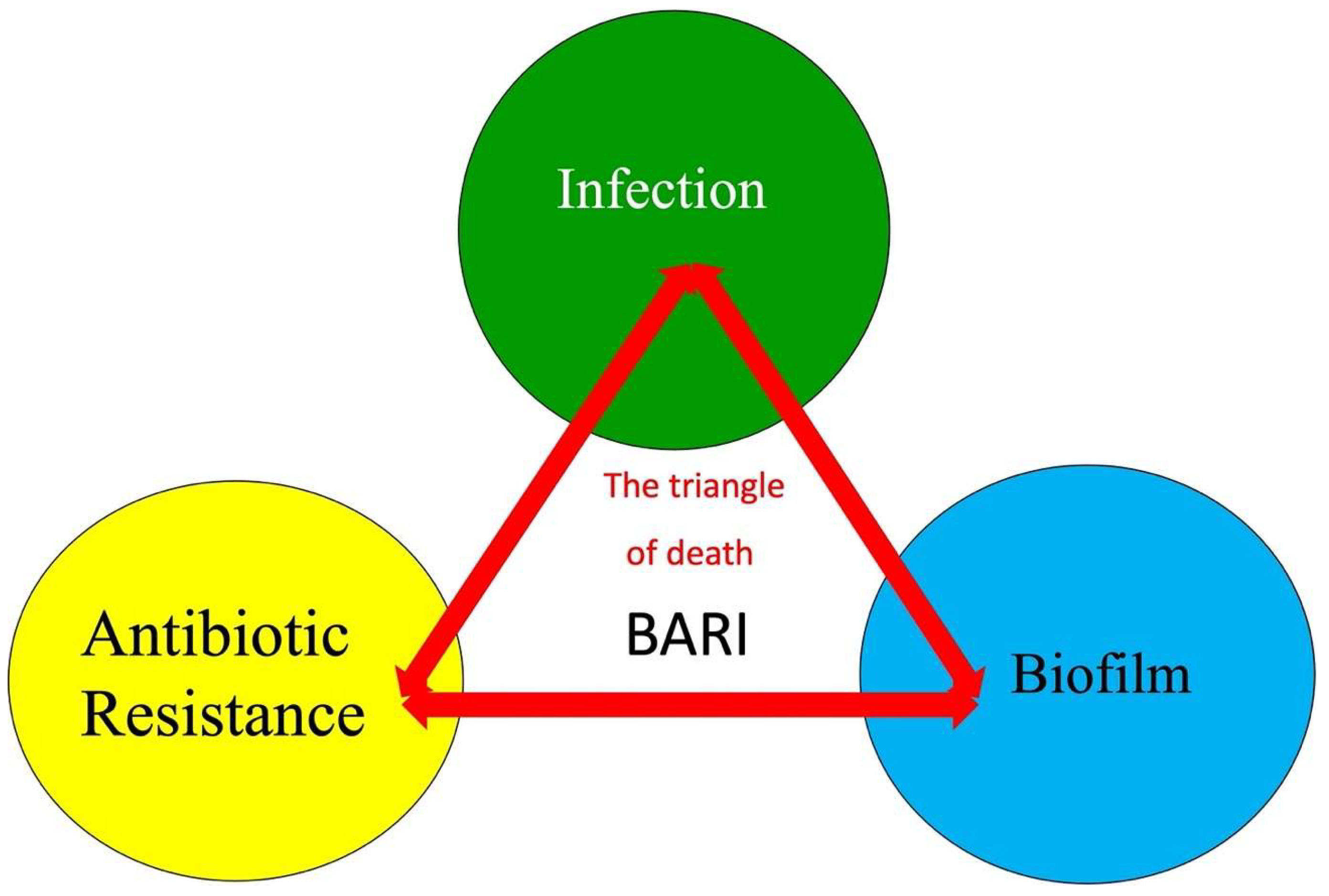
Biofilm Formation, Antibiotic Resistance, and Infection (BARI): The Triangle of Death
Abstract
1. Introduction
2. Triangle of Death of Fracture-Related Infection
2.1. Biofilm Formation
2.2. Antibiotic Resistance
2.3. Infection and Its Impact
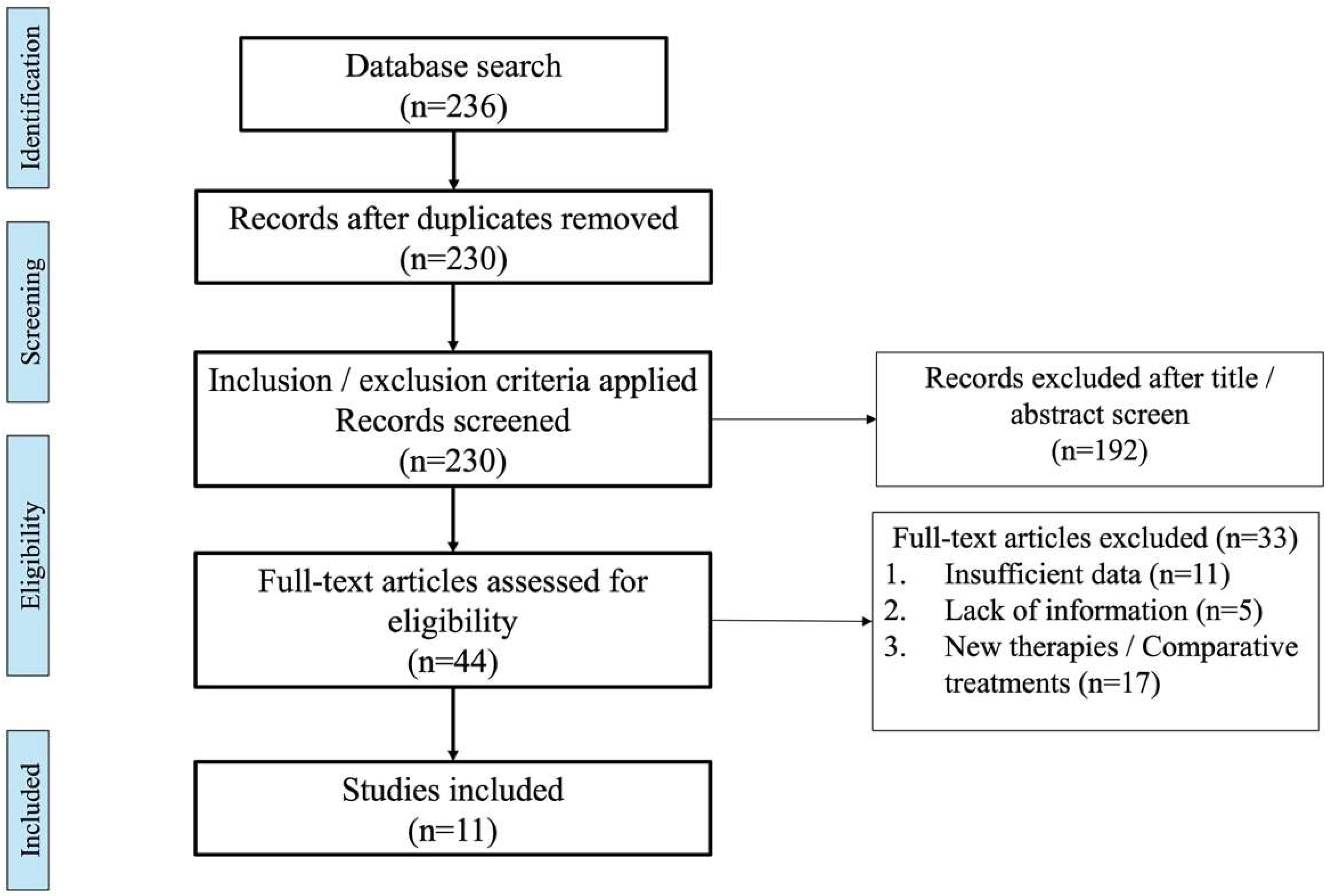
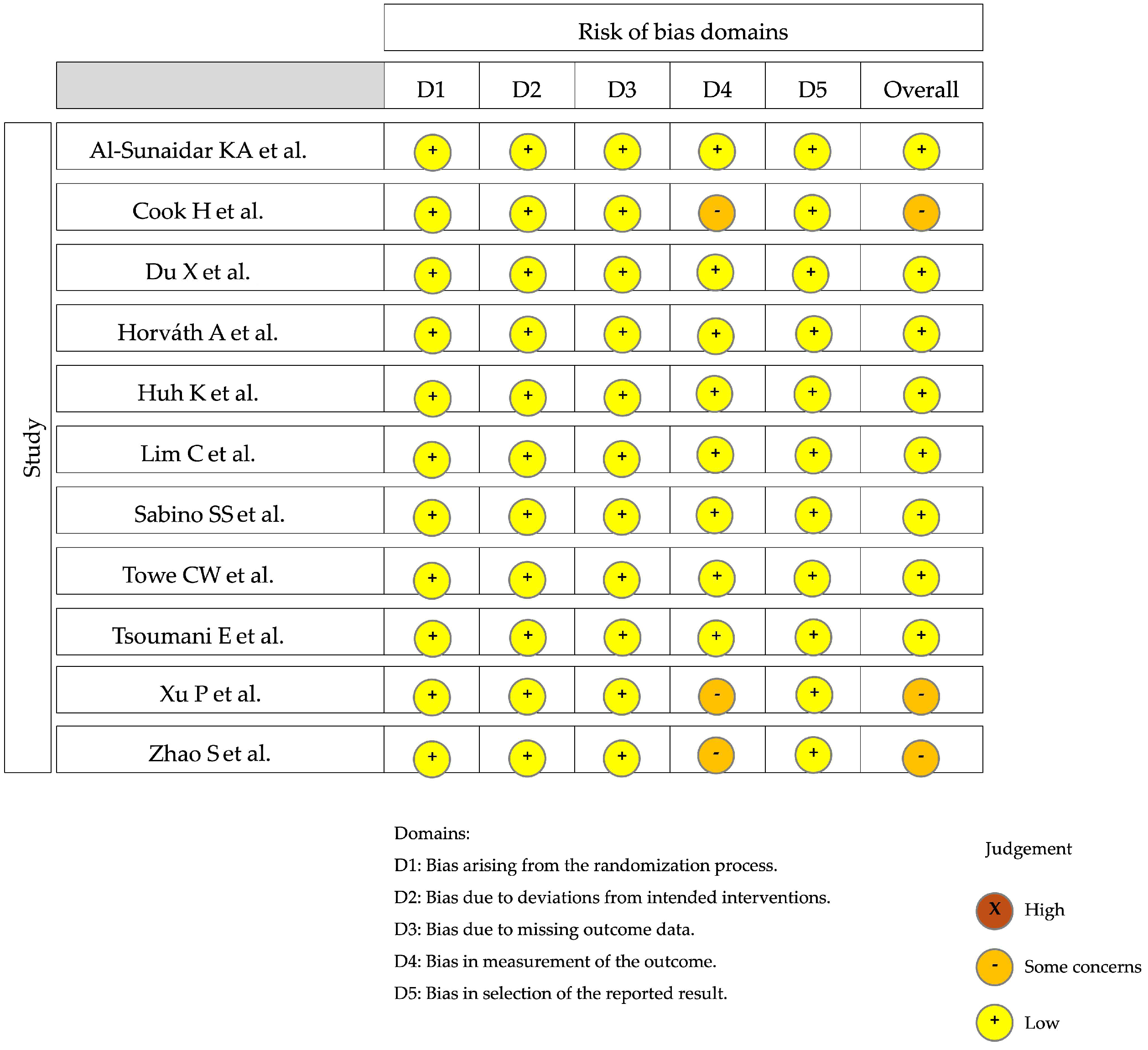
3. Materials and Methods
4. Results
5. Discussion
Prevention Strategies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baertl, S.; Metsemakers, W.J.; Morgenstern, M.; Alt, V.; Richards, R.G.; Moriarty, T.F.; Young, K. Fracture-related infection. Bone Jt. Res. 2021, 10, 351–353. [Google Scholar] [CrossRef] [PubMed]
- He, S.Y.; Yu, B.; Jiang, N. Current concepts of fracture-related infection. Int. J. Clin. Pract. 2023, 2023, 4839701. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.; Govaert, G.; Dudareva, M.; Morgenstern, M.; Metsemakers, W.J. Definition and diagnosis of fracture-related infection. EFORT Open Rev. 2020, 5, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Hellwinkel, J.E.; Working, Z.M.; Certain, L.; García, A.J.; Wenke, J.K.; Bahney, C.S. The intersection of fracture healing and infection: Orthopaedics research society workshop 2021. J. Orthop. Res. 2022, 40, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Govaert, G.A.M.; Kuehl, R.; Atkins, B.L.; Trampuz, A.; Morgenstern, M.; Obremskey, W.T.; Verhofstad, M.H.J.; McNally, M.A.; Metsemakers, W.J.; Fracture-Related Infection (FRI) Consensus Group. Diagnosing fracture-related infection: Current concepts and recommendations. J. Orthop. Trauma 2020, 34, 8–17. [Google Scholar] [CrossRef]
- Jones, J.K.; Ngo, D.; Cardon, M.; Mullis, B.H.; Weaver, B.A.; Slaven, J.E.; McCaskey, M.; Mir, H.R.; Warner, S.J.; Achor, T.S.; et al. High nonunion and amputations rates with either early intramedullary nail removal or retention for tibial shaft fracture-related infections. J. Orthop. Trauma 2023, 37, 574. [Google Scholar] [CrossRef]
- Depypere, M.; Morgenstern, M.; Kuehl, R.; Senneville, E.; Moriarty, T.F.; Obremskey, W.T.; Zimmerli, W.; Trampuz, A.; Lagrou, K.; Metsemakers, W.J. Pathogenesis and management of fracture-related infection. Clin. Microbiol. Infect. 2020, 26, 572–578. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Kuehl, R.; Moriarty, T.F.; Richards, R.G.; Verhofstad, M.H.J.; Borens, O.; Kates, S.; Morgenstern, M. Infection after fracture fixation: Current surgical and microbiological concepts. Injury 2018, 49, 511–522. [Google Scholar] [CrossRef]
- Prada, C.; Bengoa, F.; Bhandari, M. The management of fracture related infections: What practices can be supported by high-level evidence? J. Orthop. Surg. 2022, 30, 10225536221119580. [Google Scholar] [CrossRef]
- Wong, J.S.H.; Lee, A.L.H.; Fang, C.; Leung, H.C.H.; Liu, A.H.Y.; So, R.C.K.; Yung, C.S.Y.; Wong, T.M.; Leung, F. Outcomes of fracture-related infections—Do organism, depth of involvement, and temporality count? J. Orthop. Surg. 2022, 30, 10225536221118519. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial biofilm: Formation, architecture, antibiotic resistance, and control strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial biofilm: A review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Ellington, J.K.; Harris, M.; Hudson, M.C.; Vishin, S.; Webb, L.X.; Sherertz, R. Intracellular Staphylococcus aureus and antibiotic resistance: Implications for treatment of staphylococcal osteomyelitis. J. Orthop. Res. 2006, 24, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.A.; Salminen, A.T.; Begolo, S.; Luke, E.N.; Barrett, S.C.; Overby, C.T.; Gill, A.L.; de Mesy Bentley, K.L.; Awad, H.A.; Gill, S.R.; et al. An in vitro platform for elucidating the molecular genetics of S. aureus invasion of the osteocyte lacuno-canalicular network during chronic osteomyelitis. Nanomedicine 2019, 21, 102039. [Google Scholar] [CrossRef] [PubMed]
