Altered Sexual Response-Related Functional Connectivity and Morphometric Changes Influenced by Sex Hormones across Menopausal Status
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Subjects
2.3. Sex Hormone Measurements
2.4. Functional MRI Paradigm
2.5. Magnetic Resonance Imaging Acquisition
2.6. Data Preprocessing
2.6.1. Whole Brain Volumetry
2.6.2. Functional Connectivity Analysis
2.7. Statistical Analysis
3. Results
3.1. Demographic Characteristics
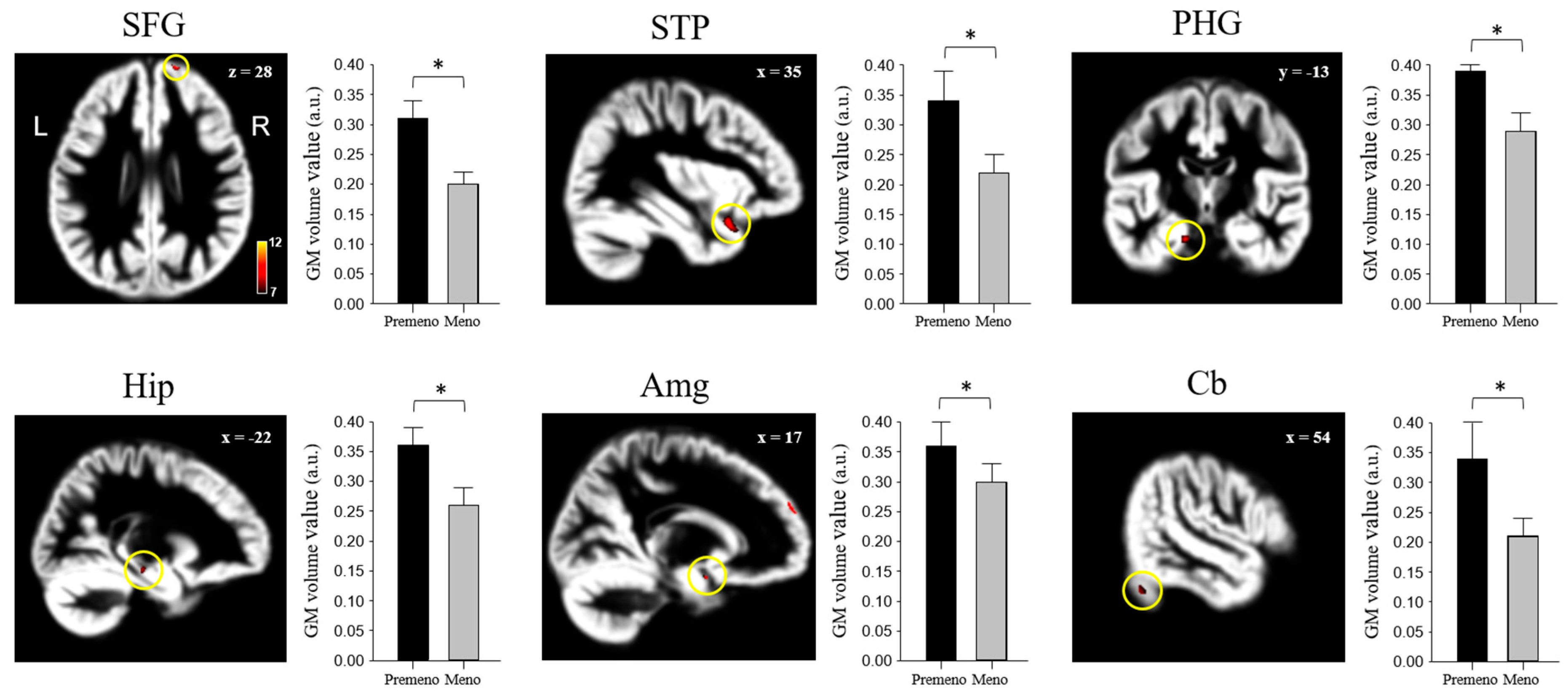
3.2. Localized Differences in Brain Volume
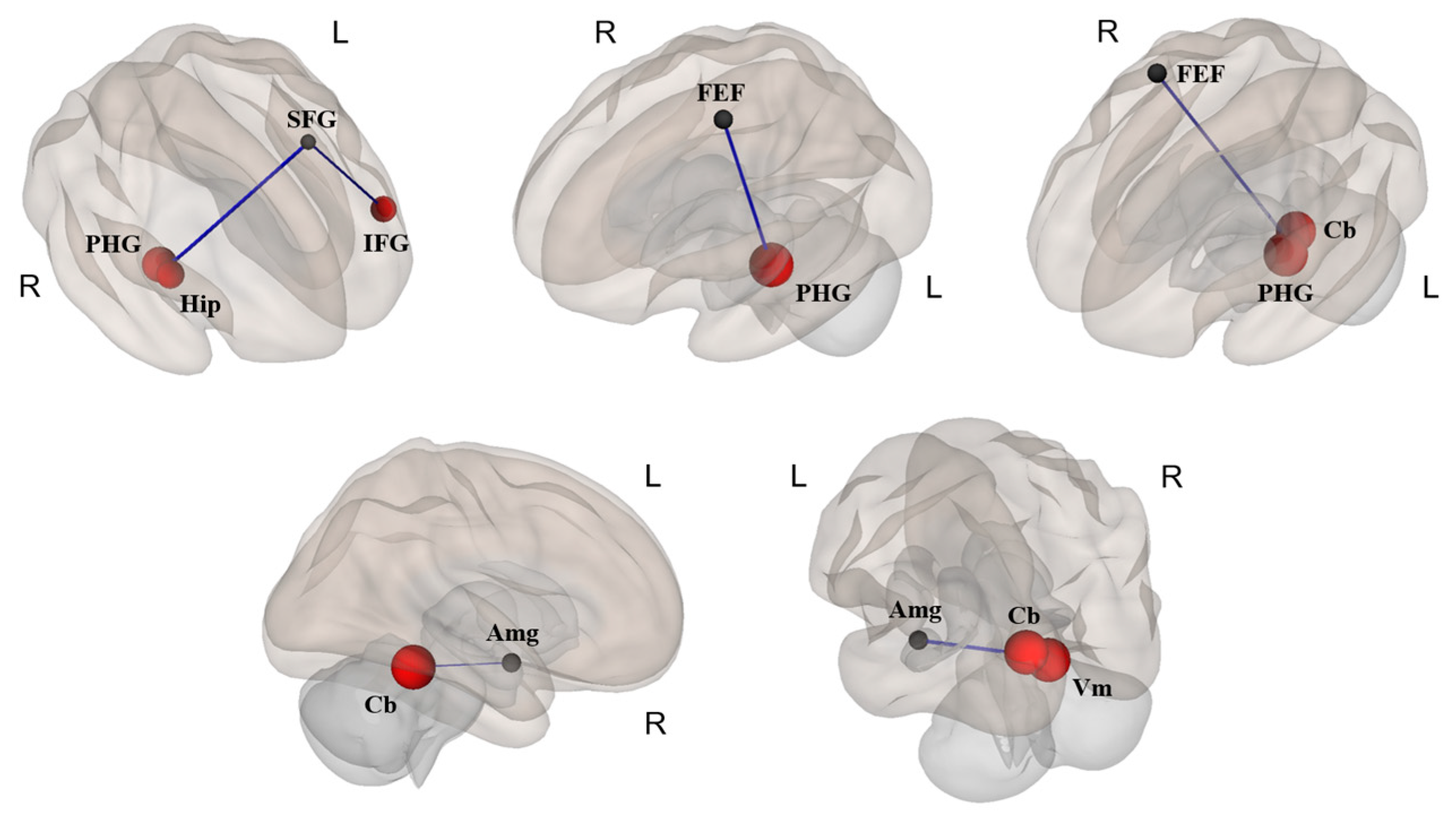
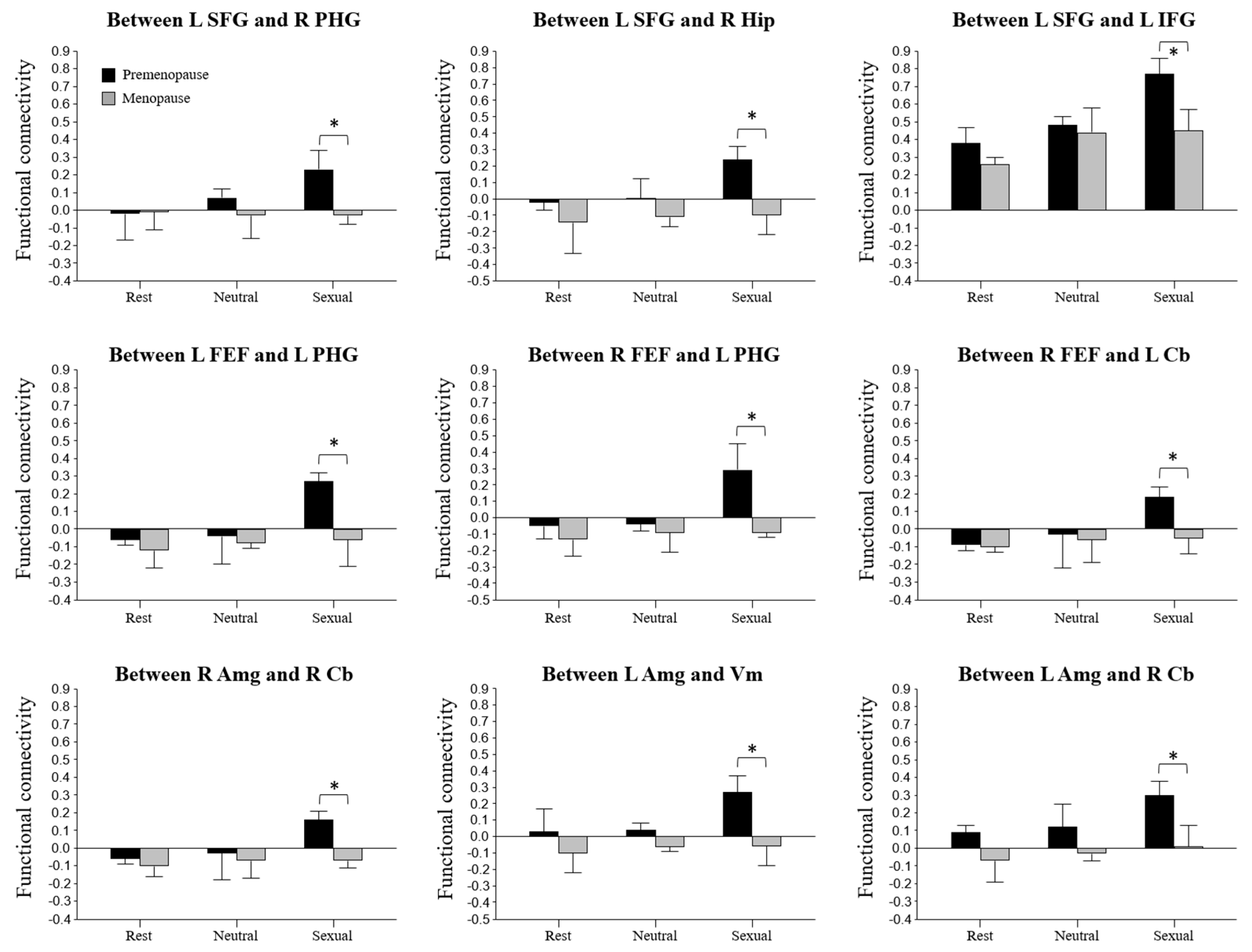
3.3. Differences in FC
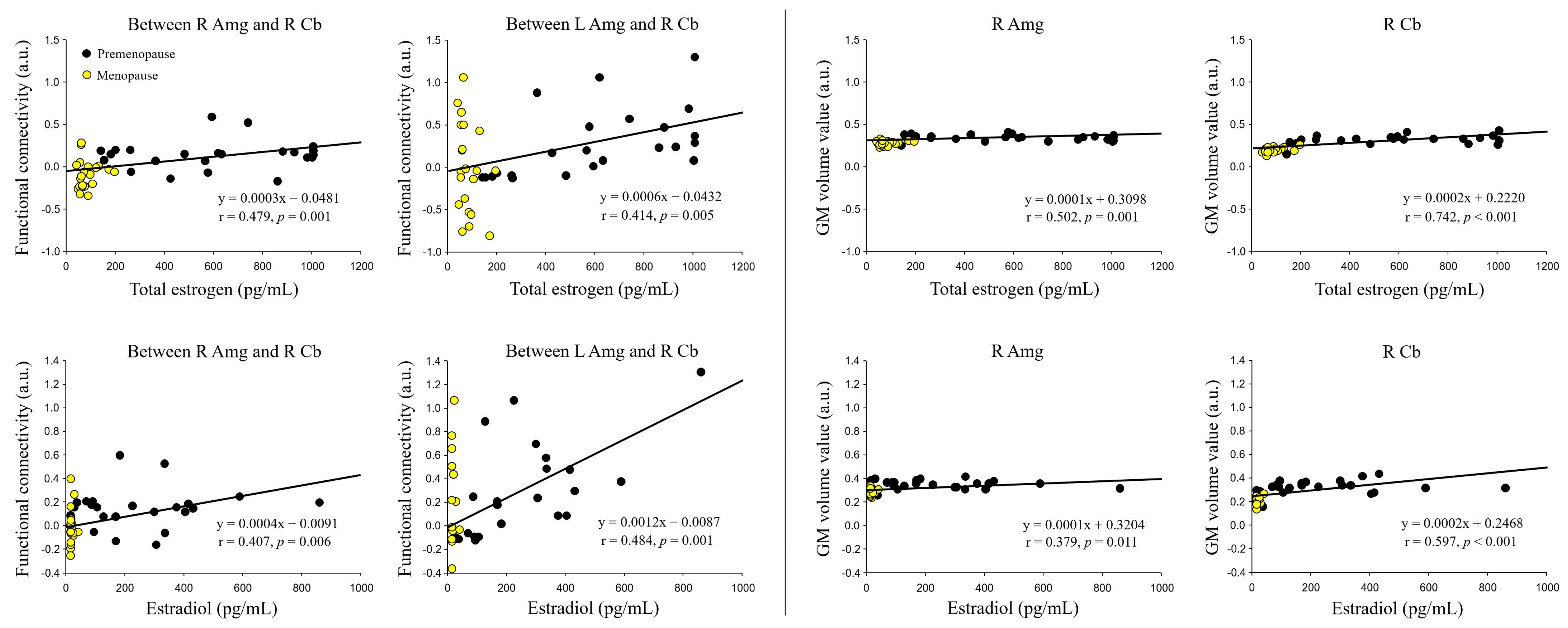
3.4. Correlation of Sex Hormone Levels with FC and Localized GM Volume
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koysombat, K.; McGown, P.; Nyunt, S.; Abbara, A.; Dhillo, W.S. New advances in menopause symptom management. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 101774. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kang, H.K.; Park, K.; Jeong, G.W. Localized brain metabolite changes during visual sexual stimulation in postmenopausal women: A pilot study using functional magnetic resonance spectroscopy. Menopause 2014, 21, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Rostami-Moez, M.; Masoumi, S.Z.; Otogara, M.; Farahani, F.; Alimohammadi, S.; Oshvandi, K. Examining the Health-Related Needs of Females during Menopause: A Systematic Review Study. J. Menopausal Med. 2023, 29, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, B.; Kim, Y.R.; Jeong, C.W.; Lee, Y.H. Gray matter differences associated with menopausal hormone therapy in menopausal women: A DARTEL-based VBM study. Sci. Rep. 2023, 13, 1401. [Google Scholar] [CrossRef] [PubMed]
