The Heart’s Function as a Pump Assessed via Impedance Cardiography and the Autonomic System Balance in Patients with Early-Stage Acromegaly
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Evaluation
2.3. Impedance Cardiography
2.4. Heart Rate Variability Assessment
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristics
3.2. Relationship between the HRV Metrics and the ICG Parameters
4. Discussion
4.1. Clinical Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleseriu, M.; Langlois, F.; Lim, D.S.T.; Varlamov, E.V.; Melmed, S. Acromegaly: Pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol. 2022, 10, 804–826. [Google Scholar] [CrossRef]
- Vitale, G.; Pivonello, R.; Galderisi, M.; D’Errico, A.; Spinelli, L.; Lupoli, G.; Lombardi, G.; Colao, A. Cardiovascular complications in acromegaly: Methods of assessment. Pituitary 2001, 4, 251–257. [Google Scholar] [CrossRef]
- Mosca, S.; Paolillo, S.; Colao, A.; Bossone, E.; Cittadini, A.; Iudice, F.L.; Parente, A.; Conte, S.; Rengo, G.; Leosco, D.; et al. Cardiovascular involvement in patients affected by acromegaly: An appraisal. Int. J. Cardiol. 2012, 167, 1712–1718. [Google Scholar] [CrossRef]
- Kahaly, G.; Olshausen, K.V.; Mohr-Kahaly, S.; Erbel, R.; Boor, S.; Beyer, J.; Meyer, J. Arrhythmia profile in acromegaly. Eur. Heart J. 1992, 13, 51–56. [Google Scholar] [CrossRef]
- Colao, A.; Pivonello, R.; Grasso, L.F.; Auriemma, R.S.; Galdiero, M.; Savastano, S.; Lombardi, G. Determinants of cardiac disease in newly diagnosed patients with acromegaly: Results of a 10 year survey study. Eur. J. Endocrinol. 2011, 165, 713–721. [Google Scholar] [CrossRef]
- Maffei, P.; Martini, C.; Milanesi, A.; Corfini, A.; Mioni, R.; de Carlo, E.; Menegazzo, C.; Scanarini, M.; Vettor, R.; Federspil, G.; et al. Late potentials and ventricular arrhythmias in acromegaly. Int. J. Cardiol. 2005, 104, 197–203. [Google Scholar] [CrossRef]
- Giustina, A.; Barkan, A.; Beckers, A.; Biermasz, N.; Biller, B.M.K.; Boguszewski, C.; Bolanowski, M.; Bonert, V.; Bronstein, M.D.; Casanueva, F.F.; et al. A Consensus on the Diagnosis and Treatment of Acromegaly Comorbidities: An Update. J. Clin. Endocrinol. Metab. 2020, 105, dgz096. [Google Scholar] [CrossRef]
- Comunello, A.; Dassie, F.; Martini, C.; De Carlo, E.; Mioni, R.; Battocchio, M.; Paoletta, A.; Fallo, F.; Vettor, R.; Maffei, P. Heart rate variability is reduced in acromegaly patients and improved by treatment with somatostatin analogues. Pituitary 2015, 18, 525–534. [Google Scholar] [CrossRef]
- Chemla, D.; Attal, P.; Maione, L.; Veyer, A.-S.; Mroue, G.; Baud, D.; Salenave, S.; Kamenicky, P.; Bobin, S.; Chanson, P. Impact of successful treatment of acromegaly on overnight heart rate variability and sleep apnea. J. Clin. Endocrinol. Metab. 2014, 99, 2925–2931. [Google Scholar] [CrossRef]
