1. Introduction
Giant intracranial aneurysms (GIAs) are cerebral aneurysms with a diameter of at least 25 mm [
1,
2]. GIAs are thought to develop from a combination of genetic and environmental factors that lead to degeneration of the vessel wall [
3,
4], resulting in weakening and dilatation of the wall. Risk factors for the development of intracranial aneurysms include hypertension, smoking, positive family history, and connective tissue disease [
5]. Elongation and enlargement of the affected artery lead to hemodynamic and hemostatic changes with possible formation of intra-aneurysmal thrombosis, brainstem compression, and increased risk of ischemic complications [
5].
The presence of perforating arteries with risk of brain stem infarctions is one of the major challenges facing the endovascular treatment of fusiform aneurysms of the basilar artery (BA) [
6]. The overall prevalence of GIAs is higher in women than in men, and they tend to occur more frequently in the anterior circulation than in the posterior circulation [
7].
The clinical presentation of GIAs can vary widely depending on the location, size, and associated complications. Common symptoms include headaches, visual disturbances, cranial nerve deficits, seizures, and neurological deficits such as hemiparesis, dysarthria, and cognitive impairment [
3,
5]. Subarachnoid hemorrhage (SAH) and intracerebral hemorrhage (ICH) are the most serious complications associated with GIAs and are responsible for high morbidity and mortality [
2].
Treatment of GIAs is difficult and there is no consensus on the optimal treatment strategy. Traditional treatment options include surgical clipping and endovascular coiling [
8,
9]. However, these methods have their limitations, especially for large and complex aneurysms. Recently, new endovascular techniques such as flow diversion and stent-assisted coiling have shown promising results in the treatment of GIAs [
9,
10].
The giant fusiform aneurysms of the BA have specific features compared to aneurysms of the anterior circulation. First, the BA as well as the distal vertebral artery (VA) have to be considered as fully perforator bearing, and second, the diameter of the parent vessel can reach 10 mm or even more, making them untreatable with commonly available intracranial stents.
The Accero
®-Rex-Stents are intended for use with embolization materials in the treatment of intracranial aneurysms. They consist of a Nitinol composite wire with a platinum core and a central X-ray marker in the stent mesh. Compared to the established standard Accero
®-Stents with length between 10–25 mm and a diameter between 2.5–4 mm, they have a higher amount of platinum in the wire core. Despite its size with a length of 30–60 mm and a diameter of 7–10 mm, it possesses the same resheathability. It is intended for the use in vessels with diameters between 5.5–10 mm and can be delivered via 0.039″ NeuroSlider 39
® microcatheters (Acandis, Pforzheim, Germany) delivery catheters (see
Figure 1).
We report here on the world’s first five cases with overall 12 deployments of the Accero®-Rex-Stents in the treatment of fusiform GIAs of the posterior circulation. Our objective is to discuss the technical feasibility of the implantation of these stents in the endovascular treatment of GIA.
2. Materials and Methods
In this retrospective, multicentre study, we investigated the interventional treatment of five patients with giant aneurysms of the posterior circulation by using Accero®-Rex-Stents between November 2022–August 2023. The patients were treated at three specialized tertiary hospitals with a neurointerventional unit and expertise in aneurysm treatment.
We report on the pre-interventional diagnosis and clinical state of the patients, including clinical and baseline imaging characteristics, as well as technical aspects of the interventional procedures with emphasis on the used material and the feasibility of the procedures in all patients (
Table 1). Moreover, we report of the first treated patient in detail as an illustrative case (referred to as patient #1).
2.1. Study Population
Inclusion criteria were the presence of giant aneurysms of the posterior circulation and the interventional treatment of these aneurysms with Accero®-Rex-Stents. We included n = 5 patients, all male (100%), with a mean age of 54.4 ± 8.1 years. Initial symptoms included diplopia, ataxia, hemiparesis, cephalgia, with gait disturbances being the most prevalent one. All patients showed a worsening of symptoms between initial diagnosis and time of treatment, with the mean time period being 3.8 years.
2.2. Image Acquisition
Magnetic Resonance Imaging (MRI)
Pre- and postinterventional magnetic resonance imaging (MRI) examinations were performed on a 3 Tesla scanner (Philips Healthcare, Best, The Netherlands) after administration of 0.1 mL/kg of a contrast agent containing gadolinium (Gadovist, Bayer Vital GmbH, Leverkusen, Germany).