- Jett, S.; Dyke, J.P.; Andy, C.; Schelbaum, E.; Jang, G.; Boneu Yepez, C.; Pahlajani, S.; Diaz, I.; Diaz Brinton, R.; Mosconi, L. Sex and menopause impact (31)P-Magnetic Resonance Spectroscopy brain mitochondrial function in association with (11)C-PiB PET amyloid-beta load. Sci. Rep. 2022, 12, 22087. [Google Scholar] [CrossRef]
- Albert, K.M.; Boyd, B.D.; Taylor, W.D.; Newhouse, P.A. Differential effects of estradiol on neural and emotional stress response in postmenopausal women with remitted Major Depressive Disorder. J. Affect. Disord. 2021, 293, 355–362. [Google Scholar] [CrossRef]
- Vinehout, K.; Schmit, B.D.; Schindler-Ivens, S. Lower Limb Task-Based Functional Connectivity Is Altered in Stroke. Brain Connect. 2019, 9, 365–377. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Friston, K.J. Functional and effective connectivity: A review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef]
- Lentini, E.; Kasahara, M.; Arver, S.; Savic, I. Sex differences in the human brain and the impact of sex chromosomes and sex hormones. Cereb. Cortex 2013, 23, 2322–2336. [Google Scholar] [CrossRef]
- Berent-Spillson, A.; Marsh, C.; Persad, C.; Randolph, J.; Zubieta, J.K.; Smith, Y. Metabolic and hormone influences on emotion processing during menopause. Psychoneuroendocrinology 2017, 76, 218–225. [Google Scholar] [CrossRef]
- Robertson, D.; Craig, M.; van Amelsvoort, T.; Daly, E.; Moore, C.; Simmons, A.; Whitehead, M.; Morris, R.; Murphy, D. Effects of estrogen therapy on age-related differences in gray matter concentration. Climacteric 2009, 12, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, M.; Ghidoni, R.; Govoni, S.; Testa, C.; Benussi, L.; Bonetti, M.; Binetti, G.; Frisoni, G.B. Effects of hormone therapy on brain morphology of healthy postmenopausal women: A Voxel-based morphometry study. Menopause 2006, 13, 584–591. [Google Scholar] [CrossRef]