- Dural, M.; Kabakcı, G.; Çınar, N.; Erbaş, T.; Canpolat, U.; Gürses, K.M.; Tokgözoğlu, L.; Oto, A.; Kaya, E.B.; Yorgun, H.; et al. Assessment of cardiac autonomic functions by heart rate recovery, heart rate variability and QT dynamicity parameters in patients with acromegaly. Pituitary 2013, 17, 163–170. [Google Scholar] [CrossRef]
- Resmini, E.; Casu, M.; Patrone, V.; Murialdo, G.; Bianchi, F.; Giusti, M.; Ferone, D.; Minuto, F. Sympathovagal imbalance in acromegalic patients. J. Clin. Endocrinol. Metab. 2006, 91, 115–120. [Google Scholar] [CrossRef]
- Bilchick, K.C.; Fetics, B.; Djoukeng, R.; Fisher, S.G.; Fletcher, R.D.; Singh, S.N.; Nevo, E.; Berger, R.D. Prognostic value of heart rate variability in chronic congestive heart failure (veterans affairs’ survival trial of antiarrhythmic therapy in congestive heart failure). Am. J. Cardiol. 2002, 90, 24–28. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef]
- Chattipakorn, N.; Incharoen, T.; Kanlop, N.; Chattipakorn, S. Heart rate variability in myocardial infarction and heart failure. Int. J. Cardiol. 2007, 120, 289–296. [Google Scholar] [CrossRef]
- Iellamo, F.; Perrone, M.A.; Cimini, A.; Caminiti, G.; Chiaravalloti, A.; Parisi, A.; Schillaci, O. Complementary Role of Combined Indirect and Direct Cardiac Sympathetic (Hyper)Activity Assessment in Patients with Heart Failure by Spectral Analysis of Heart Rate Variability and Nuclear Imaging: Possible Application in the Evaluation of Exercise Training Effects. J. Cardiovasc. Dev. Dis. 2022, 9, 181. [Google Scholar]
- Goldenberg, I.; Goldkorn, R.; Shlomo, N.; Einhorn, M.; Levitan, J.; Kuperstein, R.; Klempfner, R.; Johnson, B. Heart Rate Variability for Risk Assessment of Myocardial Ischemia in Patients Without Known Coronary Artery Disease: The HRV-DETECT (Heart Rate Variability for the Detection of Myocardial Ischemia) Study. J. Am. Heart Assoc. 2019, 8, e014540. [Google Scholar] [CrossRef]
- Jurek, A.; Krzesiński, P.; Gielerak, G.; Witek, P.; Zieliński, G.; Kazimierczak, A.; Wierzbowski, R.; Banak, M.; Uziębło-Życzkowska, B. Acromegaly: The Research and Practical Value of Noninvasive Hemodynamic Assessments via Impedance Cardiography. Front. Endocrinol. 2022, 12, 793280. [Google Scholar] [CrossRef]
- Katznelson, L.; Laws, E.R., Jr.; Melmed, S.; Molitch, M.E.; Murad, M.H.; Utz, A.; Wass, J.A. Acromegaly: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3933–3951. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [PubMed]