MRI protocols included the following sequences: 3D T1 weighted sequences pre- and post-contrast administration, 3D FLAIR sequences, diffusion and susceptibility weighted sequences, and time-of-flight (ToF) sequences.
The MRI images were evaluated by at least two radiologists with at least 6 years of experience in neuroradiological diagnostics.
2.3. Computed Tomography (CT), CT-Angiography (CT-A), and CT-Perfusion (CT-P)
Examination was performed with dual source CT (SOMATOM Definition Flash, Siemens Healthineers, Erlangen, Germany, 128 × 0.6 mm) with iodinated non-ionic contrast medium (ULTRAVIST, Bayer Vital GmbH, Leverkusen, Germany) delivered by a power injector after non-enhanced CT of the brain. The examination protocol consists of a non-contrast head CT scan followed by a CT-angiography of the supra-aortic and cerebral vasculature (80 cc contrast medium, 4 cc/s flow rate) while the patients’ arms are lowered. CT-P images were acquired after a delay of 180 s followed by an injection of 30 cc contrast medium at a flow rate of 5 cc/s. Acquisition parameters were 120 kV and 175 mA, the rotation time was 0.5 s, and the pitch was 0.5. Syngovia NeuroPerfusion Software (Siemens Healthineers, Version 5.1) was used for analyzing raw perfusion data. The arterial input function and the venous output function were determined from the middle cerebral artery and the superior sagittal sinus. Maps of relative cerebral-blood-flow (CBF), cerebral-blood-volume (CBV), mean-transit-time (MTT), and time-to-drain (TTD) were calculated automatically by software-based default settings as used in clinical routine.
2.4. Neurointerventional Procedure Exemplified by the Intervention in Patient #1
Diagnostic angiography of the cerebral arteries was performed via an arterial transfemoral approach with general anesthesia.
The following materials were used as standard for diagnostic angiography, stent, and flow-diverter implantation in patient #1:
7F Cook sheath (Cook Medical, Bloomington, IN, USA), Sofia EX DAC (Microvention, Saint-Germain-en-Laye, France), NeuroSlider 39® microcatheter (Acandis, Pforzheim, Germany), Terumo standard wire (Radiofocus, Tokyo, Japan), Rebar 18 microcatheter (Medtronic, Irvine, CA, USA, 0.021-inch inner diameter), Traxcess 14 microwire (Microvention, Tustin, CA, USA), Syncro support microwire (Stryker, Salt Lake City, UT, USA), Derivo-Heal-Flow-Diverter 8 × 25 mm (Acandis, Pforzheim, Germany), Eclipse 2L 6 × 20 mm double lumen balloon catheter (Balt, Montmorency, France), and pRESET 6 × 30 mm stent retriever (Phenox, Bochum, Germany).
2.5. Drug Therapy during Intervention and after Stent Implantation in Patient #1
Pre-interventional drug administration
Clopidogrel 75 mg (1-0-0/d) for five days.
2.6. Peri-interventional Drug Administration in Patient #1
An amount of 5000 I.E. Heparin, 500 mg ASA + eptifibatid bolus and infusion (b.w. adapted). Fortecotin: 4 mg (1-1-1/d).
2.7. Postinterventional Drug Administration Patient #1
Clopidogrel 75 mg (1-0-0/d) + 100 mg (1-0-0/d) ASA for twelve months. Additionally, 100 mg (1-0-0/d) ASA life-long.
3. Results
The patient collective consisted of five patients with large fusiform aneurysms of the posterior circulation. All patients were treated successfully with 2–3 implanted Accero
®-Rex-Stents of different sizes (
Table 1). In all cases, the implantation of the stents was achieved without technical complications. The post-interventional CT or MRI controls showed no/only minor focal ischemia in three out of five cases (patient #2–#4), the neurological status of these patients showed no marked clinical deterioration.