- Song, S.H.; Jeon, H.; Kim, S.W.; Paick, J.S.; Son, H. The prevalence and risk factors of female sexual dysfunction in young korean women: An internet-based survey. J. Sex. Med. 2008, 5, 1694–1701. [Google Scholar] [CrossRef]
- Kim, G.W.; Jeong, G.W. Menopause-related brain activation patterns during visual sexual arousal in menopausal women: An fMRI pilot study using time-course analysis. Neuroscience 2017, 343, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kang, H.K.; Jeong, G.W. Assessment of brain metabolites change during visual sexual stimulation in healthy women using functional MR spectroscopy. J. Sex. Med. 2013, 10, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.M.; Kim, B.C.; Jeong, G.W. Corrigendum to "Associations of neurofunctional, morphometric and metabolic abnormalities with clinical symptom severity and recognition deficit in obsessive-compulsive disorder" [J. Affect. Disord. 227 (2018) 603-612]. J. Affect. Disord. 2018, 236, 318. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zhang, Y.; Cai, X.; Peng, Z.; Zhang, L.; Shao, Y.; Wang, C. Effects of Sleep Deprivation on Working Memory: Change in Functional Connectivity Between the Dorsal Attention, Default Mode, and Fronto-Parietal Networks. Front. Hum. Neurosci. 2020, 14, 360. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Zhang, Y.; Chen, Y.; Peng, J.; Shao, Y.; Zhang, X. Decreased Functional Connectivity Between the Right Precuneus and Middle Frontal Gyrus Is Related to Attentional Decline Following Acute Sleep Deprivation. Front. Neurosci. 2020, 14, 530257. [Google Scholar] [CrossRef]
- He, L.; Guo, W.; Qiu, J.; An, X.; Lu, W. Altered Spontaneous Brain Activity in Women During Menopause Transition and Its Association With Cognitive Function and Serum Estradiol Level. Front. Endocrinol. 2021, 12, 652512. [Google Scholar] [CrossRef]