- Bhalla, V.; Isakson, S.; Bhalla, M.A.; Lin, J.P.; Clopton, P.; Gardetto, N.; Maisel, A.S. Diagnostic Ability of B-Type Natriuretic Peptide and Impedance Cardiography: Testing to Identify Left Ventricular Dysfunction in Hypertensive Patients. Am. J. Hypertens. 2005, 18 Pt 2, 73S–81S. [Google Scholar] [CrossRef]
- Krzesiński, P.; Gielerak, G.; Stańczyk, A.; Uziębło-Życzkowska, B.; Smurzyński, P.; Piotrowicz, K.; Skrobowski, A. What Does Impedance Cardiography Add More to the Assessment of Left Ventricular Diastolic Function in Essential Hypertension? Pol. Merkur. Lek. 2015, 39, 352–358. [Google Scholar]
- Parrott, C.W.; Burnham, K.M.; Quale, C.; Lewis, D.L. Comparison of Changes in Ejection Fraction to Changes in Impedance Cardiography Cardiac Index and Systolic Time Ratio. Congest. Heart Fail. 2004, 10 (Suppl. 2), 11–13. [Google Scholar] [CrossRef]
- Packer, M.; Abraham, W.T.; Mehra, M.R.; Yancy, C.W.; Lawless, C.E.; Mitchell, J.E.; Smart, F.W.; Bijou, R.; O’Connor, C.M.; Massie, B.M.; et al. Prospective Evaluation and Identification of Cardiac Decompensation by ICG Test (PREDICT) Study Investigators and Coordinators. Utility of Impedance Cardiography for the Identification of Short-Term Risk of Clinical Decompensation in Stable Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 2006, 47, 2245–2252. [Google Scholar] [PubMed]
- Malik, M. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart Rate Variability. Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Sassi, R.; Cerutti, S.; Lombardi, F.; Malik, M.; Huikuri, H.V.; Peng, C.K.; Schmidt, G.; Yamamoto, Y. Advances in heart rate variability signal analysis: Joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1341–1353. [Google Scholar] [CrossRef]
- Xhyheri, B.; Manfrini, O.; Mazzolini, M.; Pizzi, C.; Bugiardini, R. Heart rate variability today. Prog. Cardiovasc. Dis. 2012, 55, 321–331. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart rate variability: Measurement and clinical utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Parolin, M.; Dassie, F.; Vettor, R.; Steeds, R.P.; Maffei, P. Electrophysiological features in acromegaly: Re-thinking the arrhythmic risk? J. Endocrinol. Invest. 2021, 44, 209–221. [Google Scholar] [CrossRef]
- Ito, M. Recent progress in electrocardiography. Rinsho Byori 1999, 47, 255–261. (In Japanese) [Google Scholar] [PubMed]
- Orosz, A.; Csajbók, É.; Czékus, C.; Gavallér, H.; Magony, S.; Valkusz, Z.; Várkonyi, T.T.; Nemes, A.; Baczkó, I.; Forster, T.; et al. Increased Short-Term Beat-To-Beat Variability of QT Interval in Patients with Acromegaly. PLoS ONE 2015, 10, e0125639. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, M.K.; Kannankeril, P.J.; Goldberger, J.J. Assessment of autonomic function in cardiovascular disease: Physiological basis and prognostic implications. J. Am. Coll. Cardiol. 2008, 51, 1725–1733. [Google Scholar] [CrossRef]
