Amyloid Burden Correlates with Electrocardiographic Findings in Patients with Cardiac Amyloidosis—Insights from Histology and Cardiac Magnetic Resonance Imaging
Abstract
1. Introduction
2. Methods
2.1. Setting and Study Design
2.2. Diagnosis of Cardiac Transthyretin Amyloidosis
2.3. Diagnosis of Cardiac Light Chain Amyloidosis
2.4. Electrocardiogram
2.5. Cardiac Magnetic Resonance Imaging
2.6. Endomyocardial Biopsy and Histological Analysis
2.7. Endpoints and Outcome Measures
2.8. Statistical Analysis
3. Results
3.1. Study Population
3.2. Baseline Characteristics
3.3. Correlation and Regression Analyses
3.4. Electrocardiographic Parameters Stratified According to Extracellular Volume Median
3.5. Survival Analysis
4. Discussion
4.1. Correlation of Amyloid Burden with Electrocardiographic Findings in Cardiac Amyloidosis
4.2. Prevalence of Electrocardiographic Findings in Cardiac Amyloidosis
4.3. Prognostic Significance of Electrocardiographic Findings in Cardiac Amyloidosis
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2021, 23, 512–526. [Google Scholar] [CrossRef]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Palladini, G.; Minnema, M.C.; Wechalekar, A.D.; Jaccard, A.; Lee, H.C.; Sanchorawala, V.; Gibbs, S.; Mollee, P.; Venner, C.P.; et al. Daratumumab-Based Treatment for Immunoglobulin Light-Chain Amyloidosis. N. Engl. J. Med. 2021, 385, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Dispenzieri, A.; Sher, T. Pathophysiology and treatment of cardiac amyloidosis. Nat. Rev. Cardiol. 2015, 12, 91–102. [Google Scholar] [CrossRef]
- Tanskanen, M.; Peuralinna, T.; Polvikoski, T.; Notkola, I.L.; Sulkava, R.; Hardy, J.; Singleton, A.; Kiuru-Enari, S.; Paetau, A.; Tienari, P.J.; et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: A population-based autopsy study. Ann. Med. 2008, 40, 232–239. [Google Scholar] [CrossRef] [PubMed]
- González-López, E.; Gallego-Delgado, M.; Guzzo-Merello, G.; de Haro-Del Moral, F.J.; Cobo-Marcos, M.; Robles, C.; Bornstein, B.; Salas, C.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015, 36, 2585–2594. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.; Bokhari, S.; Maurer, M.S. Unveiling wild-type transthyretin cardiac amyloidosis as a significant and potentially modifiable cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015, 36, 2595–2597. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Frantz, J.G.; Hershberger, R.E.; Maurer, M.S.; et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient With Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2023, 81, 1076–1126. [Google Scholar] [CrossRef]