- Lu, W.; Guo, W.; Hou, K.; Zhao, H.; Shi, L.; Dong, K.; Qiu, J. Grey matter differences associated with age and sex hormone levels between premenopausal and perimenopausal women: A voxel-based morphometry study. J. Neuroendocrinol. 2018, 30, e12655. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.M. Estrogens, estrogen receptors, and female cognitive aging: The impact of timing. Horm. Behav. 2013, 63, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Voytko, M.L.; Tinkler, G.P.; Browne, C.; Tobin, J.R. Neuroprotective effects of estrogen therapy for cognitive and neurobiological profiles of monkey models of menopause. Am. J. Primatol. 2009, 71, 794–801. [Google Scholar] [CrossRef]
- Brown, A.M.C.; Gervais, N.J. Role of Ovarian Hormones in the Modulation of Sleep in Females Across the Adult Lifespan. Endocrinology 2020, 161, bqaa128. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Adams, L.F.; Schmidt, P.J.; Rubinow, D.R.; Wassermann, E.M. Effects of ovarian hormones on human cortical excitability. Ann. Neurol. 2002, 51, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Guo, W.; Cui, D.; Dong, K.; Qiu, J. Effect of Sex Hormones on Brain Connectivity Related to Sexual Function in Perimenopausal Women: A Resting-State fMRI Functional Connectivity Study. J. Sex. Med. 2019, 16, 711–720. [Google Scholar] [CrossRef]
- Goldstein, I.; Traish, A.; Kim, N.; Munarriz, R. The role of sex steroid hormones in female sexual function and dysfunction. Clin. Obstet. Gynecol. 2004, 47, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, J.R.; Kringelbach, M.L. The human sexual response cycle: Brain imaging evidence linking sex to other pleasures. Prog. Neurobiol. 2012, 98, 49–81. [Google Scholar] [CrossRef]