- Baertl, S.; Gens, L.; Nehrbass, D.; Sumrall, E.T.; Zeiter, S.; Mannala, G.K.; Rupp, M.; Walter, N.; Richards, R.G.; Moriarty, T.F.; et al. Staphylococcus aureus from an acute fracture-related infection displays important bacteriological and histopathologic differences from a chronic equivalent in a murine bone infection model. Clin. Orthop. Relat. Res. 2023, 481, 2044–2060. [Google Scholar] [CrossRef]
- Haggag, W. The role of biofilm exopolysaccharides on biocontrol of plant diseases. Biopolymers 2010, 14, 271–284. [Google Scholar] [CrossRef][Green Version]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef]
- Gu, H.; Hou, S.; Yongyat, C.; De Tore, S.; Ren, D. Patterned biofilm formation reveals a mechanism for structural heterogeneity in bacterial biofilms. Langmuir 2013, 29, 11145–11153. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2011, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.K.; Greenwood, P.F.; Poropat, S.F.; Tripp, M.; Elson, A.; Vijay, H.; Brosnan, L.; Holman, A.I.; Campbell, M.; Hopper, P.; et al. Microbially mediated fossil concretions and their characterization by the latest methodologies: A review. Front. Microbiol. 2023, 14, 1225411. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Josse, J.; Velard, F.; Gangloff, S.C. Staphylococcus aureus vs. osteoblast: Relationship and consequences in osteomyelitis. Front. Cell Infect. Microbiol. 2015, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Bollen, C.; Louwagie, E.; Verstraeten, N.; Michiels, J.; Ruelens, P. Environmental, mechanistic and evolutionary landscape of antibiotic persistence. EMBO Rep. 2023, 24, e57309. [Google Scholar] [CrossRef]
- Khan, J.; Tarar, S.M.; Gul, I.; Nawaz, U.; Arshad, M. Challenges of antibiotic resistance biofilms and potential combating strategies: A review. 3 Biotech 2021, 11, 169. [Google Scholar] [CrossRef]
- Santos-Lopez, A.; Marshall, C.W.; Scribner, M.R.; Snyder, D.J.; Cooper, V.S. Evolutionary pathways to antibiotic resistance are dependent upon environmental structure and bacterial lifestyle. eLife 2019, 8, e47612. [Google Scholar] [CrossRef]
- Usui, M.; Yoshii, Y.; Thiriet-Rupert, S.; Ghigo, J.M.; Beloin, C. Intermittent antibiotic treatment of bacterial biofilms favors the rapid evolution of resistance. Commun. Biol. 2023, 6, 275. [Google Scholar] [CrossRef]
- Lemos Azi, M.; Valderrama-Molina, C.O.; Vallejo-Diaz, A.; Días-Belangero, W.; Giordano, V.; Carabelli, G.; Sancineto, C.F. Surgical treatment of fracture-related infection. Part. I Bone and soft tissue debridement. Rev. Colomb. Ortop traumatol. 2024, 38, e57. [Google Scholar] [CrossRef]
- Berkes, M.; Obremskey, W.T.; Scannell, B.; Ellington, J.K.; Hymes, R.A.; Bosse, M.; Southeast Fracture Consortium. Maintenance of hardware after early postoperative infection following fracture internal fixation. J. Bone Jt. Surg. Am. 2010, 92, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Lu, V.; Zhang, J.; Patel, R.; Zhou, A.K.; Thahir, A.; Krkovic, M. Fracture related infections and their risk factors for treatment failure-a major trauma centre perspective. Diagnostics 2022, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, M.; Kuehl, R.; Zalavras, C.G.; McNally, M.; Zimmerli, W.; Burch, M.A.; Vandendriessche, T.; Obremskey, W.T.; Verhofstad, M.H.J.; Metsemakers, W.J. The influence of duration of infection on outcome of debridement and implant retention in fracture-related infection. Bone Jt. J. 2021, 103-B, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Sabbatucci, M.; Boccellino, M.; Ponzo, A.; Langella, R.; Zovi, A. Therapeutic and unconventional strategies to contrast antimicrobial resistance: A literature review. Discov. Med. 2023, 35, 750–756. [Google Scholar] [CrossRef]