- González-López, E.; Gagliardi, C.; Dominguez, F.; Quarta, C.C.; de Haro-del Moral, F.J.; Milandri, A.; Salas, C.; Cinelli, M.; Cobo-Marcos, M.; Lorenzini, M.; et al. Clinical characteristics of wild-type transthyretin cardiac amyloidosis: Disproving myths. Eur. Heart J. 2017, 38, 1895–1904. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Hanna, M.; Elshazly, M.B.; Puri, R.; Saliba, W.; Kanj, M.; Vakamudi, S.; Patel, D.R.; Baranowski, B.; et al. Atrial Fibrillation in Transthyretin Cardiac Amyloidosis. JACC Clin. Electrophysiol. 2020, 6, 1118–1127. [Google Scholar] [CrossRef]
- Cipriani, A.; De Michieli, L.; Porcari, A.; Licchelli, L.; Sinigiani, G.; Tini, G.; Zampieri, M.; Sessarego, E.; Argirò, A.; Fumagalli, C.; et al. Low QRS Voltages in Cardiac Amyloidosis: Clinical Correlates and Prognostic Value. JACC Cardio Oncol. 2022, 4, 458–470. [Google Scholar] [CrossRef]
- Alpert, M.A.; Terry, B.E.; Cohen, M.V.; Fan, T.M.; Painter, J.A.; Massey, C.V. The electrocardiogram in morbid obesity. Am. J. Cardiol. 2000, 85, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Comenzo, R.; Falk, R.H.; Fermand, J.P.; Hazenberg, B.P.; Hawkins, P.N.; Merlini, G.; Moreau, P.; Ronco, P.; Sanchorawala, V.; et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am. J. Hematol. 2005, 79, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.A.; Meo Neto, J.d.P.; Canham, R.M.; Uddin, F.; Toto, K.H.; Nelson, L.L.; Kaiser, P.A.; de Lemos, J.A.; Drazner, M.H. Low voltage on the electrocardiogram is a marker of disease severity and a risk factor for adverse outcomes in patients with heart failure due to systolic dysfunction. Am. Heart J. 2006, 152, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Kammerlander, A.A.; Marzluf, B.A.; Zotter-Tufaro, C.; Aschauer, S.; Duca, F.; Bachmann, A.; Knechtelsdorfer, K.; Wiesinger, M.; Pfaffenberger, S.; Greiser, A.; et al. T1 Mapping by CMR Imaging: From Histological Validation to Clinical Implication. JACC Cardiovasc. Imaging 2016, 9, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Kyle, R.A.; Lie, J.T. Clinical significance of histopathologic patterns of cardiac amyloidosis. Mayo Clin. Proc. 1984, 59, 547–555. [Google Scholar] [CrossRef]
- De Gaspari, M.; Sinigiani, G.; De Michieli, L.; Della Barbera, M.; Rizzo, S.; Thiene, G.; Iliceto, S.; Perazzolo Marra, M.; Mele, D.; Basso, C.; et al. Relative apical sparing in cardiac amyloidosis is not always explained by an amyloid gradient. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1258–1268. [Google Scholar] [CrossRef]