- Komisaruk, B.R.; Whipple, B. Functional MRI of the brain during orgasm in women. Annu. Rev. Sex. Res. 2005, 16, 62–86. [Google Scholar]
- Rasgon, N.L.; Silverman, D.; Siddarth, P.; Miller, K.; Ercoli, L.M.; Elman, S.; Lavretsky, H.; Huang, S.C.; Phelps, M.E.; Small, G.W. Estrogen use and brain metabolic change in postmenopausal women. Neurobiol. Aging 2005, 26, 229–235. [Google Scholar] [CrossRef]
- Mastropasqua, A.; Dowsett, J.; Dieterich, M.; Taylor, P.C.J. Right frontal eye field has perceptual and oculomotor functions during optokinetic stimulation and nystagmus. J. Neurophysiol. 2020, 123, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Dennerstein, L.; Randolph, J.; Taffe, J.; Dudley, E.; Burger, H. Hormones, mood, sexuality, and the menopausal transition. Fertil. Steril. 2002, 77 (Suppl. S4), S42–S48. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.; Pritschet, L.; Santander, T.; Grafton, S.T.; Jacobs, E.G. Cerebellar network organization across the human menstrual cycle. Sci. Rep. 2020, 10, 20732. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Park, K.; Jeong, G.W. Effects of Sex Hormones and Age on Brain Volume in Post-Menopausal Women. J. Sex. Med. 2018, 15, 662–670. [Google Scholar] [CrossRef]
| Premenopausal Women (n = 23) | Menopausal Women (n = 21) | p-Value * | |
|---|---|---|---|
| Age (year) | 41.52 ± 7.38 | 55.52 ± 2.80 | <0.001 |
| Period after menopause (year) | − | 6.00 ± 2.63 | |
| Sex hormone levels | |||
| Estradiol (E2) a (pg/mL) | 244.98 ± 204.41 | 13.31 ± 6.45 | <0.001 |
| Estriol (E3) b (pg/mL) | 2.74 ± 1.66 | 2.44 ± 1.40 | 0.519 |
| Total estrogen c (pg/mL) | 597.30 ± 312.69 | 77.97 ± 41.15 | <0.001 |
| Follicle-stimulating hormone d (FSH, mlU/mL) | 6.63 ± 3.82 | 64.29 ± 20.41 | <0.001 |
| Luteinizing hormone e (LH, mlU/mL) | 16.17 ± 15.47 | 35.50 ± 10.23 | <0.001 |
