Valve-in-Valve Transcatheter Aortic Valve Replacement: From Pre-Procedural Planning to Procedural Scenarios and Possible Complications
Abstract
1. Introduction
2. ViV TAVR vs. Redo-SAVR: Evidence, Indications and Patients’ Selection
- -
- patients at intermediate or low surgical risk;
- -
- young individuals with longer life expectancy (because no data are available on long-term durability of ViV TAVR);
- -
- patients with complex anatomical features for ViV TAVR, such as a high risk for coronary obstruction (without possibility of performing BASILICA) or with small anatomies.
- -
- in cases of non-structural BHV dysfunction, such as patient-prosthesis mismatch (PPM) or severe PVL (percutaneous approach might be reasonable in cases of PPM when a balloon valve fracture might be performed within a stented surgical valve or in cases of PVL suitable for a percutaneous closure).
3. Lifetime Management of Patients with AS: TAV Surgical Explant vs. Redo-TAVR for Failed THVs
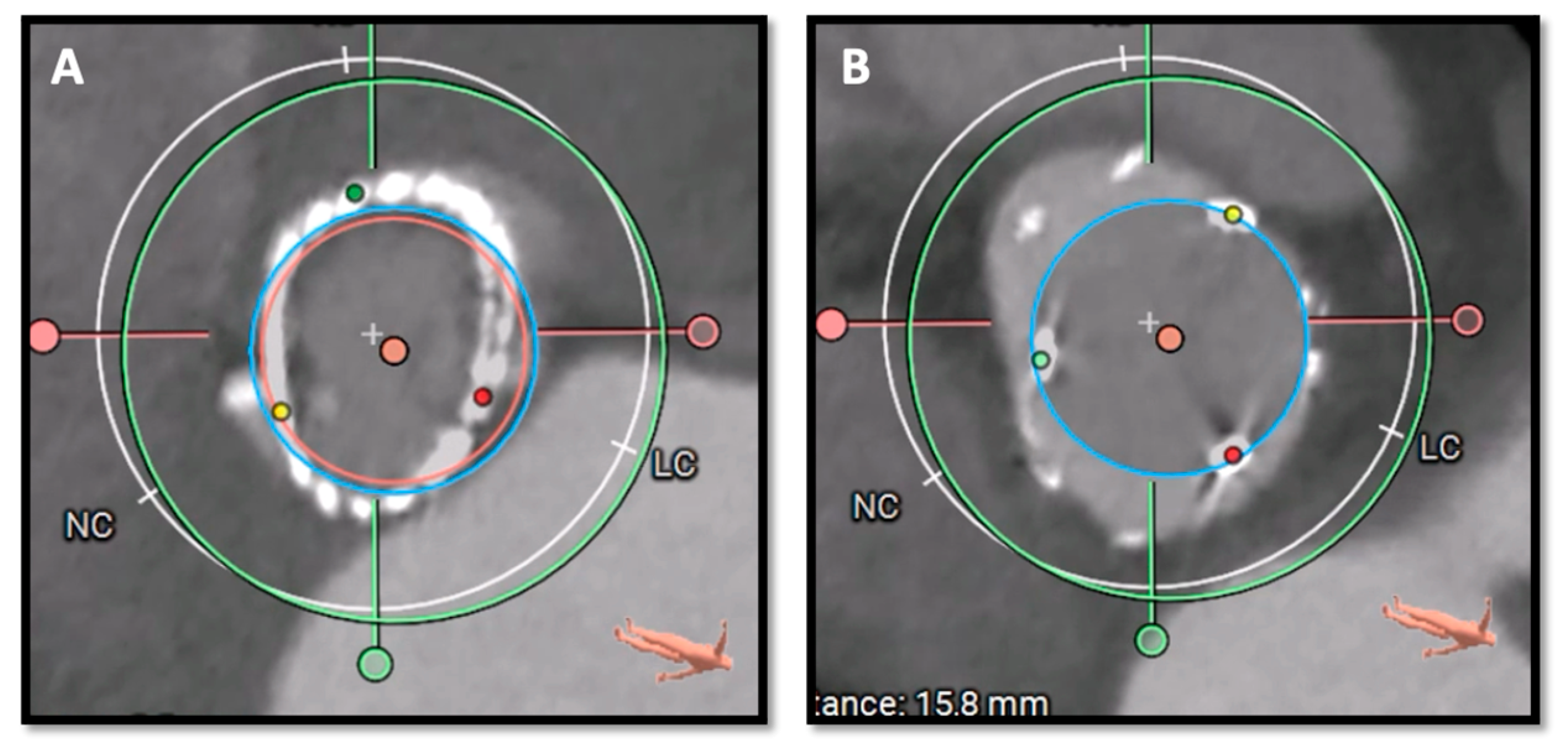