- Malliani, A.; Lombardi, F.; Pagani, M.; Cerutti, S. Power spectral analysis of cardiovascular variability in patients at risk for sudden cardiac death. J. Cardiovasc. Electrophysiol. 1994, 5, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Vanoli, E.; Schwartz, P.J. Sympathetic–parasympathetic interaction and sudden death. Basic Res. Cardiol. 1990, 85 (Suppl. 1), 305–321. [Google Scholar]
- Giustina, A.; Casanueva, F.; Cavagnini, F.; Melmed, S. Diagnosis and treatment of acromegaly complications. J. Endocrinol. Investig. 2003, 26, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Ferone, D.; Lombardi, G. Systemic complications of acromegaly: Epidemiology, pathogenesis, and management. Endocr. Rev. 2004, 25, 102–152. [Google Scholar] [CrossRef]
- Alexopoulou, O.; Bex, M.; Kamenicky, P.; Mvoula, A.B.; Chanson, P.; Maiter, D. Prevalence and risk factors of impaired glucose tolerance and diabetes mellitus at diagnosis of acromegaly: A study in 148 patients. Pituitary 2014, 17, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Pivonello, R.; Auriemma, R.S.; Guerra, E.; Milone, F.; Savastano, S.; Lombardi, G.; Colao, A. Hypertension in acromegaly and in the normal population: Prevalence and determinants. Clin. Endocrinol. 2005, 63, 470–476. [Google Scholar] [CrossRef]
- Fedrizzi, D.; Rodrigues, T.C.; Costenaro, F.; Scalco, R.; Czepielewski, M.A. Hypertension-related factors in patients with active and inactive acromegaly. Arq. Bras. Endocrinol. Metabol. 2011, 55, 468–474. [Google Scholar] [CrossRef][Green Version]
- Matta, M.F.; Caron, P. Acromegalic Cardiomyopathy: A Review of the Literature. Pituitary 2003, 6, 203–207. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef]
- Wolters, T.L.C.; Netea, M.G.; Riksen, N.P.; Hermus, A.R.M.M.; Netea-Maier, R.T. Acromegaly, inflammation and cardiovascular disease: A review. Rev. Endocr. Metab. Disord. 2020, 21, 547–568. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; Auriemma, R.S.; Grasso, L.F.; Pivonello, C.; Simeoli, C.; Patalano, R.; Galdiero, M.; Colao, A. Complications of acromegaly: Cardiovascular, respiratory and metabolic comorbidities. Pituitary 2017, 20, 46–62. [Google Scholar] [CrossRef]
- Maffei, P.; Dassie, F.; Wennberg, A.; Parolin, M.; Vettor, R. The endothelium in acromegaly. Front. Endocrinol. 2019, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Tellatin, S.; Maffei, P.; Osto, E.; Dassie, F.; Famoso, G.; Montisci, R.; Martini, C.; Fallo, F.; Marra, M.P.; Mioni, R.; et al. Coronary microvascular dysfunction may be related to IGF-1 in acromegalic patients and can be restored by therapy. Atherosclerosis 2017, 269, 100–105. [Google Scholar] [CrossRef]