- Odom, H.; Davis, J.L.; Dinh, H.; Baker, B.J.; Roberts, W.C.; Murphy, M.L. QRS voltage measurements in autopsied men free of cardiopulmonary disease: A basis for evaluating total QRS voltage as an index of left ventricular hypertrophy. Am. J. Cardiol. 1986, 58, 801–804. [Google Scholar] [CrossRef]
- Kumar, D.; Bajaj, R.; Chhabra, L.; Spodick, D.H. Refinement of total 12-lead QRS voltage criteria for diagnosing left ventricular hypertrophy. World J. Cardiovasc. Dis. 2013, 3, 5. [Google Scholar] [CrossRef]
- Gertz, M.A.; Benson, M.D.; Dyck, P.J.; Grogan, M.; Coelho, T.; Cruz, M.; Berk, J.L.; Plante-Bordeneuve, V.; Schmidt, H.H.J.; Merlini, G. Diagnosis, Prognosis, and Therapy of Transthyretin Amyloidosis. J. Am. Coll. Cardiol. 2015, 66, 2451–2466. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.; Duca, F.; Binder, T.; Rettl, R.; Dachs, T.M.; Seirer, B.; Camuz Ligios, L.; Dusik, F.; Capelle, C.; Qin, H.; et al. Prognostic implications of pericardial and pleural effusion in patients with cardiac amyloidosis. Clin. Res. Cardiol. 2021, 110, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Martini, N.; Sinigiani, G.; De Michieli, L.; Mussinelli, R.; Perazzolo Marra, M.; Iliceto, S.; Zorzi, A.; Perlini, S.; Corrado, D.; Cipriani, A. Electrocardiographic features and rhythm disorders in cardiac amyloidosis. Trends Cardiovasc. Med. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, J.; Tian, Z.; Fang, Q. Clinical correlates and prognostic values of pseudoinfarction in cardiac light-chain amyloidosis. J. Cardiol. 2016, 68, 426–430. [Google Scholar] [CrossRef]
- Duca, F.; Kammerlander, A.A.; Panzenböck, A.; Binder, C.; Aschauer, S.; Loewe, C.; Agis, H.; Kain, R.; Hengstenberg, C.; Bonderman, D.; et al. Cardiac Magnetic Resonance T(1) Mapping in Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2018, 11, 1924–1926. [Google Scholar] [CrossRef]
- Kristen, A.V.; Perz, J.B.; Schonland, S.O.; Hegenbart, U.; Schnabel, P.A.; Kristen, J.H.; Goldschmidt, H.; Katus, H.A.; Dengler, T.J. Non-invasive predictors of survival in cardiac amyloidosis. Eur. J. Heart Fail. 2007, 9, 617–624. [Google Scholar] [CrossRef]
- Sperry, B.W.; Vranian, M.N.; Hachamovitch, R.; Joshi, H.; McCarthy, M.; Ikram, A.; Hanna, M. Are classic predictors of voltage valid in cardiac amyloidosis? A contemporary analysis of electrocardiographic findings. Int. J. Cardiol. 2016, 214, 477–481. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Garcia-Pavia, P.; Grogan, M.; Hanna, M.A.; Heitner, S.B.; Jacoby, D.; Maurer, M.S.; Rapezzi, C.; Shah, S.J.; Ganju, J.; et al. Abstract 14214: ATTRibute-CM: A Randomized, Double-Blind, Placebo-Controlled, Multi-Center, Global Phase 3 Study of AG10 in Patients with Transthyretin Amyloid Cardiomyopathy (ATTR-CM). Circulation 2019, 140, A14214. [Google Scholar] [CrossRef]