4. Pre-Procedural Planning
4.1. TAVR in SAVR
4.2. TAVR in TAVR
5. Procedural Scenarios and Possible Complications Management
- Deployment
6. Main Concerns to Consider during VIV Procedures
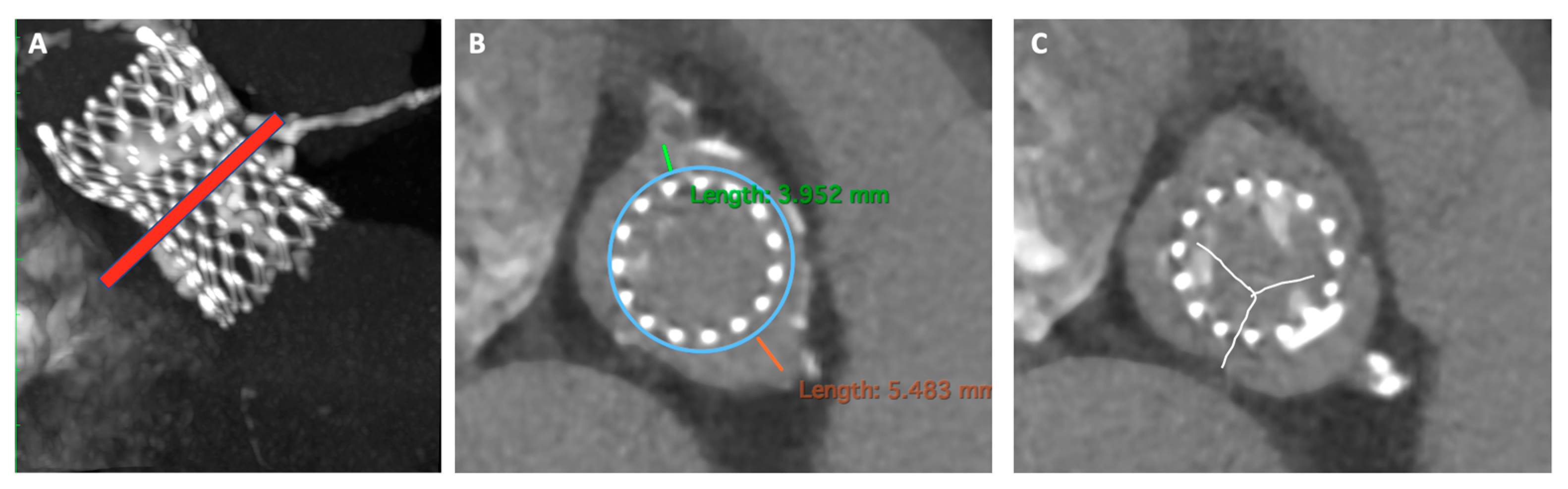
- Patient prosthesis mismatch (PPM)
- -
- Self-expanding THV with supra-annular design: When feasible, are the preferred choice in small SHV. Rodés-Cabau et al. [37], in their randomized trial comparing balloon vs. self-expanding valve systems in failed small SHV, founded an association between supra-annular SEV and improved hemodynamics with lower PPM.
- -
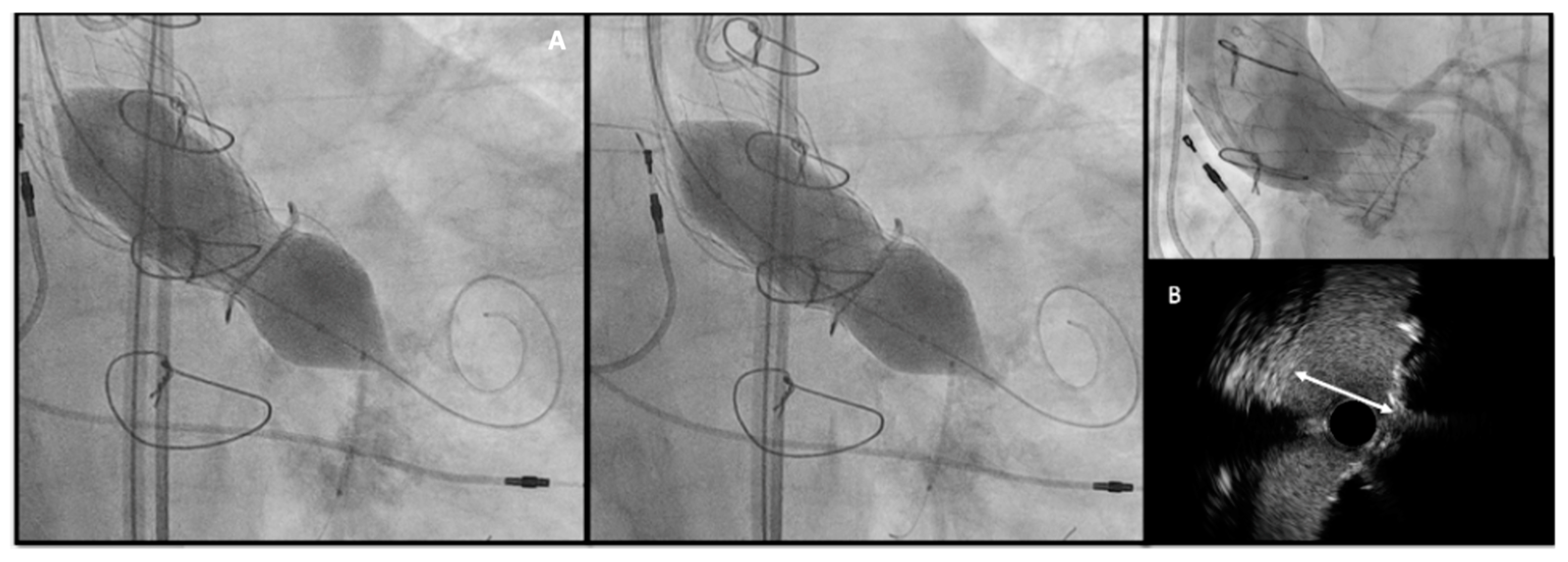
- Higher THV deployment (even if with a mild higher risk of coronary obstruction or valve embolization): As suggested in the VIVID registry [27] and confirmed by Simonato et al. [38] with their in vitro analysis. Considering the two most used THVs, the optimal implantation depth is 0 to 5 mm for the Corevalve and 0–2 mm up to 3.5 mm (0%–10% device frame) for Sapien THV.
