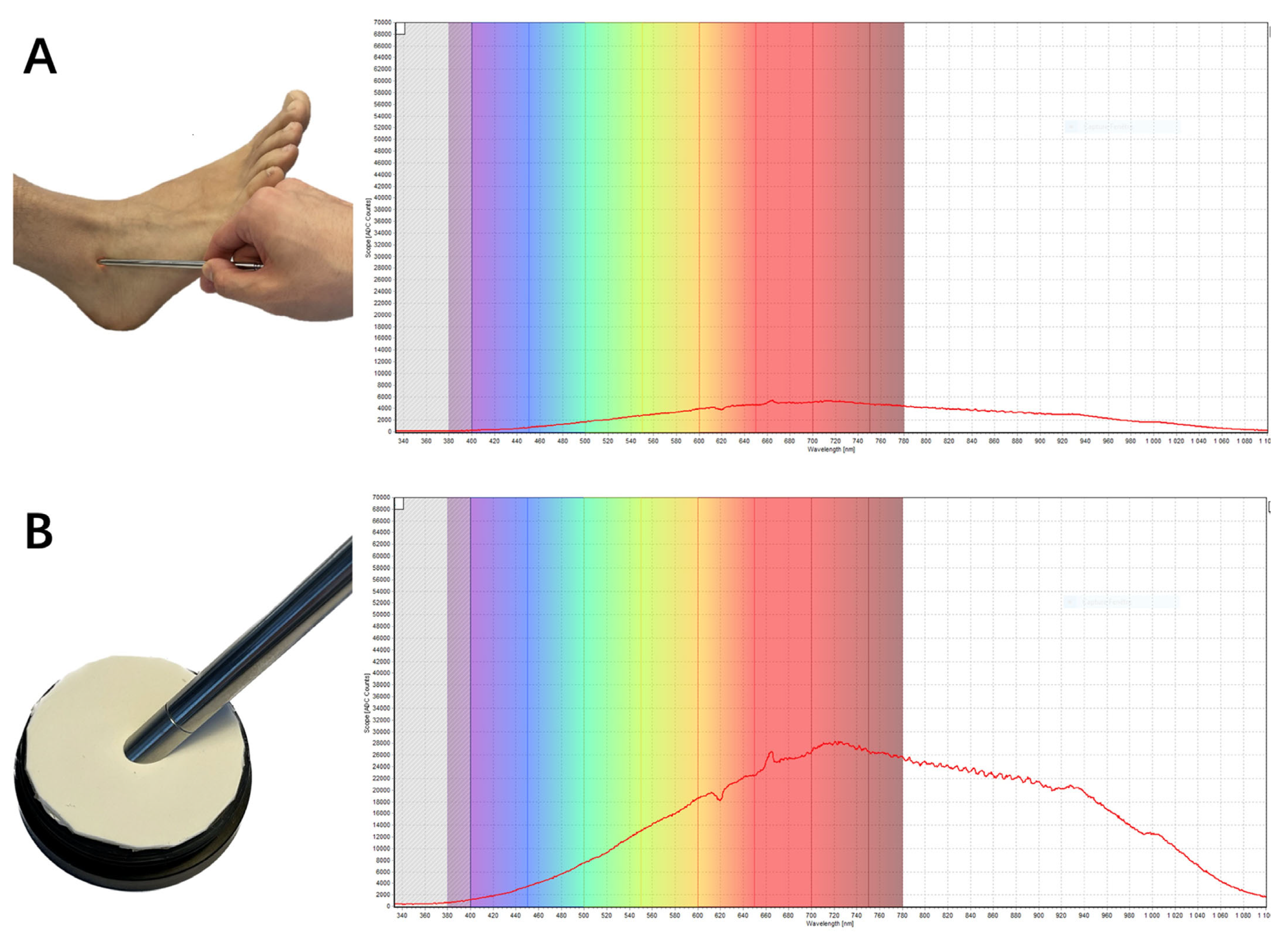
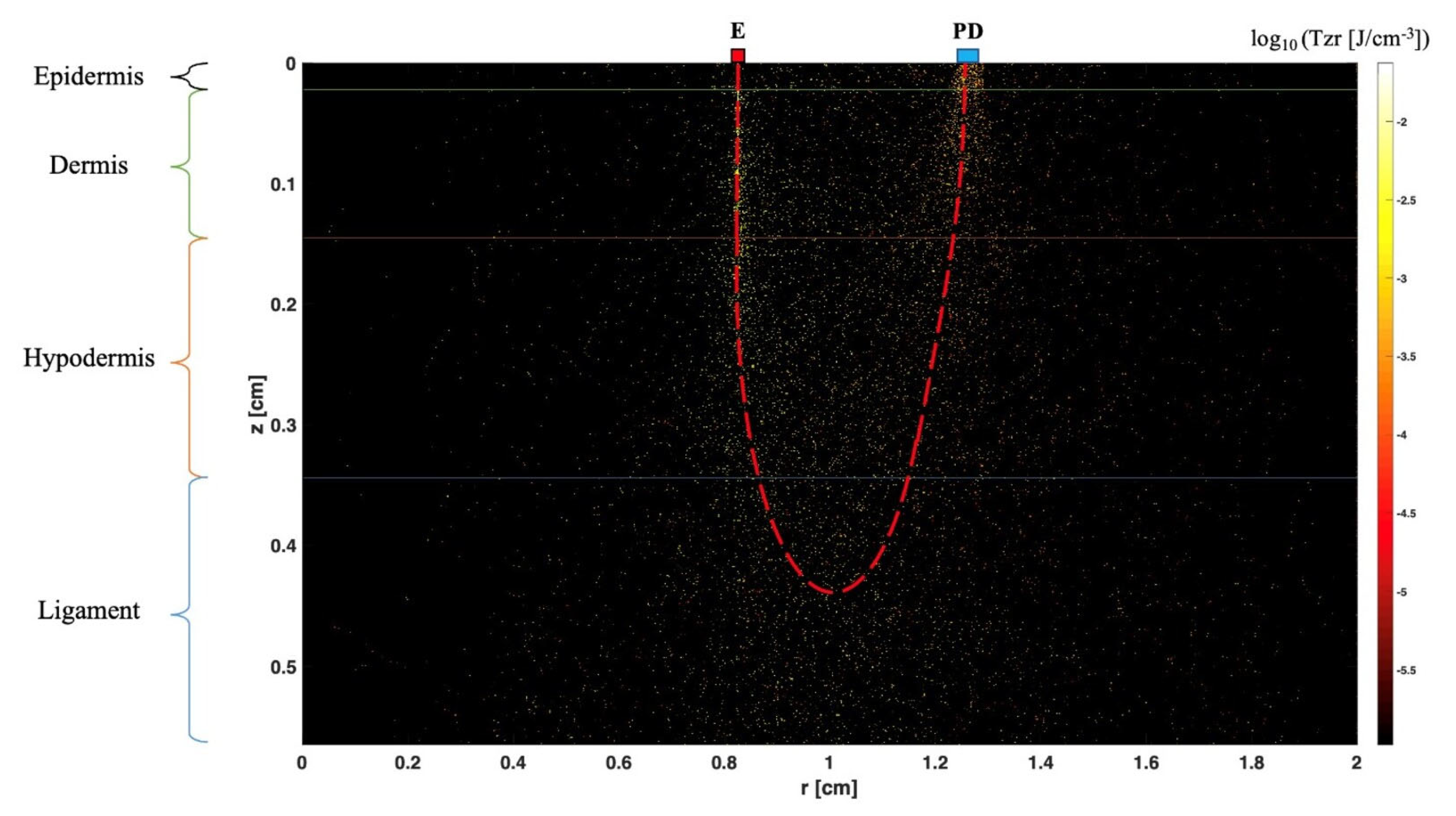
12. Near-Infrared Spectroscopy (NIRS)
13. Summary
- Ankle sprain represents a frequent pathology among athletes and the general population. Inversion-type is the most common mechanism of injury. Patients that do not respond to conservative management may develop CLAI.
- CLAI is diagnosed following a thorough anamnesis, physical examination and medical imaging including stress radiographs, MRI and ultrasonography. AD testing should be replaced by ALD testing in clinical practice because it allows detection of both anteroposterior and rotational talar instability. Stress radiographs are limited by their low sensitivity. MRI may be better used to rule out injuries that could be associated with CLAI. Dynamic US is showing good outcomes in recent published studies, but these results are yet to be validated.