| Variable | All-Comer Study Cohort n = 105 |
|---|---|
| Clinical parameters | |
| Age, years (IQR) | 77 (70–82) |
| Male sex, n (%) | 78 (74.3) |
| Transthyretin amyloidosis, n (%) | 87 (82.9) |
| Wild-type transthyretin amyloidosis, n (%) | 78 (74.3) |
| Variant transthyretin amyloidosis, n (%) | 9 (8.6) |
| Light chain amyloidosis, n (%) | 18 (17.1) |
| Body mass index, kg/m2 (IQR) | 27.0 (23.9–29.0) |
| New York Heart Association class ≥ III, n (%) | 45 (42.9) |
| 6-minute walk distance, m (IQR) | 390 (285–473) |
| Laboratory parameters | |
| N-terminal pro brain natriuretic peptide, pg/mL (IQR) | 2020 (849–3999) |
| Troponin t, ng/L (IQR) | 43.0 (27.0–68.0) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 (IQR) | 54.6 (42.3–71.0) |
| National Amyloidosis Center stage I | 49 (46.7) |
| National Amyloidosis Center stage II | 24 (22.9) |
| National Amyloidosis Center stage III | 14 (13.3) |
| Histologically quantified amyloid area | |
| Amyloid area, cm2 (IQR) | |
| Electrocardiographic parameters | |
| Total voltage, mm (IQR) | 102 (81.5–123) |
| Total peripheral lead voltage, mm (IQR) | 34.0 (23.8–41.8) |
| Total precordial lead voltage, mm (IQR) | 67.0 (54.3–78.5) |
| Low voltage criteria, n (%) | 29 (27.6) |
| Sinus rhythm, n (%) | 66 (62.9) |
| Atrial fibrillation, n (%) | 36 (34.3) |
| Atrial flutter, n (%) | 3 (2.9) |
| Heart rate, bpm (IQR) | 71 (62–82.5) |
| PR interval, ms (IQR) | 195 (166–212) |
| QRS width, ms (IQR) | 102 (86–134) |
| QT time, ms (IQR) | 462 (438–484) |
| AV-Block 1°, n (%) | 18 (17.1) |
| AV-Block 2° (Mobitz type 1), n (%) | 1 (1.0) |
| Incomplete left bundle branch block, n (%) | 7 (6.7) |
| Complete left bundle branch block, n (%) | 17 (16.2) |
| Complete right bundle branch block, n (%) | 8 (7.6) |
| Left anterior fascicular block, n (%) | 8 (7.6) |
| Bifascicular block n (%) | 9 (8.6) |
| Anterior pseudoinfarct pattern, n (%) | 21 (20.0) |
| Ventricular premature complexes, n (%) | 8 (7.6) |
| Cardiac magnetic resonance imaging parameters | |
| Extracellular volume, % (IQR) | 43.9 (36.4–54.4) |
| LVEDD, mm (IQR) | 44.0 (40.0–48.0) |
| RVEDD, mm (IQR) | 39.0 (35.0–44.5) |
| Interventricular septum, mm (IQR) | 18.0 (15.0–21.0) |
| Left atrial area, cm2 (IQR) | 31.0 (27.0–35.0) |
| Right atrial area, cm2 (IQR) | 28.0 (23.0–35.5) |
| Ascending aorta, mm (IQR) | 36.0 (33.0–39.0) |
| Pulmonary artery, mm (IQR) | 28.0 (25.0–31.0) |
| Left ventricular ejection fraction, % (IQR) | 54.3 (45.0–61.3) |
| Left ventricular end-diastolic volume, mL (IQR) | 157 (127–189) |