- Valderrama-Molina, C.O.; Pesántez, R. Fracture-Related infection—The role of the surgeon and surgery in prevention and treatment. J. Orthop. Surg. 2022, 30, 10225536221118520. [Google Scholar] [CrossRef]
- Galvain, T.; Chitnis, A.; Paparouni, K.; Tong, C.; Holy, C.E.; Giannoudis, P.V. The economic burden of infections following intramedullary nailing for a tibial shaft fracture in England. BMJ Open 2020, 10, e035404. [Google Scholar] [CrossRef]
- Iliaens, J.; Onsea, J.; Hoekstra, H.; Nijs, S.; Peetermans, W.E.; Metsemakers, W.J. Fracture-related infection in long bone fractures: A comprehensive analysis of the economic impact and influence on quality of life. Injury 2021, 52, 3344–3349. [Google Scholar] [CrossRef]
- Walter, N.; Rupp, M.; Hierl, K.; Pfeifer, C.; Kerschbaum, M.; Hinterberger, T.; Alt, V. Long-term patient-related quality of life after fracture-related infections of the long bones. Bone Jt. Res. 2021, 10, 321–327. [Google Scholar] [CrossRef]
- Woffenden, H.; Yasen, Z.; Burden, E.; Douthwaite, A.; Elcock, S.B.; Mclean, L.; Hoven, P.J.V.; Fenton, P. Fracture-related infection: Analysis of healthcare utilisation and associated costs. Injury 2023, 54, 111109. [Google Scholar] [CrossRef]
- Panteli, M.; Giannoudis, P.V. Chronic osteomyelitis: What the surgeon needs to know. EFORT Open Rev. 2017, 1, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Hotchen, A.J.; Dudareva, M.; Corrigan, R.A.; Ferguson, J.Y.; McNally, M.A. Can we predict outcome after treatment of long bone osteomyelitis? Bone Jt. J. 2020, 102-B, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Bezstarosti, H.; Van Lieshout, E.M.M.; Voskamp, L.W.; Kortram, K.; Obremskey, W.; McNally, M.A.; Metsemakers, W.J.; Verhofstad, M.H.J. Insights into treatment and outcome of fracture-related infection: A systematic literature review. Arch. Orthop. Trauma Surg. 2019, 139, 61–72. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.; Corrigan, R.; Sliepen, J.; Dudareva, M.; Rentenaar, R.; IJpma, F.; Atkins, B.L.; Wouthuyzen-Bakker, M.; Govaert, G. What factors affect outcome in the treatment of fracture-related infection? Antibiotics 2022, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.J.; Morgenstern, M.; Senneville, E.; Borens, O.; Govaert, G.A.M.; Onsea, J.; Depypere, M.; Richards, R.G.; Trampuz, A.; Verhofstad, M.H.J.; et al. General treatment principles for fracture-related infection: Recommendations from an international expert group. Arch. Orthop. Trauma Surg. 2020, 140, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Tribble, D.R.; Krauss, M.R.; Murray, C.K.; Warkentien, T.E.; Lloyd, B.A.; Ganesan, A.; Greenberg, L.; Xu, J.; Li, P.; Carson, M.L.; et al. Epidemiology of trauma-related infections among a combat casualty cohort after initial hospitalization: The Trauma Infectious Disease Outcomes Study. Surg. Infect. 2018, 19, 494–503. [Google Scholar] [CrossRef]
- Wimalan, B.; Rupp, M.; Alt, V.; Walter, N. The patients’ perspective—A qualitative analysis of experiencing a fracture-related infection. Front. Psychol. 2023, 14, 1126826. [Google Scholar] [CrossRef]