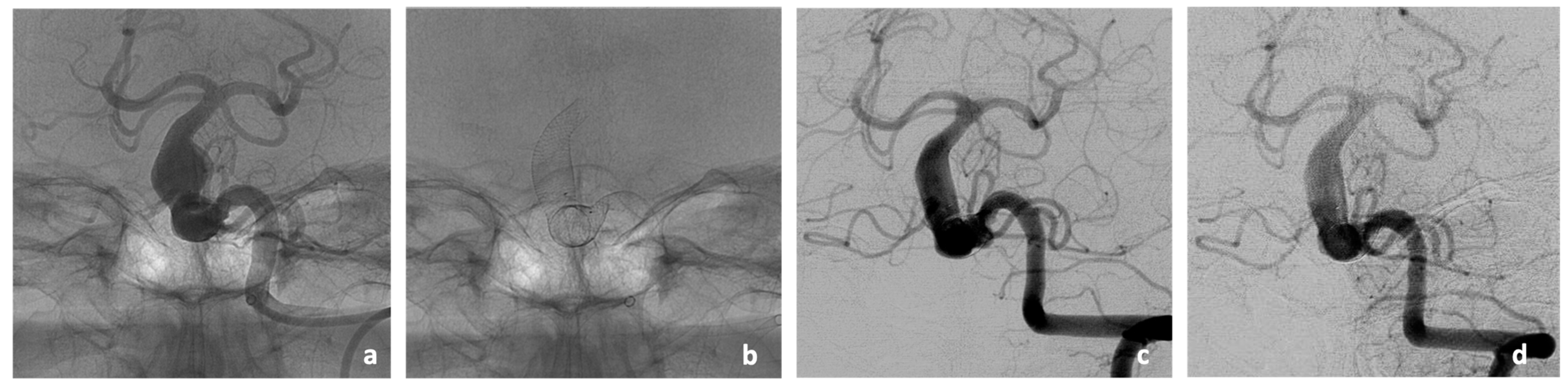
As an illustrative case of the vessel remodeling effect of the implanted stents, the DSA imaging of patient #2 in the three- and eight-months post-interventional course is given (
Figure 2). In this case, the stent-covered aneurysm of the BA showed a significant reduction in size from the previous 11 × 11 mm to only 6 × 6 mm in the follow-up imaging at 3 months and stable size at follow-up imaging at 8 months. The follow-up of patient #5 showed a stable size of the stent-treated aneurysm at 3 months. Two patients died due to pathologies that were not associated with the intervention before follow-up imaging at 3 months could be performed (cardiac co-morbidities in patient #1 and pulmonary sepsis in patient #3). Furthermore, 3 months follow-up of patient #4 was still pending at the time of manuscript submission.
3.1. Illustrative Case of the First Patient Treated with the Accero®-Rex-Stent
The interventional procedure regarding the endovascular treatment of a fusiform aneurysm of the posterior circulation as well as the postinterventional outcome are described based on the first patient as an illustrative case.
3.2. Patient #1
An adult patient presented to the neurological department of our hospital in the year 2018 with known cerebral macroangiopathy and onset of diplopia, gait disturbance, and ataxia. The patient had a pre-existing condition of severe and medicated hypertension, yet the patient was a heavy cigarette smoker (40 pack years).