| Left ventricular cardiac output, L/min (IQR) | 5.3 (4.6–6.4) |
| Left ventricular mass, g (IQR) | 177 (141–211) |
| Right ventricular ejection fraction, % (IQR) | 48.0 (41.0–56.5) |
| Right ventricular end-diastolic volume, mL (IQR) | 165 (136–203) |
| Right ventricular cardiac output, L/min (IQR) | 5.1 (4.5–6.2) |
| Variable n = 105 | Correlation Coefficient | p Value |
|---|---|---|
| Clinical and laboratory parameters | ||
| Body mass index | −0.132 | 0.179 |
| New York Heart Association class ≥III | −0.111 | 0.273 |
| 6-minute walk distance | 0.092 | 0.460 |
| Log N-terminal pro brain natriuretic peptide | −0.113 | 0.352 |
| Troponin T | −0.221 | 0.028 |
| Estimated glomerular filtration rate | 0.106 | 0.280 |
| National Amyloidosis Center stage I–III | 0.014 | 0.894 |
| Electrocardiographical parameters | ||
| Heart rate | −0.118 | 0.231 |
| PR interval | −0.104 | 0.408 |
| QRS width | 0.239 | 0.014 |
| QT time | 0.154 | 0.119 |
| Histologically quantified amyloid area | ||
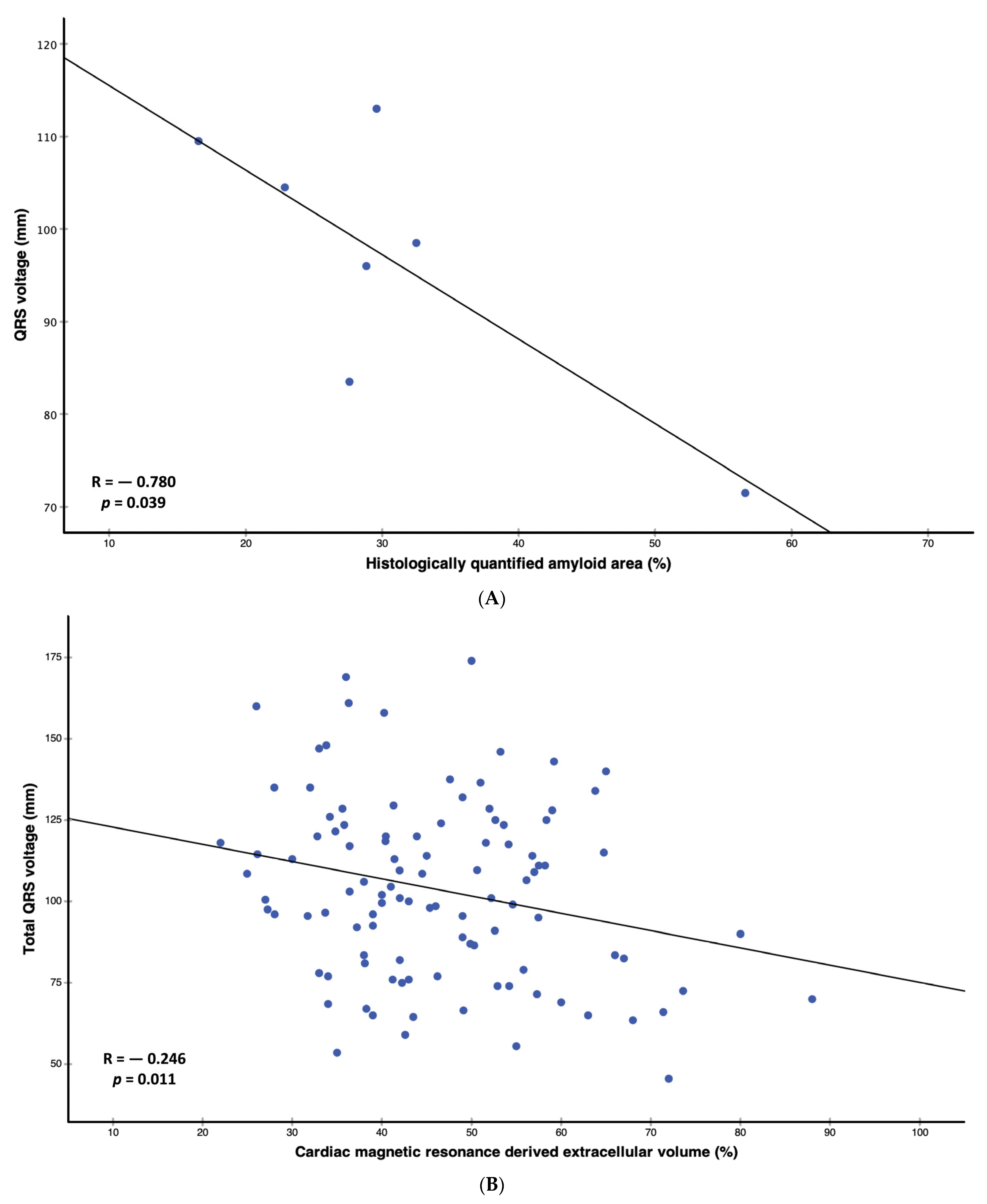
| Amyloid area, n = 7 | −0.780 | 0.039 |
| Cardiac magnetic resonance imaging parameters | ||
| Extracellular volume | −0.246 | 0.011 |
| Left ventricular end-diastolic diameter | −0.103 | 0.294 |
| Right ventricular end-diastolic diameter | −0.091 | 0.356 |
| Interventricular septum | 0.282 | 0.004 |
| Left atrial area | 0.229 | 0.019 |
| Right atrial area | −0.033 | 0.738 |
| Ascending aorta | 0.051 | 0.605 |
| Pulmonary artery | −0.061 | 0.539 |
| Left ventricular ejection fraction | 0.026 | 0.795 |
| Left ventricular end-diastolic volume | 0.104 | 0.2090 |
| Left ventricular cardiac output | 0.000 | 0.996 |
| Left ventricular mass | 0.174 | 0.105 |
| Right ventricular ejection fraction | 0.063 | 0.525 |
| Right ventricular end-diastolic volume | −0.021 | 0.835 |
| Right ventricular cardiac output | −0.027 | 0.784 |
| Variable | Beta | 95% Confidence Interval | p Value | Adjusted Beta * | 95% Confidence Interval | p Value |
|---|---|---|---|---|---|---|
| Total voltage | ||||||
| Body mass index | −0.966 | −2.381–0.450 | 0.179 | −1.092 | −2.510–0.326 | 0.130 |
| New York Heart Association class ≥III | −8.781 | −19.780–2.217 | 0.116 | −6.548 | −18.178–5.082 | 0.266 |
| 6-minute walk distance | 0.018 | −0.030–0.066 | 0.460 | 0.005 | −0.051–0.062 | 0.857 |
| Log N-terminal pro brain natriuretic peptide | −2.721 | −7.398–1.956 | 0.251 | −1.129 | −6.002–3.745 | 0.647 |
| Troponin T | −0.136 | −0.257–−0.015 | 0.028 | −0.115 | −0.240–0.011 | 0.073 |
| Estimated glomerular filtration rate | 0.129 | −0.107–0.366 | 0.280 | 0.051 | −0.196–0.299 | 0.681 |