- Wright, J.G.; Swiontkowski, M.F.; Heckman, J.D. Introducing levels of evidence to the journal. J. Bone Jt. Surg. Am. 2003, 85, 1–3. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Al-Sunaidar, K.A.; Abd Aziz, N.; Hassan, Y. Appropriateness of empirical antibiotics: Risk factors of adult patients with sepsis in the ICU. Int. J. Clin. Pharm. 2020, 42, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Cook, H.; Kennedy, C.; Delijani, K.; Popovsky, D.; Elmarsafi, T.; Zarick, C.; Attinger, C.; Steinberg, J. Early clinical, functional, and mortality outcomes for heel ulcers treated with a vertical contour calcanectomy. J. Foot Ankle Surg. 2022, 61, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Xu, X.; Yao, J.; Deng, K.; Chen, S.; Shen, Z.; Yang, L.; Feng, G. Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: A systematic review and meta-analysis. Am. J. Infect. Control 2019, 47, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Horváth, A.; Dobay, O.; Sahin-Tóth, J.; Juhász, E.; Pongrácz, J.; Iván, M.; Fazakas, E.; Kristóf, K. Characterisation of antibiotic resistance, virulence, clonality and mortality in MRSA and MSSA bloodstream infections at a tertiary-level hospital in Hungary: A 6-year retrospective study. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.; Chung, D.R.; Ha, Y.E.; Ko, J.H.; Kim, S.H.; Kim, M.J.; Huh, H.J.; Lee, N.Y.; Cho, S.Y.; Kang, C.I.; et al. Impact of difficult-to-treat resistance in Gram-negative bacteremia on mortality: Retrospective analysis of nationwide surveillance data. Clin. Infect. Dis. 2020, 71, e487–e496. [Google Scholar] [CrossRef]
- Lim, C.; Hantrakun, V.; Klaytong, P.; Rangsiwutisak, C.; Tangwangvivat, R.; Phiancharoen, C.; Doung-Ngern, P.; Kripattanapong, S.; Hinjoy, S.; Yingyong, T.; et al. Frequency and mortality rate following antimicrobial-resistant bloodstream infections in tertiary-care hospitals compared with secondary-care hospitals. PLoS ONE 2024, 19, e0303132. [Google Scholar] [CrossRef]
- Sabino, S.S.; Lima, C.A.; Machado, L.G.; Campos, P.A.; Fontes, A.M.S.; Gontijo-Filho, P.P.; Ribas, R.M. Infections and antimicrobial resistance in an adult intensive care unit in a Brazilian hospital and the influence of drug resistance on the thirty-day mortality among patients with bloodstream infections. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190106. [Google Scholar] [CrossRef]
- Towe, C.W.; Srinivasan, S.; Ho, V.P.; Bachmann, K.; Worrell, S.G.; Perry, Y.; Argote-Green, L.M.; Linden, P.A. Antibiotic resistance is associated with morbidity and mortality after decortication for empyema. Ann. Thorac. Surg. 2021, 111, 206–213. [Google Scholar] [CrossRef]
- Tsoumani, E.; Carter, J.A.; Salomonsson, S.; Stephens, J.M.; Bencina, G. Clinical, economic, and humanistic burden of community acquired pneumonia in Europe: A systematic literature review. Expert. Rev. Vaccines 2023, 22, 876–884. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, X.; Chen, Q.; Si, Q.; Luo, X.; Zhang, C.; He, Z.; Lin, R.; Zheng, C. Clinical features and risk factors for mortality in patients with Klebsiella pneumoniae bloodstream infections. J. Infect. Dev. Ctries. 2024, 18, 843–850. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, Y.; Dai, Z.; Chen, Y.; Zhou, X.; Zhao, J. Risk factors for antibiotic resistance and mortality in patients with bloodstream infection of Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance: Accelerating National and Global Responses. WHO Strategic and Operational Priorities to Address Drug-Resistant Bacterial Infections in the Human Health Sector, 2025–2035. Available online: https://apps.who.int/gb/ebwha/pdf_files/EB154/B154_13-en.pdf (accessed on 31 July 2024).
