Biomarkers as Predictors of Mortality in Sepsis and Septic Shock for Patients Admitted to Emergency Department: Who Is the Winner? A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Design
2.3. Data Collection
2.4. Sample Collection and Biomarker Assays
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, A.; Rudinsky, D.; Han, G.; Elliott, J.O.; Jordan, K. Multidisciplinary Approach to Improve Sepsis Outcomes. J. Health Qual. 2019, 41, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.J.; van Wijk, X.M.R.; Chambliss, A.B. Chapter Four—Advances in sepsis biomarkers. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 119, pp. 117–166. [Google Scholar]
- Uffen, J.W.; Oosterheert, J.J.; Schweitzer, V.A.; Thursky, K.; Kaasjager, H.A.H.; Ekkelenkamp, M.B. Interventions for rapid recognition and treatment of sepsis in the emergency department: A narrative review. Clin. Microbiol. Infect. 2021, 27, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Chen, M.; Dai, J.; Zhong, H.; Chen, R.; Shi, F. Evaluation of qSOFA combined with inflammatory mediators for diagnosing sepsis and predicting mortality among emergency department. Clin. Chim. Acta 2023, 544, 117352. [Google Scholar] [CrossRef]
- Hassan, E.A.; Abdel Rehim, A.S.; Ahmed, A.O.; Abdullahtif, H.; Attia, A. Clinical Value of Presepsin in Comparison to hsCRP as a Monitoring and Early Prognostic Marker for Sepsis in Critically Ill Patients. Medicina 2019, 55, 36. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, Y.; Liang, X.; Gui, S.; Ren, D.; Liu, Y. Elevations in presepsin, PCT, hs-CRP, and IL-6 levels predict mortality among septic patients in the ICU. J. Leukoc. Biol. 2024, 121. [Google Scholar] [CrossRef]
- Lee, S.; Song, J.; Park, D.W.; Seok, H.; Ahn, S.; Kim, J. Diagnostic and prognostic value of presepsin and procalcitonin in non-infectious organ failure, sepsis, and septic shock: A prospective observational study according to the Sepsis-3 definitions. BMC Infect. Dis. 2022, 22, 8. [Google Scholar] [CrossRef]
- Molano-Franco, D.; Arevalo-Rodriguez, I.; Muriel, A.; del Campo-Albendea, L.; Fernández-García, S.; Alvarez-Méndez, A. Basal procalcitonin, C-reactive protein, interleukin-6, and presepsin for prediction of mortality in critically ill septic patients: A systematic review and meta-analysis. Diagn. Progn. Res. 2023, 7, 15. [Google Scholar] [CrossRef]
- Afsar, I.; Sener, A.G. Is Procalcitonin a Diagnostic and/or Prognostic Marker in Sepsis? Infect. Dis. Clin. Pract. 2015, 23, 3–6. [Google Scholar] [CrossRef]