| National Amyloidosis Center stage I-III | −0.204 | −8.015–7.607 | 0.959 | 0.402 | −7.562–8.365 | 0.920 |
| Electrocardiographical parameters | ||||||
| Heart rate | −0.230 | −0.607–0.148 | 0.231 | −0.239 | −0.615–0.138 | 0.211 |
| PR interval | −0.086 | −0.292–0.120 | 0.408 | −0.114 | −0.341–0.113 | 0.319 |
| QRS width | 0.218 | 0.045–0.391 | 0.014 | 0.227 | 0.048–0.406 | 0.013 |
| QT time | 0.128 | −0.033–0.289 | 0.119 | 0.130 | −0.033–0.293 | 0.117 |
| Amyloid area | −0.913 | −1.756–−0.070 | 0.039 | 0.900 | −8.675–10.476 | 0.725 |
| Extracellular volume | −0.530 | −0.939–−0.122 | 0.011 | −0.610 | −1.017–−0.204 | 0.004 |
| Left ventricular end-diastolic diameter | −0.427 | −1.229–0.375 | 0.294 | −0.454 | −1.268–0.360 | 0.271 |
| Right ventricular end-diastolic diameter | −0.329 | −1.033–0.375 | 0.356 | −0.368 | −1.082–0.346 | 0.309 |
| Interventricular septum | 1.901 | 0.637–3.165 | 0.004 | 1.749 | 0.403–3.096 | 0.011 |
| Left atrial area | 0.767 | 0.128–1.405 | 0.019 | 0.793 | 0.160–1.426 | 0.015 |
| Right atrial area | −0.119 | −0.823–0.585 | 0.738 | −0.193 | −0.956–0.570 | 0.616 |
| Ascending aorta | 0.335 | −0.945–1.614 | 0.605 | 0.401 | −0.988–1.790 | 0.568 |
| Pulmonary artery | -0.350 | −1.475–0.776 | 0.539 | −0.002 | −1.203–1.199 | 0.997 |
| Left ventricular ejection fraction | 0.058 | −0.387–0.503 | 0.795 | 0.149 | −0.301–0.600 | 0.511 |
| Left ventricular end-diastolic volume | 0.062 | −0.054–0.178 | 0.290 | 0.053 | −0.077–0.183 | 0.419 |
| Left ventricular cardiac output | 0.009 | −3.824–3.842 | 0.996 | 1.009 | −3.040–5.058 | 0.622 |
| Left ventricular mass | 0.091 | −0.019–0.201 | 0.105 | 0.129 | 0.002–0.256 | 0.046 |
| Right ventricular ejection fraction | 0.156 | −0.329–0.640 | 0.525 | 0.242 | −0.263–0.746 | 0.344 |
| Right ventricular end-diastolic volume | −0.011 | −0.119–0.097 | 0.835 | −0.024 | −0.143–0.096 | 0.694 |
| Right ventricular cardiac output | −0.500 | −4.109–3.109 | 0.784 | 0.070 | −3.610–3.751 | 0.970 |
| Variable | ECV < Median (43.9%) | ECV ≥ Median (43.9%) | p Value |
|---|---|---|---|
| Electrocardiographic parameters | |||
| Total voltage, mm (IQR) | 103 (82.8–121) | 100 (77.5–124) | 0.495 |
| Total peripheral lead voltage, mm (IQR) | 34.5 (24.3) | 33.8 (22.6–41.0) | 0.453 |
| Total precordial lead voltage, mm (IQR) | 68.0 (55.8–80.0) | 63.8 (52.6–76.6) | 0.624 |
| Low voltage criteria, n (%) | 12 (22.6) | 17 (32.7) | 0.249 |
| Sinus rhythm, n (%) | 37 (69.8) | 29 (55.8) | 0.137 |
| Atrial fibrillation, n (%) | 14 (26.4) | 22 (42.3) | 0.086 |
| Atrial flutter, n (%) | 2 (3.8) | 1 (1.9) | 0.569 |
| Heart rate, bpm (IQR) | 69.0 (60.5–80.0) | 71.0 (64.8–86.0) | 0.115 |