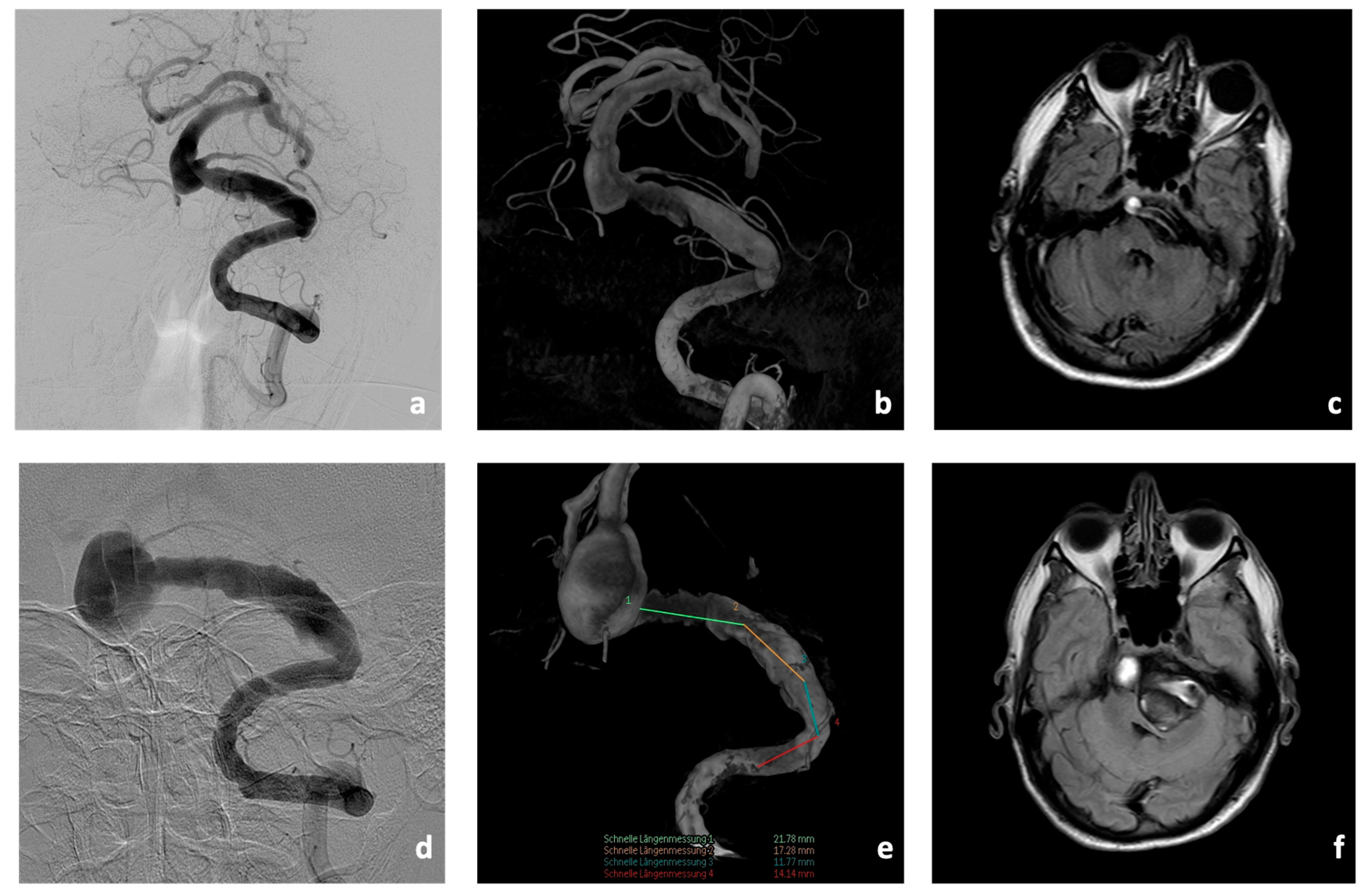
Initial cerebral imaging in 2018 with magnetic resonance imaging (MRI), computed tomography (CT), CT-angiography (CT-A), CT-perfusion (CT-P), and digital subtraction angiography (DSA) showed an aneurysm of the BA (10 × 11 mm) with concomitant fusiform caliber irregularities of the V4 segments of the left VA (35 mm in length,
Figure 3). In addition, there was a hypoplastic right VA.
At this point, an interdisciplinary consensus was reached between the departments of neurosurgery, neurology, and interventional neuroradiology to initially adopt a wait-and-see approach with optimization of the hypertension medication. Interventional treatment of the giant fusiform aneurysm was deliberately omitted.
Four years later, in 2022, the patient presented to our hospital with a significant worsening of their neurological symptoms with now existing severe dysarthria, dysphagia, hemiparesis of the left side, facial palsy of the left side, and permanent gait disturbance.
CTA showed a significant increase in the size of the fusiform aneurysm with a maximum BA diameter of 15 × 22 mm. In addition, at this time, there was already manifest compression and displacement of the brain stem to the right due to the fusiform aneurysm of the V4 segment of the left VA (8 × 50 mm,
Figure 3).
In view of these findings, endovascular therapy of the aneurysm was initiated.