- -
- Bioprosthetic valve fracture (BVF) and bioprosthetic valve remodeling (BVR) [39]: By either fracturing or stretching the surgical valve ring, providing increased THV expansion, better sealing, and post implantation hemodynamics. BVF is performed using a non-compliant balloon such as True Dilatation or Atlas Gold (Bard, Murry Hill, NJ, USA); (II) a high-pressure stopcock and tubing; (III) an indeflator and; (IV) a 60-mL syringe with dilute contrast. After initiating rapid ventricular pacing, the non-compliant balloon is rapidly inflated with 60 mL dilute contrast until fracture occurs. The best confirmation that BVF has occurred is by (A) angiographic modification of balloon waist and THV geometry, (B) a pressure drop in the in deflator or (C) an audible click concomitantly with the fracture. The balloon size should be determined by the THV used, the true ID of the SHV and the desired increase in diameter after fracture, the anatomy of the aortic root and LVOT and the height of coronary arteries. Generally, the balloon size should be 1 mm higher than the SHV diameter.
- -
- The timing of BVF, either before or after TAVR, remains controversial. BVF after THV implantation leads to better hemodynamics but carries a risk of damaging new prosthesis; BVF before THV implantation ensures better sealing, but may cause embolization of SHV, acute valvular regurgitation and hemodynamic instability [40,41]. The general practice is to do BVF after THV if using BEV so the NC balloon simultaneously fully expand the THV and fracture the surgical prosthesis while SEVs may not have enough force to fully expand a degenerated SHV and will benefit from balloon fracture before and if needed, even after implantation (Figure 6).
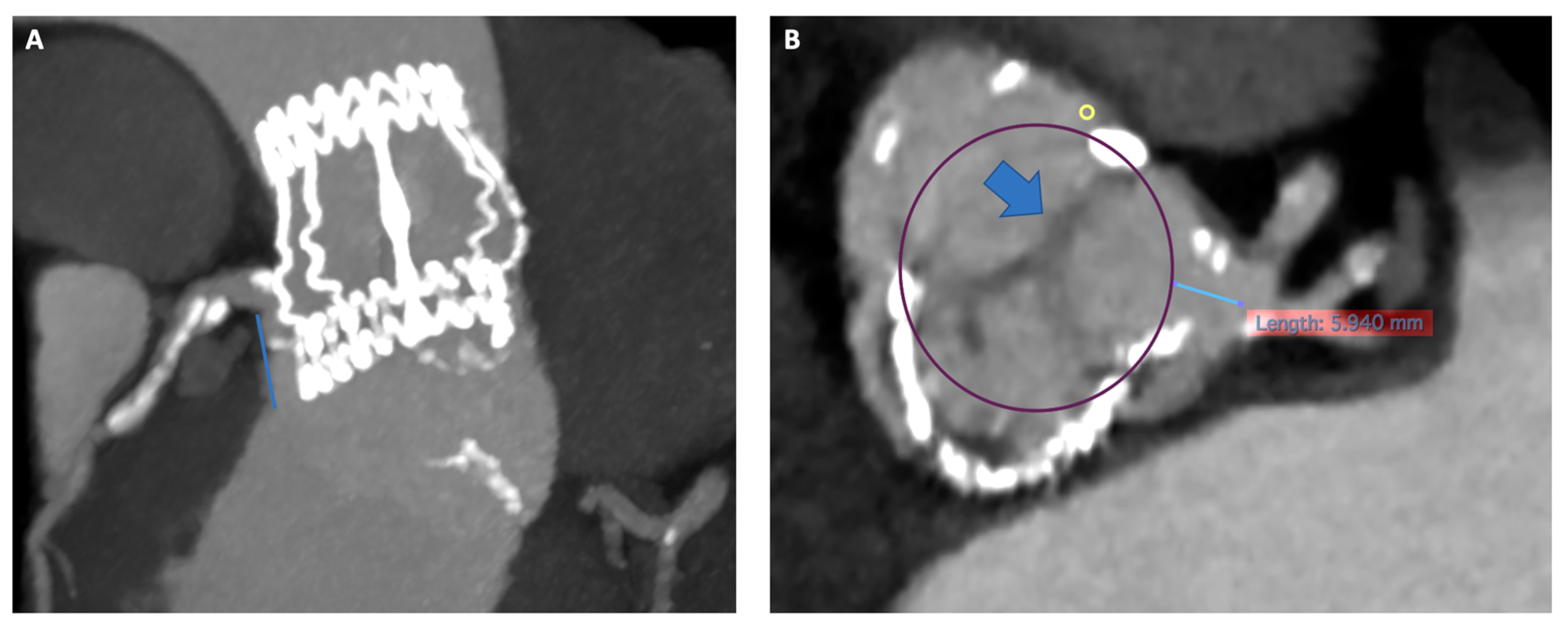
- Risk of coronary artery obstruction (CAO)
- -
- Lower implantation of the THV: Preferring a SEF due to the possibility of re-capturing or checking coronary flow before definite deployment;
- -
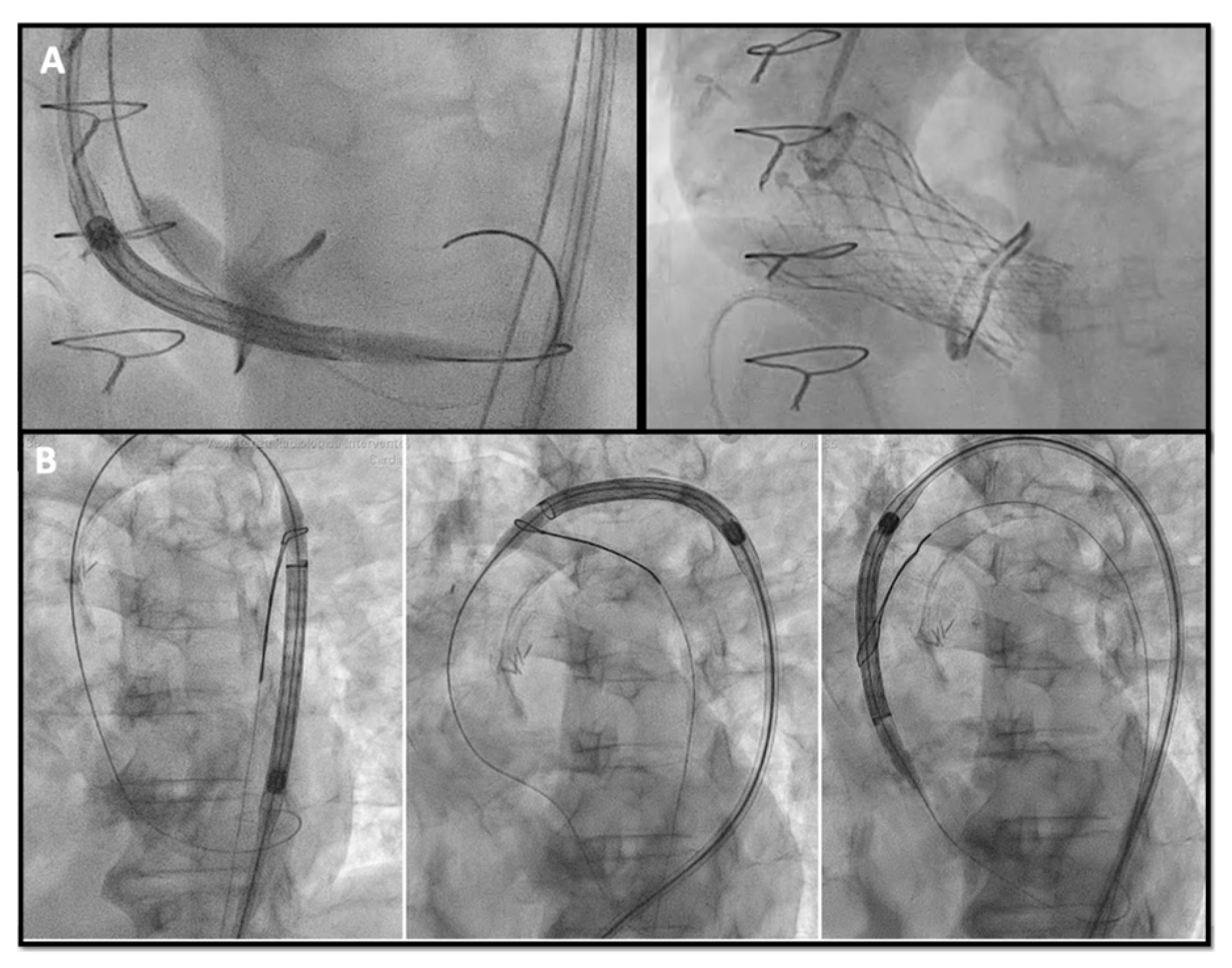
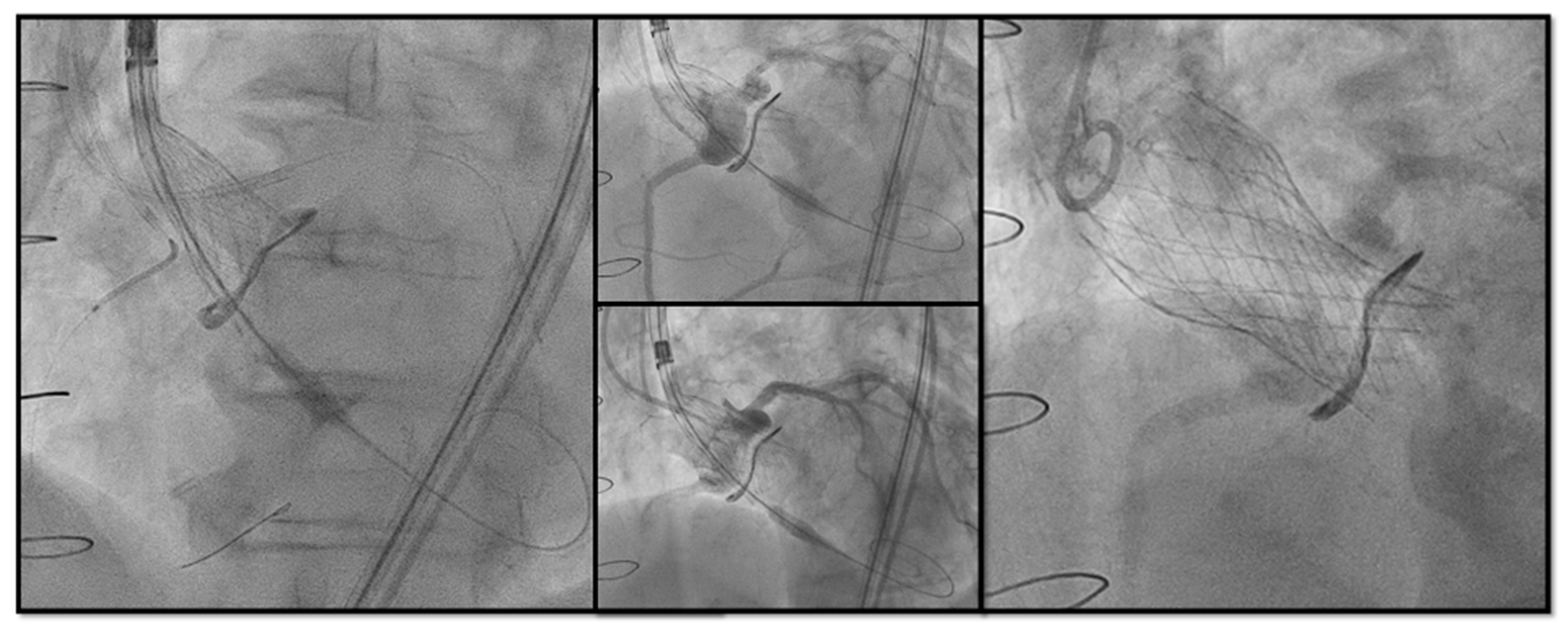
- Chimney snorkel stenting technique: Wiring the coronary artery and putting a stent on standby to be eventually implanted after THV deployment if the coronary flow is inadequate at angiographic control (Figure 7);
- -
- Orthotopic Snorkel Stenting Technique: Re-cannulation and wiring after THV release to have a more physiologic stenting through the prosthesis valve frame structure [42]
- -
- BASILICA: Intentional laceration of surgical leaflets with an electrified guidewire to create a communication between the sinus and neo-sinus [43]. Abdel-Wahab et al. [44], in the their multicentre EURO-BASILICA registry, showed a 99% of technical success and 88.3% of procedural success with an encouraging rate of freedom from any target leaflet-related CAO (90.6%) with a low rate of total coronary obstruction (2.4%). However, it is still considered a complex interventional procedure requiring meticulous preprocedural planning, dedicated material (sometimes the use of cerebral embolic protection, CEP) and high operator expertise.
7. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Woo, Y.J. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N. Engl. J. Med. 2017, 377, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Herrmann, H.C.; Wu, C.; Hahn, R.T.; Otto, C.M.; Abbas, A.E.; Chambers, J.; Dweck, M.R.; Leipsic, J.A.; Simonato, M.; et al. Standardized Definitions for Bioprosthetic Valve Dysfunction Following Aortic or Mitral Valve Replacement: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 545–561. [Google Scholar] [CrossRef]
- Leontyev, S.; Borger, M.A.; Davierwala, P.; Walther, T.; Lehmann, S.; Kempfert, J.; Mohr, F.W. Redo aortic valve surgery: Early and late outcomes. Ann. Thorac. Surg. 2011, 91, 1120–1126. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Tarantini, G.; Nai Fovino, L.; Gersh, B.J. Transcatheter aortic valve implantation in lower-risk patients: What is the perspective? Eur. Heart J. 2018, 39, 658–666. [Google Scholar] [CrossRef]
- Ciardetti, N.; Ciatti, F.; Nardi, G.; Di Muro, F.M.; Demola, P.; Sottili, E.; Stolcova, M.; Ristalli, F.; Mattesini, A.; Meucci, F.; et al. Advancements in Transcatheter Aortic Valve Implantation: A Focused Update. Medicina 2021, 57, 711. [Google Scholar] [CrossRef]
- Latib, A.; Ielasi, A.; Montorfano, M.; Maisano, F.; Chieffo, A.; Cioni, M.; Mussardo, M.; Bertoldi, L.; Shannon, J.; Sacco, F.; et al. Transcatheter valve-in-valve implantation with the Edwards Sapien in patients with bioprosthetic heart valve failure: The Milan experience. EuroIntervention 2012, 7, 1275–1284. [Google Scholar] [CrossRef]
- Gurvitch, R.; Cheung, A.; Ye, J.; Wood, D.A.; Willson, A.B.; Toggweiler, S.; Binder, R.; Webb, J.G. Transcatheter valve-in-valve implantation for failed surgical bioprosthetic valves. J. Am. Coll. Cardiol. 2011, 58, 2196–2209. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, F.; Laudisa, M.L.; Pizzocri, S.; Tamburino, C.; Ussia, G.P.; Petronio, A.S.; Napodano, M.; Ramondo, A.; Presbitero, P.; Ettori, F.; et al. Transcatheter valve-in-valve implantation using Corevalve Revalving System for failed surgical aortic bioprostheses. JACC Cardiovasc. Interv. 2011, 4, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, H.; Schäfer, U.; Treede, H.; Boekstegers, P.; Babin-Ebell, J.; Ferrari, M.; Möllmann, H.; Baumgartner, H.; Carrel, T.; Kahlert, P.; et al. Valve-in-valve transcatheter aortic valve implantation for degenerated bioprosthetic heart valves. JACC Cardiovasc. Interv. 