- There is a wide variety of surgical procedures that address CLAI, ranging from simple repairs to more complex reconstructions. Besides non-anatomic procedures, which are increasingly abandoned due to their association with alteration of normal joint biomechanics, there is no superiority demonstrated of one technique over the others if the potential contraindications are taken into account.
- The ideal postoperative management remains unknown. There is still a lack of accurate outcome assessment methods which can reflect the healing process’s histological stage and the mechanical stability of the repaired or reconstructed ligament. In most of the published studies regarding rehabilitation protocols and management of patients after CLAI surgery, the outcomes are being measured using a wide range of functional assessment scores and stress radiographs with the absence of meaningful, precise, reliable and uniform methods to assess those outcomes.
- MRI is limited by its static character, and it seems that it cannot be a useful tool for postoperative management of patients after CLAI surgery because recent evidence showed that SNQ failed to predict the histological phase of the repaired or reconstructed healing ligament.
- The integration of BEImp, MImp and NIRS into clinical practice holds immense promise for CLAI patients. These non-invasive and robust measurement methods, widely established across medical domains, show considerable potential in assessing ligament, tendon, cartilage, and joint pathologies. While existing literature hints at their effectiveness, comprehensive studies are imperative to confirm their individual and combined accuracy. Validating these modalities could establish them as pivotal tools for surgeons, providing precise biomechanical data and enabling the objective assessment of lateral ankle ligaments’ stiffness, maturation, and function postoperatively. Moreover, these technologies extend their utility beyond surgical scenarios; they could be valuable in evaluating patients undergoing medical management and even serve diagnostic purposes. Envisioning these advancements within a wearable, easily portable device not only enhances their clinical utility but also empowers physicians to employ objective measurement tools for comprehensive assessment and management of CLAI patients. This departure from exclusive reliance on subjective measurement tools, such as Patient-Related Outcome Measures (PROMs), signifies a substantial stride toward bolstering the precision and objectivity of assessments in clinical practice.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drakos, M.; Hansen, O.; Kukadia, S. Ankle Instability. Foot Ankle Clin. 2022, 27, 371–384. [Google Scholar] [CrossRef]
- Chang, S.H.; Morris, B.L.; Saengsin, J.; Tourné, Y.; Guillo, S.; Guss, D.; DiGiovanni, C.W. Diagnosis and Treatment of Chronic Lateral Ankle Instability: Review of Our Biomechanical Evidence. J. Am. Acad. Orthop. Surg. 2021, 29, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.J.; Fuld, R.S.; Sutphin, B.S.; Pereira, H.; D’Hooghe, P. Return to sport following lateral ankle ligament repair is under-reported: A systematic review. J. ISAKOS Jt. Disord. Orthop. Sports Med. 2017, 2, 234–240. [Google Scholar] [CrossRef]
- Vopat, M.L.; Tarakemeh, A.; Morris, B.; Hassan, M.; Garvin, P.; Zackula, R.; Mullen, S.; Schroeppel, P.; Vopat, B.G. Early versus Delayed Mobilization Post-Operative Protocols for Primary Lateral Ankle Ligament Repair: A Systematic Review and Meta-analysis. Foot Ankle Orthop. 2019, 4, 2473011419S0007. [Google Scholar] [CrossRef]