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655, Erratum in: Lancet 2022, 400, 1102. https://doi.org/10.1016/S0140-6736(21)02653-2. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.B., Jr.; Espinal, M.; Ramón-Pardo, P. Antimicrobial resistance: Time for action. Rev. Panam. Salud Publica 2020, 44, e131. [Google Scholar] [CrossRef] [PubMed]
- Egorov, A.M.; Ulyashova, M.M.; Rubtsova, M.Y. Bacterial enzymes and antibiotic resistance. Acta Nat. 2018, 10, 33–48. [Google Scholar] [CrossRef]
- Martinez, J.L.; Baquero, F. Mutation frequencies and antibiotic resistance. Antimicrob. Agents Chemother. 2000, 44, 1771–1777. [Google Scholar] [CrossRef]
- Woodford, N.; Ellington, M.J. The emergence of antibiotic resistance by mutation. Clin. Microbiol. Infect. 2007, 13, 5–18. [Google Scholar] [CrossRef]
- Chen, Q.; Li, D.; Beiersmann, C.; Neuhann, F.; Moazen, B.; Lu, G.; Müller, O. Risk factors for antibiotic resistance development in healthcare settings in China: A systematic review. Epidemiol. Infect. 2021, 149, e141. [Google Scholar] [CrossRef]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef]
- Ibáñez de Aldecoa, A.L.; Zafra, O.; González-Pastor, J.E. Mechanisms and regulation of extracellular DNA release and its biological roles in microbial communities. Front. Microbiol. 2017, 8, 1390. [Google Scholar] [CrossRef]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, C.; Zhang, H.; Wu, Y.; Lin, Y.; Cheng, J.; Lu, K.; Li, L.; Zhang, Y.; Chen, H.; et al. Three lines of defense: A multifunctional coating with anti-adhesion, bacteria-killing and anti-quorum sensing properties for preventing biofilm formation of Pseudomonas aeruginosa. Acta Biomater. 2022, 151, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Thomsen, T.R.; Winkler, H.; Xu, Y. Influence of biofilm growth age, media, antibiotic concentration and exposure time on Staphylococcus aureus and Pseudomonas aeruginosa biofilm removal in vitro. BMC Microbiol. 2020, 20, 264. [Google Scholar] [CrossRef] [PubMed]
- Heitzmann, L.G.; Battisti, R.; Rodrigues, A.F.; Lestingi, J.V.; Cavazzana, C.; Queiroz, R.D. Postoperative Chronic Osteomyelitis in the Long Bones—Current Knowledge and Management of the Problem. Rev. Bras. Ortop. 2019, 54, 627–635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, C.C.; Tsai, K.T.; Weng, S.F.; Lin, H.J.; Huang, H.S.; Wang, J.J.; Guo, H.R.; Hsu, C.C. Chronic osteomyelitis increases long-term mortality risk in the elderly: A nationwide population-based cohort study. BMC Geriatr. 2016, 16, 72. [Google Scholar] [CrossRef]
- Yagdiran, A.; Otto-Lambertz, C.; Lingscheid, K.M.; Sircar, K.; Samel, C.; Scheyerer, M.J.; Zarghooni, K.; Eysel, P.; Sobottke, R.; Jung, N.; et al. Quality of life and mortality after surgical treatment for vertebral osteomyelitis (VO): A prospective study. Eur. Spine J. 2021, 30, 1721–1731. [Google Scholar] [CrossRef]
- Dudareva, M.; Ferguson, J.; Riley, N.; Stubbs, D.; Atkins, B.; McNally, M. Osteomyelitis of the pelvic bones: A multidisciplinary approach to treatment. J. Bone Jt. Infect. 2017, 2, 184–193. [Google Scholar] [CrossRef]
- Serra-Burriel, M.; Keys, M.; Campillo-Artero, C.; Agodi, A.; Barchitta, M.; Gikas, A.; Palos, C.; López-Casasnovas, G. Impact of multi-drug resistant bacteria on economic and clinical outcomes of healthcare-associated infections in adults: Systematic review and meta-analysis. PLoS ONE 2020, 15, e0227139. [Google Scholar] [CrossRef]
- Poudel, A.N.; Zhu, S.; Cooper, N.; Little, P.; Tarrant, C.; Hickman, M.; Yao, G. The economic burden of antibiotic resistance: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0285170. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Onsea, J.; Neutjens, E.; Steffens, E.; Schuermans, A.; McNally, M.; Nijs, S. Prevention of fracture-related infection: A multidisciplinary care package. Int. Orthop. 2017, 41, 2457–2469. [Google Scholar] [CrossRef] [PubMed]
- Boot, W.; Foster, A.L.; Guillaume, O.; Eglin, D.; Schmid, T.; D’Este, M.; Zeiter, S.; Richards, R.G.; Moriarty, T.F. An antibiotic-loaded hydrogel demonstrates efficacy as prophylaxis and treatment in a large animal model of orthopaedic device-related infection. Front. Cell Infect. Microbiol. 2022, 12, 826392. [Google Scholar] [CrossRef]
- Li, J.; Cheung, W.H.; Chow, S.K.; Ip, M.; Leung, S.Y.S.; Wong, R.M.Y. Current therapeutic interventions combating biofilm-related infections in orthopaedics: A systematic review of in vivo animal studies. Bone Jt. Res. 2022, 11, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, M.; Zhou, B.; Jiang, X.; Zhang, D.; Luo, H. Graphene oxide/gallium nanoderivative as a multifunctional modulator of osteoblastogenesis and osteoclastogenesis for the synergistic therapy of implant-related bone infection. Bioact. Mater. 2022, 25, 594–614. [Google Scholar] [CrossRef] [PubMed]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef]
- Joshi, M.; O’Toole, R.V.; Carlini, A.R.; Gary, J.L.; Obremskey, W.T.; Murray, C.K.; Gaski, G.; Reid, J.S.; Degani, Y.; Taylor, T.J.; et al. Does topical vancomycin powder use in fracture surgery change bacteriology and antibiotic susceptibilities? An analysis of the VANCO Trial. J. Orthop. Trauma 2024, 38, 183–189. [Google Scholar] [CrossRef]
- O’Hara, N.N.; Castillo, R.C.; Carlini, A.R.; Joshi, M.; Murray, C.K.; Allen, L.E.; Huang, Y.; Gary, J.L.; Bosse, M.J.; Obremskey, W.T.; et al. Application of Bayesian methods to help interpret the VANCO Trial results. J. Orthop. Trauma 2023, 37, 1–7. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, J.; Qian, Y.; Zhao, L. Antibacterial coatings on orthopedic implants. Mater. Today Bio 2023, 19, 100586. [Google Scholar] [CrossRef]