2011, 4, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Deharo, P.; Bisson, A.; Herbert, J.; Lacour, T.; Etienne, C.S.; Porto, A.; Theron, A.; Collart, F.; Bourguignon, T.; Cuisset, T.; et al. Transcatheter Valve-in-Valve Aortic Valve Replacement as an Alternative to Surgical Re-Replacement. J. Am. Coll. Cardiol. 2020, 76, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.Y.; Dharma, C.; Rocha, R.V.; Ouzounian, M.; Wijeysundera, H.C.; Austin, P.C.; Chikwe, J.; Gaudino, M.; Fremes, S.E. Transcatheter ViV Versus Redo Surgical AVR for the Management of Failed Biological Prosthesis: Early and Late Outcomes in a Propensity-Matched Cohort. JACC Cardiovasc. Interv. 2020, 13, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Al-Abcha, A.; Saleh, Y.; Boumegouas, M.; Prasad, R.; Herzallah, K.; Baloch, Z.Q.; Abdelkarim, O.; Rayamajhi, S.; Abela, G.S. Meta-Analysis of Valve-in-Valve Transcatheter Aortic Valve Implantation Versus Redo-surgical Aortic Valve Replacement in Failed Bioprosthetic Aortic Valve. Am. J. Cardiol. 2021, 146, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Hirji, S.A.; Percy, E.D.; McGurk, S.; Malarczyk, A.; Harloff, M.T.; Yazdchi, F.; Sabe, A.A.; Bapat, V.N.; Tang, G.H.L.; Bhatt, D.L.; et al. Incidence, Characteristics, Predictors, and Outcomes of Surgical Explantation after Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 1848–1859. [Google Scholar] [CrossRef]
- Jawitz, O.K.; Gulack, B.C.; Grau-Sepulveda, M.V.; Matsouaka, R.A.; Mack, M.J.; Holmes, D.R., Jr.; Carroll, J.D.; Thourani, V.H.; Brennan, J.M. Reoperation After Transcatheter Aortic Valve Replacement: An Analysis of the Society of Thoracic Surgeons Database. JACC Cardiovasc. Interv. 2020, 13, 1515–1525. [Google Scholar] [CrossRef]
- Bapat, V.N.; Zaid, S.; Fukuhara, S.; Saha, S.; Vitanova, K.; Kiefer, P.; Squiers, J.J.; Voisine, P.; Pirelli, L.; von Ballmoos, M.W.; et al. Surgical Explantation After TAVR Failure: Mid-Term Outcomes From the EXPLANT-TAVR International Registry. JACC Cardiovasc. Interv. 2021, 14, 1978–1991. [Google Scholar] [CrossRef]
- Percy, E.D.; Harloff, M.T.; Hirji, S.; McGurk, S.; Yazdchi, F.; Newell, P.; Malarczyk, A.; Sabe, A.; Landes, U.; Webb, J.; et al. Nationally Representative Repeat Transcatheter Aortic Valve Replacement Outcomes: Report From the Centers for Medicare and Medicaid Services. JACC Cardiovasc. Interv. 2021, 14, 1717–1726. [Google Scholar] [CrossRef]
- Landes, U.; Webb, J.G.; De Backer, O.; Sondergaard, L.; Abdel-Wahab, M.; Crusius, L.; Kim, W.K.; Hamm, C.; Buzzatti, N.; Montorfano, M.; et al. Repeat Transcatheter Aortic Valve Replacement for Transcatheter Prosthesis Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G.; Dvir, D.; Tang, G.H.L. Transcatheter aortic valve implantation in degenerated surgical aortic valves. EuroIntervention 2021, 17, 709–719. [Google Scholar] [CrossRef]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef] [PubMed]