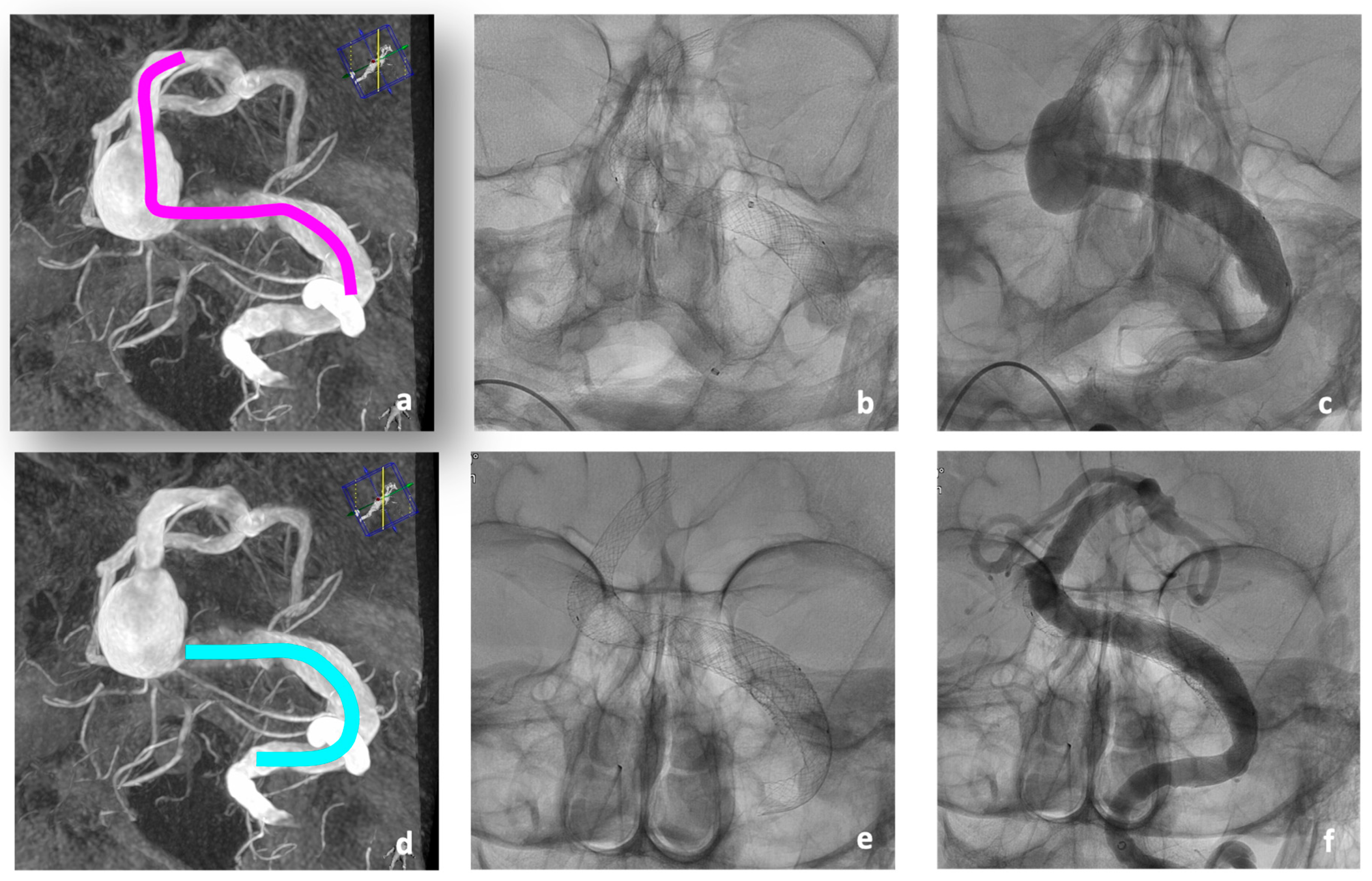
After extensive study of a re-performed DSA, a treatment concept was developed that provided for a combined therapy using telescopic braided Accero
®-Rex-Stents in a first intervention, followed by flow diverter treatment of the aneurysm remnant in a second intervention (
Figure 4).