- Casagranda, I.; Vendramin, C.; Callegari, T.; Vidali, M.; Calabresi, A.; Ferrandu, G. Usefulness of suPAR in the risk stratification of patients with sepsis admitted to the emergency department. Intern. Emerg. Med. 2015, 10, 725–730. [Google Scholar] [CrossRef]
- Nasr El-Din, A.; Abdel-Gawad, A.R.; Abdelgalil, W.; Fahmy, N.F. Evaluation of sTREM1 and suPAR Biomarkers as Diagnostic and Prognostic Predictors in Sepsis Patients. Infect. Drug Resist. 2021, 14, 3495–3507. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Xiong, H.; Yan, P.; Shuai, T.; Liu, J.; Zhu, L. The Diagnostic and Prognostic Value of suPAR in Patients with Sepsis: A Systematic Review and Meta-Analysis. Shock 2020, 53, 416–425. [Google Scholar] [CrossRef]
- Dholariya, S.; Parchwani, D.N.; Singh, R.; Radadiya, M.; Katoch, C.D.S. Utility of P-SEP, sTREM-1 and suPAR as Novel Sepsis Biomarkers in SARS-CoV-2 Infection. Ind. J. Clin. Biochem. 2022, 37, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Smok, B.; Domagalski, K.; Pawłowska, M. Diagnostic and Prognostic Value of IL-6 and sTREM-1 in SIRS and Sepsis in Children. Mediat. Inflamm. 2020, 2020, 8201585. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhuang, D.; Chen, H.; Zou, S.; Chen, W.; Chen, Y. 28-day sepsis mortality prediction model from combined serial interleukin-6, lactate, and procalcitonin measurements: A retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Park, D.W.; Moon, S.; Cho, H.-J.; Park, J.H.; Seok, H. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: A prospective controlled study according to the Sepsis-3 definitions. BMC Infect. Dis. 2019, 19, 968. [Google Scholar] [CrossRef]
- Katsaros, K.; Renieris, G.; Safarika, A.; Adami, E.-M.; Gkavogianni, T.; Giannikopoulos, G. Heparin Binding Protein for the Early Diagnosis and Prognosis of Sepsis in the Emergency Department: The Prompt Multicenter Study. Shock 2022, 57, 518–525. [Google Scholar] [CrossRef]
- Tydén, J.; Herwald, H.; Sjöberg, F.; Johansson, J. Increased Plasma Levels of Heparin-Binding Protein on Admission to Intensive Care Are Associated with Respiratory and Circulatory Failure. PLoS ONE 2016, 11, e0152035. [Google Scholar] [CrossRef]