- Keshishi, M.; Fatima, R.; Seidman, M.A.; Butany, J.; Ouzounian, M.; Chung, J. Comparison of modes of failure and clinical outcomes between explanted porcine and bovine pericardial bioprosthetic valves. Cardiovasc. Pathol. 2023, 65, 107516. [Google Scholar] [CrossRef] [PubMed]
- Bapat, V.N.; Attia, R.; Thomas, M. Effect of valve design on the stent internal diameter of a bioprosthetic valve: A concept of true internal diameter and its implications for the valve-in-valve procedure. JACC Cardiovasc. Interv. 2014, 7, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Leipsic, J.; Blanke, P.; Ribeiro, H.B.; Kornowski, R.; Pichard, A.; Rodés-Cabau, J.; Wood, D.A.; Stub, D.; Ben-Dor, I.; et al. Coronary obstruction in transcatheter aortic valve-in-valve implantation: Preprocedural evaluation, device selection, protection, and treatment. Circ. Cardiovasc. Interv. 2015, 8, e002079. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.B.; Rodés-Cabau, J.; Blanke, P.; Leipsic, J.; Kwan Park, J.; Bapat, V.; Makkar, R.; Simonato, M.; Barbanti, M.; Schofer, J.; et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: Insights from the VIVID registry. Eur. Heart J. 2018, 39, 687–695. [Google Scholar] [CrossRef]
- Landes, U.; Sathananthan, J.; Witberg, G.; De Backer, O.; Sondergaard, L.; Abdel-Wahab, M.; Holzhey, D.; Kim, W.K.; Hamm, C.; Buzzatti, N.; et al. Transcatheter Replacement of Transcatheter Versus Surgically Implanted Aortic Valve Bioprostheses. J. Am. Coll. Cardiol. 2021, 77, 1–14. [Google Scholar] [CrossRef]
- Tarantini, G.; Sathananthan, J.; Fabris, T.; Landes, U.; Bapat, V.N.; Khan, J.M.; Nai Fovino, L.; Zaid, S.; Van Mieghem, N.M.; Latib, A.; et al. Transcatheter Aortic Valve Replacement in Failed Transcatheter Bioprosthetic Valves. JACC Cardiovasc. Interv. 2022, 15, 1777–1793. [Google Scholar] [CrossRef]
- Akodad, M.; Sellers, S.; Gulsin, G.S.; Tzimas, G.; Landes, U.; Chatfield, A.G.; Chuang, A.; Meier, D.; Leipsic, J.; Blanke, P.; et al. Leaflet and Neoskirt Height in Transcatheter Heart Valves: Implications for Repeat Procedures and Coronary Access. JACC Cardiovasc. Interv. 2021, 14, 2298–2300. [Google Scholar] [CrossRef]
- Tarantini, G.; Delgado, V.; de Backer, O.; Sathananthan, J.; Treede, H.; Saia, F.; Blackman, D.; Parma, R. Redo-Transcatheter Aortic Valve Implantation Using the SAPIEN 3/Ultra Transcatheter Heart Valves-Expert Consensus on Procedural Planning and Techniques. Am. J. Cardiol. 2023, 192, 228–244. [Google Scholar] [CrossRef]
- Hayashida, K.; Lefèvre, T.; Chevalier, B.; Hovasse, T.; Romano, M.; Garot, P.; Mylotte, D.; Uribe, J.; Farge, A.; Donzeau-Gouge, P.; et al. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc. Interv. 2011, 4, 851–858. [Google Scholar] [CrossRef]