- Spadaro, O.; Camell, C.D.; Bosurgi, L.; Nguyen, K.Y.; Youm, Y.-H.; Rothlin, C.V.; Dixit, V.D. IGF1 Shapes macrophage activation in response to immunometabolic challenge. Cell Rep. 2017, 19, 225–234. [Google Scholar] [CrossRef]
- Boero, L.; Manavela, M.; Rosso, L.G.; Insua, C.; Berardi, V.; Fornari, M.C.; Brites, F. Alterations in biomarkers of cardiovascular disease (CVD) in active acromegaly. Clin. Endocrinol. 2009, 70, 88–95. [Google Scholar] [CrossRef]
- Boero, L.; Manavela, M.; Merono, T.; Maidana, P.; Rosso, L.G.; Brites, F. GH levels and insulin sensitivity are differently associated with biomarkers of cardiovascular disease in active acromegaly. Clin. Endocrinol. 2012, 77, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ortiga, R.; Climent, V.; Sanchez-Tejada, L.; Candela, A.; Pico, A. Severe sleep apnea-hypopnea syndrome is related to left ventricle dysfunction and hypertrophy in acromegalic patients. Endocrinol. Nutr. 2015, 62, 366–372. [Google Scholar] [CrossRef] [PubMed]
| VARIABLE | Mean ± SD (Median; Interquartile Interval) or n (%) |
|---|---|
| DEMOGRAPHIC DATA | |
| Age, years | 47.0 ± 13.5 (47.0; 38.0–61.0) |
| Male sex | 18 (54.5) |
| BMI, kg/m2 | 27.8 ± 4.1 (27.7; 25.3–30.1) |
| BMI 18.5–24.9, kg/m2 | 7 (21.2) |
| BMI 25–29.9, kg/m2 | 17 (51.5) |
| BMI ≥ 30, kg/m2 | 9 (27.3) |
| HR, bpm | 75.6 ± 10.7 (77.0; 67.0–82.0) |
| SBP, mm Hg | 121.0 ± 11.2 (123.0; 115.0–127.0) |
| DBP, mm Hg | 77.0 ± 9.7 (77.0; 72.0–81.0) |
| CLINICAL DATA | |
| HTN | 18 (54.5) |
| T2DM | 6 (18.2) |
| Prediabetes | 10 (33.3) |
| Creatinine, mg/dL | 0.76 ± 0.20 (0.80; 0.60–0.80) |
| LVEF, % | 62.5 ± 5.1 (62.9; 60.8–66.0) |
| Correlations: R (p) | ||||||||
|---|---|---|---|---|---|---|---|---|
| VARIABLE | SDNN Day | rMSSD_day | SDSD_day | pNN50_day | SDNN_night | rMSSD_night | SDSD_night | pNN50_night |
| Age | 0.14 (0.445) | −0.39 (0.028) | −0.34 (0.059) | −0.42 (0.016) | −0.22 (0.228) | −0.34 (0.056) | −0.37 (0.038) | −0.28 (0.109) |
| BMI | 0.27 (0.138) | 0.124 (0.499) | 0.121 (0.511) | 0.27 (0.131) | 0.19 (0.290) | −0.11 (0.559) | 0.19 (0.302) | 0.16 (0.359) |
| HR, bpm | −0.50 (0.003) | −0.16 (0.381) | −0.14 (0.428) | −0.20 (0.264) | −0.35 (0.046) | −0.19 (0.274) | −0.21 (0.251) | −0.21 (0.246) |
| SBP, mm Hg | 0.13 (0.490) | 0.26 (0.158) | 0.28 (0.123) | 0.13 (0.457) | 0.06 (0.739) | 0.08 (0.666) | 0.08 (0.648) | 0.09 (0.060) |
| DBP, mm Hg | 0.004 (0.982) | 0.01 (0.945) | 0.02 (0.918) | −0.09 (0.585) | −0.07 (0.698) | −0.01 (0.934) | −0.03 (0.869) | 0.01 (0.952) |