- Machado, M.; Amado, P.; Babulal, J. Ankle instability—Review and new trends. J. Orthop. Trauma Rehabil. 2021, 28, 221049172110355. [Google Scholar] [CrossRef]
- Bestwick-Stevenson, T.; Wyatt, L.A.; Palmer, D.; Ching, A.; Kerslake, R.; Coffey, F.; Batt, M.E.; Scammell, B.E. Incidence and risk factors for poor ankle functional recovery, and the development and progression of posttraumatic ankle osteoarthritis after significant ankle ligament injury (SALI): The SALI cohort study protocol. BMC Musculoskelet. Disord. 2021, 22, 362. [Google Scholar] [CrossRef] [PubMed]
- Tourné, Y.; Besse, J.-L.; Mabit, C. Chronic ankle instability. Which tests to assess the lesions? Which therapeutic options? Orthop. Traumatol. Surg. Res. 2010, 96, 433–446. [Google Scholar] [CrossRef]
- Dromzée, E.; Granger, B.; Rousseau, R.; Steltzlen, C.; Stolz, H.; Khiami, F. Long-Term Results for Treatment of Chronic Ankle Instability with Fibular Periosteum Ligamentoplasty and Extensor Retinaculum Flap. J. Foot Ankle Surg. 2019, 58, 674–678. [Google Scholar] [CrossRef]
- Cho, B.-K.; Kim, Y.-M.; Shon, H.-C.; Park, K.-J.; Cha, J.-K.; Ha, Y.-W. A Ligament Reattachment Technique for High-Demand Athletes with Chronic Ankle Instability. J. Foot Ankle Surg. 2015, 54, 7–12. [Google Scholar] [CrossRef]
- Porter, D.A.; Kamman, K.A. Chronic Lateral Ankle Instability. Foot Ankle Clin. 2018, 23, 539–554. [Google Scholar] [CrossRef]
- Clements, A.D.; Belilos, E.B.; Keeling, L.; Kelly, M.; Casscells, N. Postoperative Rehabilitation of Chronic Lateral Ankle Instability: A Systematic Review. Sports Med. Arthrosc. Rev. 2021, 29, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Faure, C.; Deplus, F.; Besse, J.L.; Moyen, B.; Bochu, M. Chronic external instability of the ankle. Contribution of dynamic radiographies, x-ray computed tomography and x-ray computed tomographic arthrography. J. Radiol. 1997, 78, 629–634. [Google Scholar]
- van Groningen, B.; van der Steen, M.C.; Janssen, D.M.; van Rhijn, L.W.; van der Linden, A.N.; Janssen, R.P.A. Assessment of Graft Maturity After Anterior Cruciate Ligament Reconstruction Using Autografts: A Systematic Review of Biopsy and Magnetic Resonance Imaging studies. Arthrosc. Sports Med. Rehabil. 2020, 2, e377–e388. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.; Barzilay, Y.; Hasharoni, A.; Kaplan, L. Long-term outcome of surgical correction of congenital kyphosis in patients with myelomeningocele (MMC) with segmental spino-pelvic fixation. Evid. Based Spine-Care J. 2011, 2, 17–22. [Google Scholar] [CrossRef][Green Version]
- Vega, J.; Malagelada, F.; Céspedes, M.-C.M.; Dalmau-Pastor, M. The lateral fibulotalocalcaneal ligament complex: An ankle stabilizing isometric structure. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Umans, H.; Cerezal, L.; Linklater, J.; Fritz, J. Postoperative MRI of the Ankle and Foot. Magn. Reson. Imaging Clin. North Am. 2022, 30, 733–755. [Google Scholar] [CrossRef]
- Brantigan, J.; Pedegana, L.; Lippert, F. Instability of the subtalar joint. Diagnosis by stress tomography in three cases. Minerva Anestesiol. 1977, 59, 321–324. [Google Scholar] [CrossRef]
- Tochigi, Y.; Amendola, A.; Rudert, M.J.; Baer, T.E.; Brown, T.D.; Hillis, S.L.; Saltzman, C.L. The role of the interosseous talocalcaneal ligament in subtalar joint stability. Foot Ankle Int. 2004, 25, 588–596. [Google Scholar] [CrossRef]
- Ozeki, S.; Yasuda, K.; Kaneda, K.; Yamakoshi, K.; Yamanoi, T. Simultaneous strain measurement with determination of a zero strain reference for the medial and lateral ligaments of the ankle. Foot Ankle Int. 2002, 23, 825–832. [Google Scholar] [CrossRef]