3.3. Interventional Procedures
For the first treatment, a Neuron Max® 80 cm 6 F Sheath (Penumbra, Alameda, CA, USA) was directed into the left VA. Coaxially, a Sofia EX intermediate catheter (Microvention, Aliso Viejo, CA, USA) was navigated to the V3 segment. Passage of the aneurysm with a 0.0014” Guidewire was easily possible. Tracking of the deployment catheter, the Neuroslider 39® (Acandis, Pforzheim, Germany), over a wire was only possible up to the proximal BA due to relative rigidity of this large deployment catheter. Instead, the intermediate catheter could be tracked up to the left P1 segment using a tetra-axial approach utilizing a Rebar 18 microcatheter and different guidewires. In this stable position, the Neuroslider 39® could be brought up to the tip of the intermediate catheter, facilitating the deployment of the first and most distal stent. In total, three stents were implanted (one 10 × 60 mm and two 10 × 30 mm) to cover the entire diseased vessel.
No peri-interventional technical complications occurred (
Figure 4a–c).
Further, 5 days later, the aneurysm of the BA already showed a partial thrombosis in the post-interventional MRI with regular perfusion of the implanted stents. Several embolic microinfarcts were detected on both sides of the occipital, pontine, and cerebellar region, and the post-interventional neurological status of the patient showed a deterioration with a manifest incomplete tetraparesis.
In the second intervention 7 days later, the treatment was completed by implanting a Derivo-Heal
®-Flow-Diverter (8 × 25 mm) with coverage of the large aneurysm of the left VA compressing the brain stem (
Figure 4d–f). The Accero
®-Rex-Stents previously implanted in the BA had already led to remodeling of the BA, and extensive thrombosis of the treated BA aneurysm had occurred (
Figure 4f). The post-interventional CT showed no new ischemia. The patient showed a persistence of the known tetraparesis.
The patient could be discharged for rehabilitation with antiplatelet therapy of ticagrelor (90/0/90 mg/d) and ASA (100/0/0 mg/d) on the eighth day after the second intervention. Unfortunately, the patient died two months after endovascular intervention due to his cardiovascular co-morbidities.
4. Discussion
In this multicentre study, we were able to demonstrate the world’s first and technically successful use of Accero®-Rex-Stents in the treatment of five fusiform giant aneurysms of the posterior circulation. Despite the difficult conditions with parent vessel diameters above 7 mm in all cases, the stents were implanted safely, partly in combination with the use of flow-diverters. The first post-interventional MRI or CT scans follow-up showed a remodeling effect with significant reduction in the perfused aneurysm portion and regular perfusion of the inserted stents. The available three-month post-interventional DSA showed a significant reduction in the size of the stent-treated BA aneurysm (patient #2) and a stable size of the stent-treated aneurysm, respectively (patient #5).
Fusiform aneurysms of the cerebral vessels belong to the rarest vascular diseases, representing only 2–3% of all cerebral aneurysms [
2]. There are correspondingly few reports of successful treatments available and there is also no consensus on the best therapeutic approach. Therapeutic concepts range from watch-and-wait strategy to endovascular and combined endovascular-surgical approaches [
9,
11].
With increasing size, aneurysms often show accompanying space-occupying effects with compression of the brain stem as well as smaller infarcts from partially thrombosed aneurysms, which lead to an aggravation of the neurological symptoms [
4,
5,
12,
13].
In this context, giant aneurysms of the posterior circulation in particular are very difficult to treat successfully in terms of their outcome and their risk of bleeding; these procedures in particular show a frequent occurrence of peri-interventional complications such as aneurysm rupture [
14]. For example, regarding peri-interventional complications, previous studies have shown the occurrence of peri-interventional strokes in up to 23% of treated patients [
15].
The treatment of such large aneurysms of the posterior circulation certainly remains challenging and must be questioned very critically regarding its risks [
8,
10].
Previous limitations in the endovascular treatment options were often the very large diameter of the parent BA or VA. Commercially available braided stents usable and approved for intracranial use have a maximum diameter of 6 mm.
Cases #1 and #2 in our study show the importance of treating such giant aneurysms as early as possible to prevent further growth of the aneurysm, which ultimately increases the complexity and risks of interventional treatment at a later stage [
4]. Apparently, it seems particularly important to prevent an aggravation of the neurological symptoms, as the outcome is clearly worse in such courses of disease. In our opinion, it is important to treat a patient as early as possible. Not least because of the very small database, almost every treatment must be considered as an individual healing attempt.