| MBP, mm Hg | −0.007 (0.967) | 0.001 (0.994) | 0.01 (0.933) | −0.11 (0.531) | −0.11 (0.555) | −0.03 (0.851) | −0.05 (0.777) | 0.0008 (0.996) |
| PP, mm Hg | 0.13 (0.472) | 0.19 (0.301) | 0.23 (0.195) | 0.18 (0.313) | −0.10 (0.569) | −0.07 (0.699) | −0.03 (0.858) | −0.07 (0.708) |
| SI, mL/m2 | 0.16 (0.385) | 0.14 (0.427) | 0.14 (0.439) | 0.26 (0.147) | 0.28 (0.124) | 0.33 (0.064) | 0.35 (0.049) | 0.27 (0.129) |
| CI, mL × m−2 × min−1 | −0.16 (0.387) | 0.02 (0.909) | 0.03 (0.860) | 0.11 (0.553) | 0.03 (0.888) | 0.19 (0.287) | 0.21 (0.252) | 0.12 (0.493) |
| VI, 1 × 1000−1 × s−1 | −0.03 (0.854) | −0,05 (0.788) | −0.03 (0.868) | 0.01 (0.944) | 0.13 (0.467) | 0.17 (0.354) | 0.16 (0.384) | 0.11 (0.529) |
| ACI, 1/100/s2 | −0.07 (0.709) | 0.19 (0.289) | 0.20 (0.266) | 0.16 (0.363) | −0.03 (0.879) | 0.24 (0.186) | 0.19 (0.291) | 0.16 (0.366) |
| HI, Ohm/s2 | −0.05 (0.792) | 0.03 (0.871) | 0.07 (0.682) | −0.04 (0.822) | −0.07 (0.721) | 0.09 (0.605) | 0.08 (0.657) | 0.05 (0.773) |
| SVRI, dyn × s × cm−5 × m2 | 0.12 (0.497) | −0.01 (0.966) | −0.02 (0.929) | −0.13 (0.473) | −0.09 (0.591) | −0.16 (0.391) | −0.18 (0.333) | −0.13 (0.473) |
| TACI, mL/mmHg × m2 | 0.0001 (0.999) | −0.07 (0.719) | −0.10 (0.587) | 0.08 (0.668) | 0.20 (0.289) | 0.34 (0.074) | 0.34 (0.073) | 0.27 (0.153) |
| TFC, 1/kOhm | 0.005 (0.977) | 0.17 (0.351) | 0.11 (0.539) | 0.31 (0.082) | 0.23 (0.212) | 0.17 (0.347) | 0.15 (0.425) | 0.19 (0.267) |
| Correlations: R (p) | ||||||||
|---|---|---|---|---|---|---|---|---|
| VARIABLE | LF_day | LF_night | HF_day | HF_night | LF/HF_day | LF/HF_night | TP_day | TP_night |
| Age | −0.37 (0.032) | −0.05 (0.778) | 0.34 (0.056) | −0.02 (0.930) | −0.40 (0.023) | −0.05 (0.789) | −0.39 (0.026) | −0.41 (0.020) |
| BMI | −0.01 (0.935) | 0.16 (0.386) | 0.01 (0.982) | −0.11 (0557) | 0.05 (0.768) | 0.16 (0.384) | 0.26 (0.143) | 0.24 (0.195) |
| HR, bpm | 0.06 (0.747) | −0.12 (0.517) | −0.217 (0.224) | 0.001 (0.995) | 0.14 (0.419) | 0.001 (0.994) | −0.38 (0.033) | −0.25 (0.163) |
| SBP, mm Hg | −0.18 (0.311) | −0.06 (0.721) | 0.187 (0.295) | 0.02 (0.922) | −0.13 (0.481) | −0.120 (0.505) | 0.11 (0.536) | 0.15 (0.417) |
| DBP, mm Hg | −0.09 (0.604) | −0.04 (0.835) | 0.01 (0.953) | −0.03 (0.878) | −0.02 (0.901) | −0.01 (0.949) | −0.07 (0.718) | 0.01 (0.961) |
| MBP, mm Hg | −0.14 (0.444) | −0.09 (0.587) | 0.06 (0.727) | 0.02 (0.918) | −0.07 (0.705) | −0.08 (0.672) | −0.08 (0.669) | −0.004 (0.981) |
| PP, mm Hg | −0.26 (0.135) | −0.09 (0.612) | 0.33 (0.06) | 0.04 (0.838) | −0.252 (0.157) | −0.13 (0.464) | 0.17 (0.359) | −0.03 (0.868) |
| SI, mL/m2 | 0.08 (0.672) | −0.181 (0.313) | 0.10 (0.557) | 0.27 (0.128) | −0.03 (0.864) | −0.24 (0.175) | 0.25 (0.165) | 0.35 (0.049) |