- Ahmadabadi, H.Y.; Yu, K.; Kizhakkedathu, J.N. Surface modification approaches for prevention of implant associated infections. Colloids Surf. B Biointerfaces 2020, 193, 111116. [Google Scholar] [CrossRef]
- Ran, B.; Jing, C.; Yang, C.; Li, X.; Li, Y. Synthesis of efficient bacterial adhesion-resistant coatings by one-step polydopamine-assisted deposition of branched polyethylenimine-g-poly(sulfobetaine methacrylate) copolymers. Appl. Surf. Sci. 2018, 450, 77–84. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhao, Y.Q.; Zhang, Y.; Wang, A.; Ding, X.; Li, Y.; Duan, S.; Ding, X.; Xu, F.J. Antimicrobial peptide-conjugated hierarchical antifouling polymer brushes for functionalized catheter surfaces. Biomacromolecules 2019, 20, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Craig, J.; Fuchs, T.; Jenks, M.; Fleetwood, K.; Franz, D.; Iff, J.; Raschke, M. Systematic review and meta-analysis of the additional benefit of local prophylactic antibiotic therapy for infection rates in open tibia fractures treated with intramedullary nailing. Int. Orthop. 2014, 38, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Greco, T.; Vitiello, R.; Cazzato, G.; Cianni, L.; Malerba, G.; Maccauro, G.; Perisano, C. Intramedullary antibiotic coated nail in tibial fracture: A systematic review. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. 2), 63–69. [Google Scholar] [PubMed]
- Franz, D.; Raschke, M.; Giannoudis, P.V.; Leliveld, M.; Metsemakers, W.J.; Verhofstad, M.H.J.; Craig, J.A.; Shore, J.; Smith, A.; Muehlendyck, C.; et al. Use of antibiotic coated intramedullary nails in open tibia fractures: A European medical resource use and cost-effectiveness analysis. Injury 2021, 52, 1951–1958. [Google Scholar] [CrossRef]
- Steflik, M.J.; Griswold, B.G.; Patel, D.V.; Blair, J.A.; Davis, J.M. Antibiotic cement-coated intramedullary nail is cost-effective for the initial treatment of GAIII open tibia fractures. Injury 2022, 53, 3471–3474. [Google Scholar] [CrossRef]
- Zamorano, Á.I.; Albarrán, C.F.; Vaccia, M.A.; Parra, R.I.; Turner, T.; Rivera, I.A.; Garrido, O.A.; Suárez, P.F.; Zecchetto, P.; Bahamonde, L.A. Gentamicincoated tibial nail is an effective prevention method for fracture-related infections in open tibial fractures. Injury 2023, 54 (Suppl. 6), 110836. [Google Scholar] [CrossRef]
- Karupiah, T.; Yong, A.P.; Ong, Z.W.; Tan, H.K.; Tang, W.C.; Salam, H.B. Use of a novel anti-infective noble metal alloy-coated titanium orthopedic nail in patients with open fractures: A case series from Malaysia. Antibiotics 2022, 11, 1763. [Google Scholar] [CrossRef]
- Kotsarinis, G.; Wakefield, S.M.; Kanakaris, N.K.; Giannoudis, P.V. Stabilization of tibial fractures at risk of complications with the Bactiguard intramedullary nail: Early to medium results with a novel metal-coated device. J. Orthop. Trauma 2023, 37, S12–S17. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Vikulova, E.S.; Chepeleva, E.V.; Vasilieva, M.B.; Nasimov, D.A.; Maksimovskii, E.A.; Tsygankova, A.R.; Basova, T.V.; Sergeevichev, D.S.; Morozova, N.B. Noble metals for modern implant materials: MOCVD of film structures and cytotoxical, antibacterial, and histological studies. Biomedicines 2021, 9, 851. [Google Scholar] [CrossRef]
- Suska, F.; Svensson, S.; Johansson, A.; Emanuelsson, L.; Karlholm, H.; Ohrlander, M.; Thomsen, P. In vivo evaluation of noble metal coatings. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Declercq, P.; Zalavras, C.; Nijssen, A.; Mertens, B.; Mesure, J.; Quintens, J.; De Ridder, T.; Belmans, A.; Nijs, S.; Spriet, I.; et al. Impact of duration of perioperative antibiotic prophylaxis on development of fracture-related infection in open fractures. Arch. Orthop. Trauma Surg. 2021, 141, 235–243. [Google Scholar] [CrossRef] [PubMed]
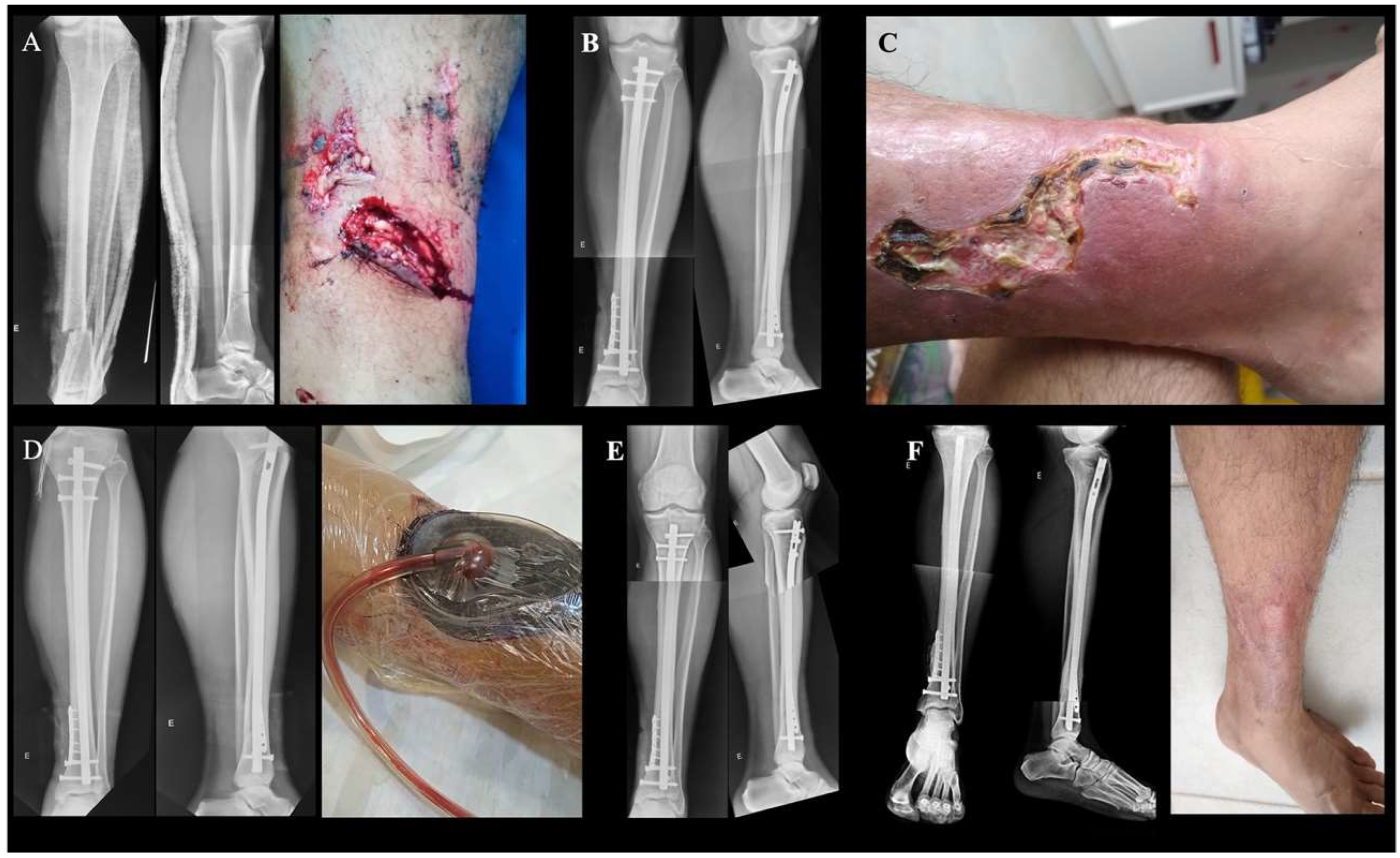
| Article Title | Publication Year | First Author | Journal | Article Type | Relevant Findings |
|---|---|---|---|---|---|
| Appropriateness of empirical antibiotics: risk factors of adult patients with sepsis in the ICU [51] | 2020 | Al-Sunaidar KA | Int J Clin Pharm | Observational retrospective | A total of 228 patients admitted to the adult ICU ward from 2011 to 2015 with a diagnosis of sepsis or who manifested sepsis symptoms were included. The most isolated microorganisms were Gram-negative bacteria (78%), and Gram-positive species comprised 22%. The total mortality rate was 193 (84.6%), with 119 males (52.2%) and 74 females (32.5%). The frequency of appropriate empirical antibiotics was 64 (28.1%). The mortality rate amongst patients who received appropriate antibiotics was 47 (20.6%), whereas amongst patients who did not was 146 (64%) (p = 0.007). Death in patients who received appropriate empirical antibiotic was 39% lower than that in patients with non-appropriate empirical antibiotics (HR 0.610, 95% CI 0.433–0.858, p = 0.005). |
| Early clinical, functional, and mortality outcomes for heel ulcers treated with a vertical contour calcanectomy [52] | 2022 | Cook H | J Foot Ankle Surg | Observational retrospective | A total of 51 patients suffering from chronic conditions and presenting heel ulcerations and calcaneal osteomyelitis were treated with a vertical contour calcanectomy. Here, 31.4% of patients had no recurrence, amputation, or mortality at 1-year follow-up. The total limb salvage rate was 68.6% and all-cause mortality was 9.8% at one year. |
| Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: a systematic review and meta-analysis [53] | 2019 | Du X | Am J Infect Control | Systematic review | This systematic review included 19 observational studies. Inappropriate empirical antimicrobial treatment was one of the major factors associated with the mortality of patients infected with Carbapenem-resistant Acinetobacter baumannii (CRAB), as well as the severity of baseline condition. Inappropriate empirical therapy increased 5-fold of the pooled mortality of 1169 patients (12 studies) with CRAB infection (p < 0.001). |
| Characterisation of antibiotic resistance, virulence, clonality and mortality in MRSA and MSSA bloodstream infections at a tertiary-level hospital in Hungary: a 6-year retrospective study [54] | 2020 | Horváth A | Ann Clin Microbiol Antimicrob | Observational retrospective | Antibiotic susceptibility, prevalence of virulence factors, genotype, and all-cause 30-day mortality of patients with MRSA and MSSA strains were compared from BSI over a 6-year period. A total of 306 S. aureus BSI isolates (153 MRSA and 153 MSSA strains) were analysed. Resistance rates of the MRSA isolates were significantly higher towards ciprofloxacin, erythromycin, clindamycin, amikacin, tobramycin, and gentamicin compared to MSSA isolates, whereas resistance rates of MSSA isolates were the highest to erythromycin and doxycycline. Almost all isolates were sensitive to sulfamethoxazole-trimethoprim and rifampicin. Of these, 81.7% of MRSA isolates were multidrug-resistant, whereas only 3.6% of MSSA isolates were multidrug-resistant. All-cause 30-day mortality was 39.9% in the MRSA and 30.7% in the MSSA group (p < 0.0001). Infections caused by SCCmec type IV isolates were associated with the highest mortality rate (42.2%), despite the similar comorbidity rates of the different patient groups. |
| Impact of difficult-to-treat resistance in Gram-negative bacteremia on mortality: retrospective analysis of nationwide surveillance data [55] | 2020 | Huh K | Clin Infect Dis | Observational retrospective | A total of 1167 episodes of monomicrobial Gram-negative BSI caused by 4 major taxa (E. coli, K. pneumoniae, P. aeruginosa, and Acinetobacter species) were identified from a nationwide surveillance database. Of these, 147 (12.6%) of the isolates were DTR (79.6% of Acinetobacter species and 17.7% of P. aeruginosa). DTR infections were associated with previous antibiotic use, healthcare contact, ventilator use, and lower respiratory tract infection. A total of 243 patients (26.3%) died in the hospital within 30 days of the onset of Gram-negative BSI. Crude mortality was significantly higher in patients with DTR Gram-negative BSI (p < 0.001). Mortality for Gram-negative BSI caused by DTR was 50.3%, whereas mortality among the other resistance categories was similar. |
| Frequency and mortality rate following antimicrobial-resistant bloodstream infections in tertiary-care hospitals compared with secondary-care hospitals [56] | 2024 | Lim C | PLoS One | Retrospective, multicentre analysis | The data of 19,665 hospitalised patients with AMR BSI caused by CRAB, CRPA, 3GCREC, 3GCRKP, CREC, CRKP, and MRSA were analysed. Of these, 10,858 (55.2%) were classified as community-origin BSI and 8807 (44.8%) were classified as hospital-origin BSI. Of 10,858 patients with community-origin AMR BSI, 2873 (27.5%) died, whereas of 8807 patients with hospital-origin AMR BSI, 3874 (38.2%) died. All-cause in-hospital mortality following hospital-origin AMR BSI was not significantly different between tertiary-care hospitals and secondary-care hospitals. CRAB had the highest mortality rate per 100,000 patient-days at risk in both tertiary-care hospitals and secondary-care hospitals. |
| Infections and antimicrobial resistance in an adult intensive care unit in a Brazilian hospital and the influence of drug resistance on the thirty-day mortality among patients with bloodstream infections [57] | 2020 | Sabino SS | Rev Soc Bras Med Trop | Retrospective cohort study | A total of 2168 patients were admitted in an ICU at a 3-year period and a total of 1979 (55.1%) healthcare-associated infection episodes were observed in these patients. Most nosocomial infections were acquired in the ICU (81.2%). Blood stream (33.4%), lung (30.5%), and urinary tract (16.6%) infections were the most observed. 1722 (87%) episodes were monomicrobial and 257 (13%) were polymicrobial. The most prevalent BSI agents were CoNS (45.2%), A. baumannii (8.7%), and P. aeruginosa (7.9%). The mortality rate among patients who developed healthcare-associated infection was 37.8%. |
| Antibiotic resistance is associated with morbidity and mortality after decortication for empyema [58] | 2021 | Towe CW | Ann Thorac Surg | Observational retrospective | A total of 185 patients who received surgical decortication for empyema were analysed for the association of microbiology and antibiotic resistance with adverse postoperative outcomes. A total of 118 (63.8%) underwent decortication for primary empyema and 67 (36.2%) for secondary empyema. Of the 185 decortications, 103 organisms were cultured from 79 (42.7%) patients. Gram-positive organisms were most common (60/79, 75.6%), being more frequently seen in patients with primary than secondary empyema (40/45 (88.9%) vs. 20/34 (58.8%), p = 0.002), while polymicrobial infections occurred in 17 patients (21.5%) and were more common among patients with secondary empyema (11/34 (32.4%) vs. 6/45 (13.3%), p = 0.042). The most common bacterial organisms were Streptococcus species (29/79, 36.7%), S. aureus (19/79, 24.1%), and Pseudomonas species (6/79, 7.6%). Antibiotic resistance was seen in 73 patients with positive cultures and was more common in patients with secondary empyema (p = 0.001). Among the 73 patients who demonstrated antibiotic resistance, 39 (53.4%) were resistant to at least one antibiotic. Mortality at 90 days occurred in 14 (7.6%) patients. |