- Nardi, G.; De Backer, O.; Saia, F.; Søndergaard, L.; Ristalli, F.; Meucci, F.; Stolcova, M.; Mattesini, A.; Demola, P.; Wang, X.; et al. Peripheral intravascular lithotripsy for transcatheter aortic valve implantation: A multicentre observational study. EuroIntervention 2022, 17, e1397–e1406. [Google Scholar] [CrossRef]
- Bapat, V. Technical pitfalls and tips for the valve-in-valve procedure. Ann. Cardiothorac. Surg. 2017, 6, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Medda, M.; Casilli, F.; Tespili, M.; Bande, M. The “Chaperone Technique”: Valve-in-Valve TAVR Procedure with a Self-Expandable Valve Inside a Degenerated Sutureless Prosthesis. JACC Cardiovasc. Interv. 2020, 13, e15–e18. [Google Scholar] [CrossRef] [PubMed]
- Bapat, V. Valve-in-valve apps: Why and how they were developed and how to use them. EuroIntervention 2014, 10 (Suppl. U), U44–U51. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; Abbas, A.E.; Serra, V.; Vilalta, V.; Nombela-Franco, L.; Regueiro, A.; Al-Azizi, K.M.; Iskander, A.; Conradi, L.; Forcillo, J.; et al. Balloon- vs Self-Expanding Valve Systems for Failed Small Surgical Aortic Valve Bioprostheses. J. Am. Coll. Cardiol. 2022, 80, 681–693. [Google Scholar] [CrossRef]
- Simonato, M.; Azadani, A.N.; Webb, J.; Leipsic, J.; Kornowski, R.; Vahanian, A.; Wood, D.; Piazza, N.; Kodali, S.; Ye, J.; et al. In vitro evaluation of implantation depth in valve-in-valve using different transcatheter heart valves. EuroIntervention 2016, 12, 909–917. [Google Scholar] [CrossRef]
- Allen, K.B.; Chhatriwalla, A.K.; Saxon, J.T.; Huded, C.P.; Sathananthan, J.; Nguyen, T.C.; Whisenant, B.; Webb, J.G. Bioprosthetic valve fracture: A practical guide. Ann. Cardiothorac. Surg. 2021, 10, 564–570. [Google Scholar] [CrossRef]
- Sreedharan, S.; Sellers, S.L.; Ihdayhid, A.R.; Landes, U.; Blanke, P.; Allen, K.B.; Chhatriwalla, A.K.; Pibarot, P.; Wood, D.A.; Webb, J.G.; et al. Bioprosthetic Valve Fracture to Facilitate Valve-in-Valve Transcatheter Aortic Valve Replacement. Structural Heart 2021, 5, 24–38. [Google Scholar] [CrossRef]
- Attisano, T.; Bellino, M.; Vigorito, F.; Maione, A.; Ravera, A.; Pierri, A.; Baldi, C.; Galasso, G.; Vecchione, C.; Bonan, R. Bioprosthetic Valve Fracture After TAVR Complicated by Balloon Rupture: Bail-Out TAVR in TAVR in SAVR. JACC Case Rep. 2022, 4, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Burzotta, F.; Kovacevic, M.; Aurigemma, C.; Shoeib, O.; Bruno, P.; Cangemi, S.; Romagnoli, E.; Trani, C. An “Orthotopic” Snorkel-Stenting Technique to Maintain Coronary Patency During Transcatheter Aortic Valve Replacement. Cardiovasc. Revasc Med. 2021, 28s, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Damlin, A.; Meduri, C.; Manouras, A.; Verouhis, D.; Linder, R.; Rück, A.; Settergren, M. BASILICA Procedure Prior to Valve-in-Valve TAVR in a Supra-Annular TAV Prosthesis. JACC Case Rep. 2023, 11, 101777. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Richter, I.; Taramasso, M.; Unbehaun, A.; Rudolph, T.; Ribichini, F.L.; Binder, R.; Schofer, J.; Mangner, N.; Dambrink, J.H.; et al. Procedural and one-year outcomes of the BASILICA technique in Europe: The multicentre EURO-BASILICA registry. EuroIntervention 2023, 19, e432–e441. [Google Scholar] [CrossRef]