| CI, mL × m−2 × min−1 | 0.11 (0.554) | −0.32 (0.069) | −0.02 (0.908) | 0.32 (0.071) | 0.05 (0.781) | −0.28 (0.108) | 0.000 (1.000) | 0.18 (0.331) |
| VI, 1 × 1000−1 × s−1 | −0.01 (0.962) | −0.27 (0.121) | 0.16 (0.357) | 0.322 (0.067) | −0.16 (0.383) | −0.29 (0.103) | −0.11 (0.537) | 0.10 (0.598) |
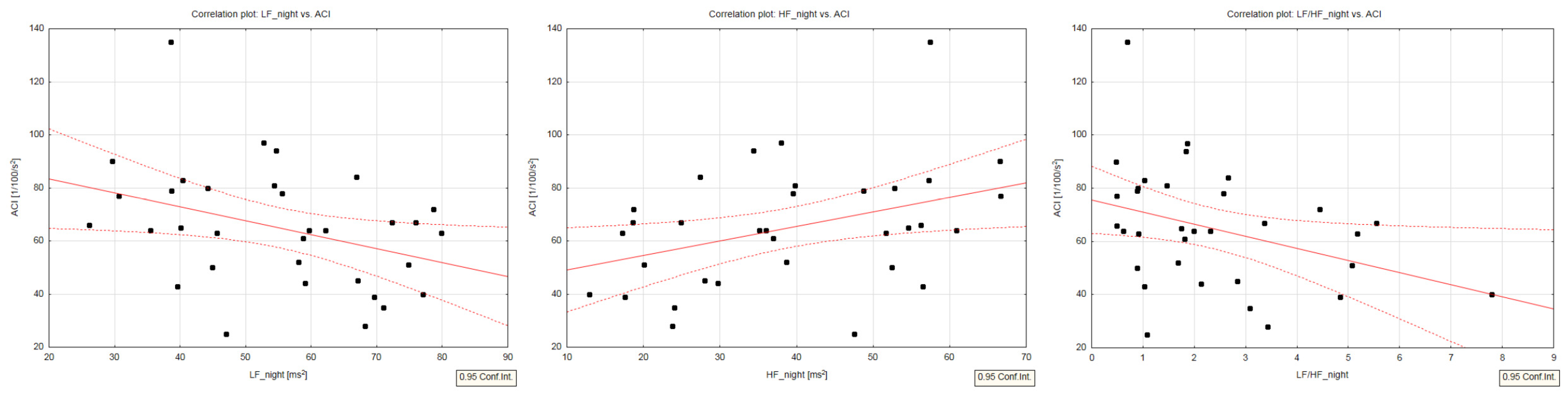
| ACI, 1/100/s2 | −0.08 (0.636) | −0.38 (0.027) | 0.19 (0.291) | 0.38 (0.026) | −0.19 (0.279) | −0.36 (0.037) | 0.16 (0.379) | 0.09 (0.610) |
| HI, Ohm/s2 | −0.22 (0.214) | −0.46 (0.007) | 0.28 (0.109) | 0.42 (0.014) | −0.33 (0.059) | −0.42 (0.014) | 0.007 (0.967) | −0.06 (0.749) |
| SVRI, dyn × s × cm−5 × m2 | −0.16 (0.359) | 0.18 (0.318) | 0.06 (0.716) | −0.21 (0.233) | −0.09 (0.632) | 0.17 (0.349) | −0.03 (0.851) | −0.16 (0.380) |
| TACI, mL/mmHg × m2 | 0.22 (0.238) | −0.26 (0.169) | −0.06 (0.738) | 0.34 (0.067) | 0.11 (0.561) | −0.25 (0.181) | 0.04 (0.834) | 0.31 (0.09) |
| TFC, 1/kOhm | 0.21 (0.231) | 0.23 (0.194) | −0.27 (0.130) | −0.17 (0.331) | 0.33 (0.06) | 0.19 (0.277) | 0.04 (0.841) | 0.28 (0.124) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurek, A.; Krzesiński, P.; Wierzbowski, R.; Uziębło-Życzkowska, B.; Witek, P.; Zieliński, G.; Kazimierczak, A.; Banak, M.; Gielerak, G. The Heart’s Function as a Pump Assessed via Impedance Cardiography and the Autonomic System Balance in Patients with Early-Stage Acromegaly. J. Clin. Med. 2024, 13, 395. https://doi.org/10.3390/jcm13020395
Jurek A, Krzesiński P, Wierzbowski R, Uziębło-Życzkowska B, Witek P, Zieliński G, Kazimierczak A, Banak M, Gielerak G. The Heart’s Function as a Pump Assessed via Impedance Cardiography and the Autonomic System Balance in Patients with Early-Stage Acromegaly. Journal of Clinical Medicine. 2024; 13(2):395. https://doi.org/10.3390/jcm13020395
Chicago/Turabian StyleJurek, Agnieszka, Paweł Krzesiński, Robert Wierzbowski, Beata Uziębło-Życzkowska, Przemysław Witek, Grzegorz Zieliński, Anna Kazimierczak, Małgorzata Banak, and Grzegorz Gielerak. 2024. "The Heart’s Function as a Pump Assessed via Impedance Cardiography and the Autonomic System Balance in Patients with Early-Stage Acromegaly" Journal of Clinical Medicine 13, no. 2: 395. https://doi.org/10.3390/jcm13020395
APA StyleJurek, A., Krzesiński, P., Wierzbowski, R., Uziębło-Życzkowska, B., Witek, P., Zieliński, G., Kazimierczak, A., Banak, M., & Gielerak, G. (2024). The Heart’s Function as a Pump Assessed via Impedance Cardiography and the Autonomic System Balance in Patients with Early-Stage Acromegaly. Journal of Clinical Medicine, 13(2), 395. https://doi.org/10.3390/jcm13020395