| Clinical, economic, and humanistic burden of community acquired pneumonia in Europe: a systematic literature review [59] | 2023 | Tsoumani E | Expert Rev Vaccines | Systematic review | This systematic review included 82 studies published from 2011 to 2021 that sought to summarize the clinical, economic, and humanistic burden of CAP in Europe. The most frequently implicated bacterial pathogen was S. pneumoniae (implicated in 43% of cases) followed by H. influenzae and S. aureus (16.1% and 9.6%, respectively). Mortality at 30 days ranged from 39 to 44.5%, with the highest mortality being reported among elderly patients who were admitted for inpatient treatment for CAP. Another factor associated with higher mortality at 30 days was antibiotic resistance. Longer lengths of stay (approximately 19–23 days) were associated with multidrug resistance and admittance through the ICU. |
| Clinical features and risk factors for mortality in patients with Klebsiella pneumoniae bloodstream infections [60] | 2024 | Xu P | J Infect Dev Ctries | Observational retrospective | The clinical features and risk factors for mortality were investigated in 145 patients (121 in the survival group and 24 in the non-survival group) with K. pneumoniae BSI infections. The main sources of K. pneumoniae BSI were liver infection (24.1%), urinary tract infection (17.9%), and biliary tract infection (17.2%). The K. pneumoniae strain had the highest rate of resistance to ticarcillin (53.8%) and ciprofloxacin (43.8%). Multidrug resistance was higher in the non-survival group than in the survival group (41.7% vs. 16.5%, p = 0.005). |
| Risk factors for antibiotic resistance and mortality in patients with bloodstream infection of Escherichia coli [61] | 2022 | Zhao S | Eur J Clin Microbiol Infect Dis | Retrospective cohort study | The clinical data of 388 patients were analysed to investigate the risk factors for BSI caused by ESBL-producing E. coli. The prevalence of ESBL-producing E. coli in BSI patients was 40.98% (159 of 388). E. coli isolates were commonly susceptible to carbapenem and β-lactam/β-lactamase inhibitor combinations. Only 0.52%, 0.93%, and 2.84% of isolates showed in vitro resistance to imipenem, ertapenem, and piperacillin/tazobactam, respectively. Regarding non-carbapenem and non–β-lactam antibiotics, the highest resistance was recorded for ampicillin/sulbactam (49.52%) and the lowest resistance was recorded for amikacin (1.55%). ESBL positivity, nosocomial infection, and cancer were independent risk factors of mortality. |
| Study | Methodological Index for Non-Randomized Studies (MINORS) Criteria |
|---|---|
| Appropriateness of empirical antibiotics: risk factors of adult patients with sepsis in the ICU [51] | 11 (non-comparative study) |
| Early clinical, functional, and mortality outcomes for heel ulcers treated with a vertical contour calcanectomy [52] | 11 (non-comparative study) |
| Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: a systematic review and meta-analysis [53] | 20 (comparative study) |
| Characterisation of antibiotic resistance, virulence, clonality and mortality in MRSA and MSSA bloodstream infections at a tertiary-level hospital in Hungary: a 6-year retrospective study [54] | 12 (non-comparative study) |
| Impact of difficult-to-treat resistance in Gram-negative bacteremia on mortality: retrospective analysis of nationwide surveillance data [55] | 11 (non-comparative study) |
| Frequency and mortality rate following antimicrobial-resistant bloodstream infections in tertiary-care hospitals compared with secondary-care hospitals [56] | 16 (comparative study) |
| Infections and antimicrobial resistance in an adult intensive care unit in a Brazilian hospital and the influence of drug resistance on the thirty-day mortality among patients with bloodstream infections [57] | 11 (non-comparative study) |
| Antibiotic resistance is associated with morbidity and mortality after decortication for empyema [58] | 13 (non-comparative study) |
| Clinical, economic, and humanistic burden of community acquired pneumonia in Europe: a systematic literature review [59] | 19 (comparative study) |
| Clinical features and risk factors for mortality in patients with Klebsiella pneumoniae bloodstream infections [60] | 15 (comparative study) |
| Risk factors for antibiotic resistance and mortality in patients with bloodstream infection of Escherichia coli [61] | 11 (non-comparative study) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, V.; Giannoudis, P.V. Biofilm Formation, Antibiotic Resistance, and Infection (BARI): The Triangle of Death. J. Clin. Med. 2024, 13, 5779. https://doi.org/10.3390/jcm13195779
Giordano V, Giannoudis PV. Biofilm Formation, Antibiotic Resistance, and Infection (BARI): The Triangle of Death. Journal of Clinical Medicine. 2024; 13(19):5779. https://doi.org/10.3390/jcm13195779
Chicago/Turabian StyleGiordano, Vincenzo, and Peter V. Giannoudis. 2024. "Biofilm Formation, Antibiotic Resistance, and Infection (BARI): The Triangle of Death" Journal of Clinical Medicine 13, no. 19: 5779. https://doi.org/10.3390/jcm13195779
APA StyleGiordano, V., & Giannoudis, P. V. (2024). Biofilm Formation, Antibiotic Resistance, and Infection (BARI): The Triangle of Death. Journal of Clinical Medicine, 13(19), 5779. https://doi.org/10.3390/jcm13195779