| Type of SAVR | Characteristic | Challenges in ViV |
|---|---|---|
| Stented - Internally mounted leaflets - Externally mounted leaflets Stentless | Smaller ID Wider ID Wider ID | High residual gradients Patient prosthesis mismatch Coronary obstruction Lack of fluoroscopic markers |
| Valve Characteristics | |
|---|---|
| Type of Valve | BEV, MEV, SEV |
| Stent frame height | Low/high |
| Leaftet position | Intra-annular/supra-annular |
| Skirt length | |
| Stent Expansion | Hypo-expanded/norm expanded |
| Leaflets deflection | |
| Anatomical characteristics | |
| Implant height | Low/high |
| Commissural alignment to ostia | |
| Distance coronary ostia-stent frame | |
| Size of native root | |
| Valve position in relation to annular plane | Straight/canted |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Muro, F.M.; Cirillo, C.; Esposito, L.; Silverio, A.; Ferruzzi, G.J.; D’Elia, D.; Formisano, C.; Romei, S.; Vassallo, M.G.; Di Maio, M.; et al. Valve-in-Valve Transcatheter Aortic Valve Replacement: From Pre-Procedural Planning to Procedural Scenarios and Possible Complications. J. Clin. Med. 2024, 13, 341. https://doi.org/10.3390/jcm13020341
Di Muro FM, Cirillo C, Esposito L, Silverio A, Ferruzzi GJ, D’Elia D, Formisano C, Romei S, Vassallo MG, Di Maio M, et al. Valve-in-Valve Transcatheter Aortic Valve Replacement: From Pre-Procedural Planning to Procedural Scenarios and Possible Complications. Journal of Clinical Medicine. 2024; 13(2):341. https://doi.org/10.3390/jcm13020341
Chicago/Turabian StyleDi Muro, Francesca Maria, Chiara Cirillo, Luca Esposito, Angelo Silverio, Germano Junior Ferruzzi, Debora D’Elia, Ciro Formisano, Stefano Romei, Maria Giovanna Vassallo, Marco Di Maio, and et al. 2024. "Valve-in-Valve Transcatheter Aortic Valve Replacement: From Pre-Procedural Planning to Procedural Scenarios and Possible Complications" Journal of Clinical Medicine 13, no. 2: 341. https://doi.org/10.3390/jcm13020341
APA StyleDi Muro, F. M., Cirillo, C., Esposito, L., Silverio, A., Ferruzzi, G. J., D’Elia, D., Formisano, C., Romei, S., Vassallo, M. G., Di Maio, M., Attisano, T., Meucci, F., Vecchione, C., Bellino, M., & Galasso, G. (2024). Valve-in-Valve Transcatheter Aortic Valve Replacement: From Pre-Procedural Planning to Procedural Scenarios and Possible Complications. Journal of Clinical Medicine, 13(2), 341. https://doi.org/10.3390/jcm13020341