- Dou, Q.-L.; Liu, J.; Zhang, W.; Wang, C.-W.; Gu, Y.; Li, N. Dynamic changes in heparin-binding protein as a prognostic biomarker for 30-day mortality in sepsis patients in the intensive care unit. Sci. Rep. 2022, 12, 10751. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- ALLEA—All European Academies. The European Code of Conduct for Research Integrity; ALLEA—All European Academies: Berlin, Germany, 2023. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Capsoni, N.; Bellone, P.; Aliberti, S.; Sotgiu, G.; Pavanello, D.; Visintin, B. Prevalence, risk factors and outcomes of patients coming from the community with sepsis due to multidrug resistant bacteria. Multidiscip. Respir. Med. 2019, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; Guia, M.C.; Vallecoccia, M.S.; Suarez, D.; Ibarz, M.; Irazabal, M. Risk factors for mortality in elderly and very elderly critically ill patients with sepsis: A prospective, observational, multicenter cohort study. Ann. Intensive Care 2019, 9, 26. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019—Results from a systematic review and meta-analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Markazi-Moghaddam, N.; Ramezankhani, A. A systematic review on risk factors associated with sepsis in patients admitted to intensive care units. Aust. Crit. Care 2019, 32, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Choi, J.-H. An Update on Sepsis Biomarkers. Infect. Chemother. 2020, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Jirak, P.; Haertel, F.; Mirna, M.; Rezar, R.; Lichtenauer, M.; Paar, V. A Comparative Analysis of Novel Biomarkers in Sepsis and Cardiovascular Disease. Appl. Sci. 2022, 12, 1419. [Google Scholar] [CrossRef]
- Velissaris, D.; Zareifopoulos, N.; Koniari, I.; Karamouzos, V.; Bousis, D.; Gerakaris, A. Soluble Urokinase Plasminogen Activator Receptor as a Diagnostic and Prognostic Biomarker in Cardiac Disease. J. Clin. Med. Res. 2021, 13, 133–142. [Google Scholar] [CrossRef]
- Xie, Y.; Li, B.; Lin, Y.; Shi, F.; Chen, W.; Wu, W. Combining Blood-Based Biomarkers to Predict Mortality of Sepsis at Arrival at the Emergency Department. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e929527-1–e929527-8. [Google Scholar] [CrossRef]
- Yu, B.; Chen, M.; Zhang, Y.; Cao, Y.; Yang, J.; Wei, B. Diagnostic and Prognostic Value of Interleukin-6 in Emergency Department Sepsis Patients. Infect. Drug Resist. 2022, 15, 5557–5566. [Google Scholar] [CrossRef]
- Said, E.A.; Al-Reesi, I.; Al-Shizawi, N.; Jaju, S.; Al-Balushi, M.S.; Koh, C.Y. Defining IL-6 levels in healthy individuals: A meta-analysis. J. Med. Virol. 2021, 93, 3915–3924. [Google Scholar] [CrossRef]