| Sexual hormone binding globulin f (SHBG, nmol/L) | 99.81 ± 31.47 | 69.87 ± 17.13 | <0.001 |
| Free testosterone g (pg/mL) | 0.46 ± 0.34 | 0.27 ± 0.17 | 0.020 |
| Female sexual function index † | |||
| Desire | 2.91 ± 1.00 | 2.51 ± 1.03 | 0.197 |
| Arousal | 4.08 ± 1.02 | 2.80 ± 1.07 | <0.001 |
| Lubrication | 4.79 ± 1.37 | 2.81 ± 1.04 | <0.001 |
| Orgasm | 4.56 ± 1.20 | 2.84 ± 1.05 | <0.001 |
| Satisfaction | 4.37 ± 1.28 | 2.86 ± 1.18 | <0.001 |
| Pain | 4.91 ± 1.20 | 2.67 ± 1.06 | <0.001 |
| Full scale score | 25.61 ± 3.23 | 16.48 ± 2.03 | <0.001 |
| Tissue | Premenopausal Women | Menopausal Women | p-Value |
|---|---|---|---|
| Gary matter (mm3) | 629.20 ± 50.44 | 582.44 ± 44.23 | 0.056 |
| White matter (mm3) | 501.33 ± 55.36 | 460.88 ± 51.07 | 0.150 |
| Cerebrospinal fluid (mm3) | 451.37 ± 102.83 | 490.02 ± 109.87 | 0.718 |
| Total volume (mm3) | 1581.90 ± 137.09 | 1533.34 ± 155.62 | 0.155 |
| Brain Area | MNI Coordinates | Voxels | t-Value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Superior frontal gyrus (SFG) | 18 | 60 | 28 | 114 | 8.70 |
| Superior temporal pole (STP) | 35 | 16 | −23 | 1371 | 10.10 |
| Parahippocampal gyrus (PHG) | −18 | −13 | −26 | 165 | 9.70 |
| Hippocampus (Hip) | −22 | −22 | −12 | 101 | 8.84 |
| Amygdala (Amg) | 17 | 4 | −15 | 86 | 8.49 |
| Cerebellum (Cb) | 54 | −63 | −33 | 192 | 7.59 |
| Brain Area | MNI Coordinates | t-Value | p-Value | |||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Seed region: Superior frontal gyrus (SFG) | −12 | 56 | 28 | |||
| Target region: | Parahippocampal gyrus (PHG) | 24 | −26 | −16 | 3.76 | 0.0005 |
| Hippocampus (Hip) | 29 | −16 | −14 | 2.98 | 0.0048 | |
| Inferior frontal gyrus (IFG) | −51 | 26 | 2 | 2.72 | 0.0048 | |
| Seed region: Frontal eye fields (FEF) | −27 | −9 | 64 | |||