- Hintermann, B.; Boss, A.; Schäfer, D. Arthroscopic Findings in Patients with Chronic Ankle Instability. Am. J. Sports Med. 2002, 30, 402–409. [Google Scholar] [CrossRef]
- Vega, J.; Allmendinger, J.; Malagelada, F.; Guelfi, M.; Dalmau-Pastor, M. Combined arthroscopic all-inside repair of lateral and medial ankle ligaments is an effective treatment for rotational ankle instability. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 132–140. [Google Scholar] [CrossRef]
- Vega, J.; Peña, F.; Golanó, P. Minor or occult ankle instability as a cause of anterolateral pain after ankle sprain. Knee Surg. Sports Traumatol. Arthrosc. 2014, 24, 1116–1123. [Google Scholar] [CrossRef]
- Guillo, S.; Bauer, T.; Lee, J.; Takao, M.; Kong, S.; Stone, J.; Mangone, P.; Molloy, A.; Perera, A.; Pearce, C.; et al. Consensus in chronic ankle instability: Aetiology, assessment, surgical indications and place for arthroscopy. Orthop. Traumatol. Surg. Res. 2013, 99, S411–S419. [Google Scholar] [CrossRef]
- van Dijk, C.N.; Lim, L.S.L.; Bossuyt, P.M.M.; Marti, R.K. Physical examination is sufficient for the diagnosis of sprained ankles. J. Bone Jt. Surg. 1996, 78, 958–962. [Google Scholar] [CrossRef]
- Phisitkul, P.; Chaichankul, C.; Sripongsai, R.; Prasitdamrong, I.; Tengtrakulcharoen, P.; Suarchawaratana, S. Accuracy of Anterolateral Drawer Test in Lateral Ankle Instability: A Cadaveric Study. Foot Ankle Int. 2009, 30, 690–695. [Google Scholar] [CrossRef]
- Frey, C.; Bell, J.; Teresi, L.; Kerr, R.; Feder, K. A Comparison of MRI and Clinical Examination of Acute Lateral Ankle Sprains. Foot Ankle Int. 1996, 17, 533–537. [Google Scholar] [CrossRef]
- Hoffman, E.; Paller, D.; Koruprolu, S.; Drakos, M.; Behrens, S.B.; Crisco, J.J.; DiGiovanni, C.W. Accuracy of Plain Radiographs Versus 3D Analysis of Ankle Stress Test. Foot Ankle Int. 2011, 32, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Peyre, M.; Rodineau, J. L’auto Varus: Une Technique D’exploration des Instabilités Externes de Cheville; 3e Journées d’imagerie ostéo-articulaire de la Pitié Salpêtrière: Paris, France, 1993. [Google Scholar]
- Jolman, S.; Robbins, J.; Lewis, L.; Wilkes, M.; Ryan, P. Comparison of Magnetic Resonance Imaging and Stress Radiographs in the Evaluation of Chronic Lateral Ankle Instability. Foot Ankle Int. 2017, 38, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Alshalawi, S.; Galhoum, A.E.; Alrashidi, Y.; Wiewiorski, M.; Herrera, M.; Barg, A.; Valderrabano, V. Medial Ankle Instability. Foot Ankle Clin. 2018, 23, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Broström, L. Sprained ankles. VI. Surgical treatment of “chronic” ligament ruptures. Acta Chir Scand 1966, 132, 551–565. [Google Scholar] [PubMed]
- Gould, N.; Seligson, D.; Gassman, J. Early and Late Repair of Lateral Ligament of the Ankle. Foot Ankle 1980, 1, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Bergsten, T.; Lansinger, O.; Peterson, L. Surgical treatment of chronic lateral instability of the ankle joint. Am. J. Sports Med. 1989, 17, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Viens, N.A.; Wijdicks, C.A.; Campbell, K.J.; LaPrade, R.F.; Clanton, T.O. Anterior Talofibular Ligament Ruptures, Part 1. Am. J. Sports Med. 2014, 42, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.J.; Pereira, H.; Kelley, J.; Anderson, N.; Fuld, R.; Baldini, T.; Kumparatana, P.; D’hooghe, P. The Role of Calcaneofibular Ligament Injury in Ankle Instability: Implications for Surgical Management. Am. J. Sports Med. 2019, 47, 431–437. [Google Scholar] [CrossRef]
- Camacho, L.D.; Roward, Z.T.; Deng, Y.; Latt, L.D. Surgical Management of Lateral Ankle Instability in Athletes. J. Athl. Train. 2019, 54, 639–649. [Google Scholar] [CrossRef]