| PR interval, ms (IQR) | 180 (157–204) | 204 (171–218) | 0.016 |
| QRS width, ms (IQR) | 96.0 (86.0–135) | 108 (92.0–132) | 0.255 |
| QT time, ms (IQR) | 454 (431–481) | 466 (451–489) | 0.079 |
| Any AV-Block, n (%) | 7 (13.2) | 12 (23.1) | 0.189 |
| AV-Block 1°, n (%) | 7 (13.2) | 11 (21.2) | 0.134 |
| AV-Block 2° (Mobitz type 1), n (%) | 0 (0.0) | 1 (1.9) | 0.310 |
| Any bundle branch block, n (%) | 22 (41.5) | 27 (23.1) | 0.285 |
| Incomplete left bundle branch block, n (%) | 3 (5.7) | 4 (7.7) | 0.676 |
| Complete left bundle branch block, n (%) | 12 (22.6) | 5 (9.6) | 0.070 |
| Complete right bundle branch block, n (%) | 2 (3.8) | 6 (11.5) | 0.134 |
| Left anterior fascicular block, n (%) | 1 (1.9) | 7 (13.5) | 0.025 |
| Bifascicular block n (%) | 4 (7.5) | 5 (9.6) | 0.705 |
| Anterior pseudoinfarct pattern, n (%) | 9 (17.0) | 12 (23.1) | 0.435 |
| Ventricular premature complexes, n (%) | 3 (5.7) | 5 (9.6) | 0.445 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duca, F.; Rettl, R.; Kronberger, C.; Poledniczek, M.; Binder, C.; Dalos, D.; Koschutnik, M.; Donà, C.; Beitzke, D.; Loewe, C.; et al. Amyloid Burden Correlates with Electrocardiographic Findings in Patients with Cardiac Amyloidosis—Insights from Histology and Cardiac Magnetic Resonance Imaging. J. Clin. Med. 2024, 13, 368. https://doi.org/10.3390/jcm13020368
Duca F, Rettl R, Kronberger C, Poledniczek M, Binder C, Dalos D, Koschutnik M, Donà C, Beitzke D, Loewe C, et al. Amyloid Burden Correlates with Electrocardiographic Findings in Patients with Cardiac Amyloidosis—Insights from Histology and Cardiac Magnetic Resonance Imaging. Journal of Clinical Medicine. 2024; 13(2):368. https://doi.org/10.3390/jcm13020368
Chicago/Turabian StyleDuca, Franz, René Rettl, Christina Kronberger, Michael Poledniczek, Christina Binder, Daniel Dalos, Matthias Koschutnik, Carolina Donà, Dietrich Beitzke, Christian Loewe, and et al. 2024. "Amyloid Burden Correlates with Electrocardiographic Findings in Patients with Cardiac Amyloidosis—Insights from Histology and Cardiac Magnetic Resonance Imaging" Journal of Clinical Medicine 13, no. 2: 368. https://doi.org/10.3390/jcm13020368
APA StyleDuca, F., Rettl, R., Kronberger, C., Poledniczek, M., Binder, C., Dalos, D., Koschutnik, M., Donà, C., Beitzke, D., Loewe, C., Nitsche, C., Hengstenberg, C., Badr-Eslam, R., Kastner, J., Bergler-Klein, J., & Kammerlander, A. A. (2024). Amyloid Burden Correlates with Electrocardiographic Findings in Patients with Cardiac Amyloidosis—Insights from Histology and Cardiac Magnetic Resonance Imaging. Journal of Clinical Medicine, 13(2), 368. https://doi.org/10.3390/jcm13020368