As our cases show, an individual therapy concept for each patient must be developed in an interdisciplinary consensus between the departments of neurology, neurosurgery, and interventional neuroradiology [
9]. In particular, the combination of different devices or different techniques must be considered pre-interventional. The results of previous studies regarding aneurysm treatments, for example with the sole use of flow diverters, also showed an improvement in the clinical symptoms for most of the patients, but in individual cases they also led to a significant worsening [
16,
17].
Our study shows the remodeling effect of the inserted stents over time, which is similar to that of a flow diverter (shown in patient #2 and #5). Unfortunately, current flow diverters are not available in the sizes necessary for the treatment of such large aneurysms. With the availability of new devices such as the Accero®-Rex-Stents, the endovascular therapy options available so far can be significantly expanded.
5. Technical Aspects
With regard to the technical implementation, we emphasize that the navigation and deployment of such large stents in the posterior circulation requires appropriate access techniques. In two of our cases, the application of an intermediate catheter (Sofia, Microvention or Red 68, Penumbra, Alameda, CA, USA) was useful in guiding the relatively rigid 0.039″ Neuroslider® delivery catheter (Patients #1–#3). The use of two parallel 0.014″ microwires to achieve the deployment position (Patients #4 and #5) is a second feasible technique.
6. Limitations
The authors are aware of the limitations of their study, and especially the fact that Accero®-Rex-Stents have only been used in five cases in the treatment of fusiform giant aneurysms of the posterior circulation.
Despite the promising preliminary results, the lack of post-interventional follow-up DSA in some patients remains a major limitation to our study.
More frequent use of these stents and further evaluation in larger patient cohorts are needed to confirm our initial positive experience with technical feasibility and promising initial results.
Another minor limitation is formed by the composition of our cohort, which entirely consists of men and GIAs of the posterior circulation, whereas women and the anterior circulation are more often affected according to the literature.
7. Conclusions
With the Accero®-Rex-Stents, a new device is available that offers another treatment option for rare cerebral fusiform giant aneurysms with very large parent vessels. In this multicentre study, we demonstrate the technical feasibility of the implantation of the stents in patients with GIA. The available follow-up imaging indicate promising results but have yet to be verified by further research.
Author Contributions
All authors contributed to the study conception and design. Endovascular interventions were performed by M.E. (Mohamed Elsharkawy), W.S., E.C., R.C. and C.P.S. Material preparation, data collection and analysis were performed by H.K., B.H.A., A.A. and C.P.S. The first draft of the manuscript was written by H.K., B.H.A., M.E. (Mostafa Ergawy) and C.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript. No funding was received to assist with the preparation of this manuscript. No funding was received for conducting this study.
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki. The ethics committee of the University of Münster has approved the study.
Informed Consent Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and wit the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. No procedures in this study were performed on animals.
Data Availability Statement