- Karlsson, J.; Rudholm, O.; Bergsten, T.; Faxen, E.; Styf, J. Early range of motion training after ligament reconstruction of the ankle joint. Knee Surg. Sports Traumatol. Arthrosc. 1995, 3, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, S.; Hersek, S.; Jeong, H.K.; Whittingslow, D.; Ganti, V.G.; Wolkoff, P.; Inan, O.T. Robust Longitudinal Ankle Edema Assessment Using Wearable Bioimpedance Spectroscopy. IEEE Trans. Biomed. Eng. 2020, 67, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Lee, K.W.; Kim, S.B.; Han, T.R.; Jung, D.K.; Roh, M.S.; Lee, J.H. Electrical impedance spectroscopy and diagnosis of tendinitis. Physiol. Meas. 2010, 31, 171–182. [Google Scholar] [CrossRef]
- Ludvig, D.; Whitmore, M.W.; Perreault, E.J. Leveraging Joint Mechanics Simplifies the Neural Control of Movement. Front. Integr. Neurosci. 2022, 16, 802608. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, K.L.; Ludvig, D.; Bujnowski, D.; Lee, S.S.M.; Perreault, E.J. Simultaneous Quantification of Ankle, Muscle, and Tendon Impedance in Humans. IEEE Trans. Biomed. Eng. 2022, 69, 3657–3666. [Google Scholar] [CrossRef]
- Jöbsis, F.F. Noninvasive, Infrared Monitoring of Cerebral and Myocardial Oxygen Sufficiency and Circulatory Parameters. Science 1977, 198, 1264–1267. [Google Scholar] [CrossRef]
- Fan, C.; Shuaib, A.; Yao, G. Path-length resolved reflectance in tendon and muscle. Opt. Express 2011, 19, 8879. [Google Scholar] [CrossRef] [PubMed]
- Torniainen, J.; Ristaniemi, A.; Sarin, J.K.; Prakash, M.; Afara, I.O.; Finnilä, M.A.J.; Stenroth, L.; Korhonen, R.K.; Töyräs, J. Near infrared spectroscopic evaluation of biochemical and crimp properties of knee joint ligaments and patellar tendon. PLoS ONE 2022, 17, e0263280. [Google Scholar] [CrossRef] [PubMed]
- Afara, I.; Prasadam, I.; Crawford, R.; Xiao, Y.; Oloyede, A. Non-destructive evaluation of articular cartilage defects using near-infrared (NIR) spectroscopy in osteoarthritic rat models and its direct relation to Mankin score. Osteoarthr. Cartil. 2012, 20, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jacques, S.L.; Zheng, L. Conv—Convolution for responses to a finite diameter photon beam incident on multi-layered tissues. Comput. Methods Programs Biomed. 1997, 54, 141–150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saliba, I.; Hardy, A.; Wang, W.; Vialle, R.; Feruglio, S. A Review of Chronic Lateral Ankle Instability and Emerging Alternative Outcome Monitoring Tools in Patients following Ankle Ligament Reconstruction Surgery. J. Clin. Med. 2024, 13, 442. https://doi.org/10.3390/jcm13020442
Saliba I, Hardy A, Wang W, Vialle R, Feruglio S. A Review of Chronic Lateral Ankle Instability and Emerging Alternative Outcome Monitoring Tools in Patients following Ankle Ligament Reconstruction Surgery. Journal of Clinical Medicine. 2024; 13(2):442. https://doi.org/10.3390/jcm13020442
Chicago/Turabian StyleSaliba, Ibrahim, Alexandre Hardy, Wenzheng Wang, Raphael Vialle, and Sylvain Feruglio. 2024. "A Review of Chronic Lateral Ankle Instability and Emerging Alternative Outcome Monitoring Tools in Patients following Ankle Ligament Reconstruction Surgery" Journal of Clinical Medicine 13, no. 2: 442. https://doi.org/10.3390/jcm13020442
APA StyleSaliba, I., Hardy, A., Wang, W., Vialle, R., & Feruglio, S. (2024). A Review of Chronic Lateral Ankle Instability and Emerging Alternative Outcome Monitoring Tools in Patients following Ankle Ligament Reconstruction Surgery. Journal of Clinical Medicine, 13(2), 442. https://doi.org/10.3390/jcm13020442