| Target region: | Parahippocampal gyrus (PHG) | −20 | −7 | −29 | 2.97 | 0.0050 |
| Seed region: Frontal eye fields (FEF) | 30 | −6 | 64 | |||
| Target region: | Parahippocampal gyrus (PHG) | −22 | −10 | −28 | 3.02 | 0.0043 |
| Cerebellum (Cb) | −26 | −30 | −30 | 2.74 | 0.0046 | |
| Seed region: Amygdala (Amg) | 26 | 1 | −15 | |||
| Target region: | Cerebellum (Cb) | 27 | −29 | −25 | 3.45 | 0.0013 |
| Seed region: Amygdala (Amg) | −25 | −1 | −18 | |||
| Target region: | Vermis (Vm) | 2 | −52 | −20 | 3.24 | 0.0023 |
| Cerebellum (Cb) | 28 | −28 | −29 | 3.21 | 0.0026 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, C.M.; Heo, S.H.; Yoon, W.; Baek, B.H.; Shin, S.S.; Kim, S.K.; Lee, Y.Y. Altered Sexual Response-Related Functional Connectivity and Morphometric Changes Influenced by Sex Hormones across Menopausal Status. J. Clin. Med. 2024, 13, 387. https://doi.org/10.3390/jcm13020387
Moon CM, Heo SH, Yoon W, Baek BH, Shin SS, Kim SK, Lee YY. Altered Sexual Response-Related Functional Connectivity and Morphometric Changes Influenced by Sex Hormones across Menopausal Status. Journal of Clinical Medicine. 2024; 13(2):387. https://doi.org/10.3390/jcm13020387
Chicago/Turabian StyleMoon, Chung Man, Suk Hee Heo, Woong Yoon, Byung Hyun Baek, Sang Soo Shin, Seul Kee Kim, and Yun Young Lee. 2024. "Altered Sexual Response-Related Functional Connectivity and Morphometric Changes Influenced by Sex Hormones across Menopausal Status" Journal of Clinical Medicine 13, no. 2: 387. https://doi.org/10.3390/jcm13020387
APA StyleMoon, C. M., Heo, S. H., Yoon, W., Baek, B. H., Shin, S. S., Kim, S. K., & Lee, Y. Y. (2024). Altered Sexual Response-Related Functional Connectivity and Morphometric Changes Influenced by Sex Hormones across Menopausal Status. Journal of Clinical Medicine, 13(2), 387. https://doi.org/10.3390/jcm13020387