The datasets and images used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We acknowledge support from the Open Access Publication Fund of the University of Münster.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Onofrj, V.; Cortes, M.; Tampieri, D. The insidious appearance of the dissecting aneurysm: Imaging findings and related pathophysiology. A report of two cases. Interv. Neuroradiol. 2016, 22, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Dengler, J.; Maldaner, N.; Glasker, S.; Endres, M.; Wagner, M.; Malzahn, U.; Heuschmann, P.U.; Vajkoczy, P.; Giant Intracranial Aneurysm Study Group. Outcome of Surgical or Endovascular Treatment of Giant Intracranial Aneurysms, with Emphasis on Age, Aneurysm Location, and Unruptured Aneuryms—A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2016, 41, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Yim, M.B.; Lee, C.Y.; Kim, E.; Son, E.I. Intracranial Fusiform Aneurysms: It’s Pathogenesis, Clinical Characteristics and Managements. J. Korean Neurosurg. Soc. 2008, 44, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, M.; Tomelleri, G.; Piovan, E.; Bovi, P.; Moretto, G.; Gulli, G. Chronic fusiform aneurysm evolving into giant aneurysm in the basilar artery. Neurol. Sci. 2012, 33, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Del Brutto, V.J.; Gutierrez, J.; Goryawala, M.Z.; Sacco, R.L.; Rundek, T.; Romano, J.G. Prevalence and Clinical Correlates of Intracranial Dolichoectasia in Individuals With Ischemic Stroke. Stroke 2021, 52, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, S.; Casaroto, E.; Bueno Alves, M.; Ierardi Goulart, L.; Annes, M.; Sampaio Silva, G. The challenge of managing fusiform basilar artery aneurysms: From acute ischemic stroke to a massive subarachnoid hemorrhage. Case Rep. Neurol. 2011, 3, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Tutuncu, F.; Schimansky, S.; Baharoglu, M.I.; Gao, B.; Calnan, D.; Hippelheuser, J.; Safain, M.G.; Lauric, A.; Malek, A.M. Widening of the basilar bifurcation angle: Association with presence of intracranial aneurysm, age, and female sex. J. Neurosurg. 2014, 121, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Amelot, A.; Smajda, S.; Terrier, L.M.; Redjem, H.; Simonneau, A.; Bekaert, O.; Piotin, M.; Lot, G.; Chauvet, D. Posterior Fossa Craniectomy with Endovascular Therapy of Giant Fusiform Basilar Artery Aneurysms: A New Approach to Consider? World Neurosurg. 2017, 98, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, M.; Gruber, A. Current Strategies in the Treatment of Intracranial Large and Giant Aneurysms. In Trends in Cerebrovascular Surgery and Interventions; Esposito, G., Regli, L., Cenzato, M., Kaku, Y., Tanaka, M., Tsukahara, T., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2021; pp. 19–26. [Google Scholar]
- Darsaut, T.E.; Darsaut, N.M.; Chang, S.D.; Silverberg, G.D.; Shuer, L.M.; Tian, L.; Dodd, R.L.; Do, H.M.; Marks, M.P.; Steinberg, G.K. Predictors of clinical and angiographic outcome after surgical or endovascular therapy of very large and giant intracranial aneurysms. Neurosurgery 2011, 68, 903–915; discussion 915. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, J. Prospects and Dilemmas of Endovascular Treatment for Vertebrobasilar Dolichoectasia. Front. Neurol. 2022, 13, 895527. [Google Scholar] [CrossRef] [PubMed]
- Kumral, E.; Kisabay, A.; Atac, C.; Kaya, C.; Calli, C. The mechanism of ischemic stroke in patients with dolichoectatic basilar artery. Eur. J. Neurol. 2005, 12, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Zis, P.; Fragkis, S.; Lykouri, M.; Bageris, I.; Kolovos, G.; Angelidakis, P.; Tavernarakis, A. From basilar artery dolichoectasia to basilar artery aneurysm: Natural history in images. J. Stroke Cerebrovasc. Dis. 2015, 24, e117–e119. [Google Scholar] [CrossRef] [PubMed]
- Greving, J.P.; Wermer, M.J.; Brown, R.D., Jr.; Morita, A.; Juvela, S.; Yonekura, M.; Ishibashi, T.; Torner, J.C.; Nakayama, T.; Rinkel, G.J.; et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef]
- Kiyofuji, S.; Graffeo, C.S.; Perry, A.; Murad, M.H.; Flemming, K.D.; Lanzino, G.; Rangel-Castilla, L.; Brinjikji, W. Meta-analysis of treatment outcomes of posterior circulation non-saccular aneurysms by flow diverters. J. Neurointerv. Surg. 2018, 10, 493–499. [Google Scholar] [CrossRef]
- Sindeev, S.; Kirschke, J.S.; Prothmann, S.; Frolov, S.; Liepsch, D.; Berg, P.; Zimmer, C.; Friedrich, B. Evaluation of flow changes after telescopic stenting of a giant fusiform aneurysm of the vertebrobasilar junction. Biomed. Eng. Online 2019, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hasegawa, H.; Ando, K.; Shibuya, K.; Takahashi, H.; Saito, S.; Oishi, M.; Fujii, Y. Possibility of Worsening Flow Diversion Effect Due to Morphological Changes of a Stented Artery With Multiple Overlapping Stents for Partially Thrombosed Vertebral Artery Aneurysms. Front. Neurol. 2020, 11, 611124. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).