- Hamilton, F.W.; Thomas, M.; Arnold, D.; Palmer, T.; Moran, E.; Mentzer, A.J. Therapeutic potential of IL6R blockade for the treatment of sepsis and sepsis-related death: A Mendelian randomisation study. PLoS Med. 2023, 20, e1004174. [Google Scholar] [CrossRef] [PubMed]
- Tomulic Brusich, K.; Juricic, K.; Bobinac, M.; Milosevic, M.; Protic, A.; Boban, A. Administration of tocilizumab in septic patients with pancytopenia and hyper-inflammatory syndrome. Ann. Hematol. 2023, 102, 2633–2634. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Jaroszewicz, J.; Rogalska, M.; Łapiński, T.; Berkan-Kawińska, A.; Bolewska, B. Tocilizumab Improves the Prognosis of COVID-19 in Patients with High IL-6. J. Clin. Med. 2021, 10, 1583. [Google Scholar] [CrossRef]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 trans-signalling: Past, present and future prospects. Nat. Rev. Immunol. 2023, 23, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Khalid, S.; Jiang, L. Diagnostic and predictive performance of biomarkers in patients with sepsis in an intensive care unit. J. Int. Med. Res. 2019, 47, 44–58. [Google Scholar] [CrossRef]
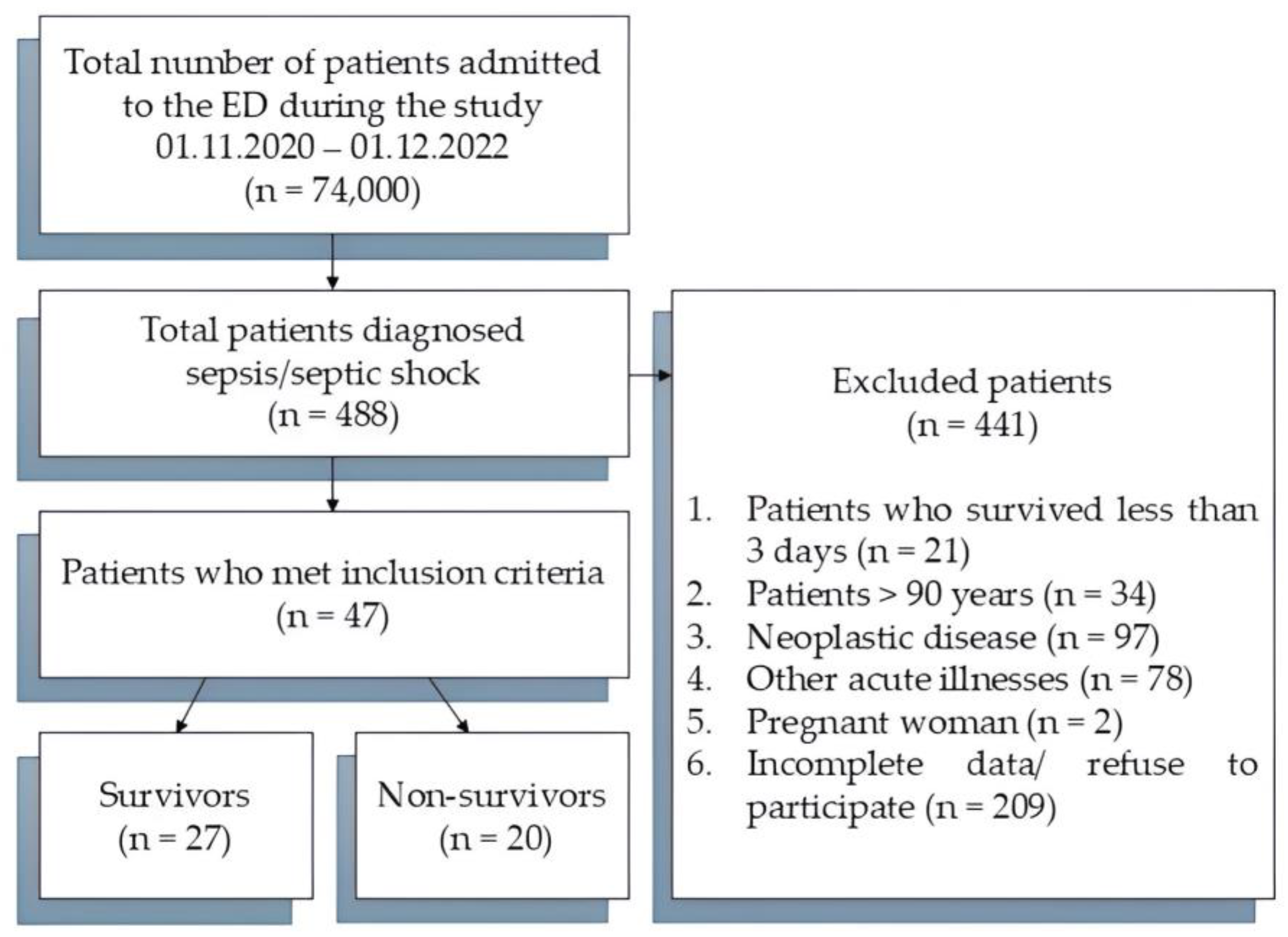
| Parameters | Time | Survivors (n = 27) | Non-Survivors (n = 20) | p |
|---|---|---|---|---|
| Age (years) † | T0 | 71.5 (63.25–81) | 74.5 (64.25–82.5) | 0.45 |
| BMI n (%) † | T0 | 27.56 (23.43–33.53) | 25.29 (22.05–30.52) | 0.16 |
| Vital signs and physiological parameters | ||||
| GCS † | T0 | 15 (13–15) | 11.5 (13–15) | 0.004 |
| T24 | 15 (12–15) | 10.5 (4.5–2.5) | <0.001 | |
| T48 | 14 (12–15) | 8 (4–10) | <0.001 | |
| Respiratory rate (resp/min) † | T0 | 27 (24–30.75) | 30 (26–34.75) | 0.20 |
| T24 | 25 (22.5–30) | 25.5 (16–32.75) | 0.64 | |
| T48 | 25 (23–28) | 22 (18–30) | 0.18 | |
| Heart Rate (beats/min) † | T0 | 108 (92.5–112.75) | 116 (107.75–126) | 0.11 |
| T24 | 101 (72.5–110) | 107 (88.25–121.25) | 0.05 | |
| T48 | 102 (70–114) | 110 (105–128) | 0.005 | |
| Glycemia (mg/dl) † | T0 | 186 (117.5–235.5) | 121 (99.25–190.5) | 0.02 |
| T24 | 118.50 (105–183.25) | 115.50 (79.25–210.25) | 0.30 | |
| T48 | 133 (106–170) | 122 (80–215.70) | 0.87 | |
| MAP (mmHg) †† | T0 | 68.2 ± 15.5 | 60.6 ± 9.9 | 0.06 |
| T24 | 76.8 ± 16 | 73.8 ± 14.9 | 0.50 | |
| T48 | 75.5 ± 14.8 | 67.6 ± 9.7 | 0.04 | |
| Lactate (mmol/L) † | T0 | 2 (1.52–3.27) | 2.85 (1.72–5.6) | 0.15 |
| T24 | 1.7 (1.10–2.22) | 2.5 (1.55–3.40) | 0.19 | |
| T48 | 1.60 (1–2) | 2 (1.8–6) | 0.003 | |
| SaO2 (%) † | T0 | 94.5 (92–97) | 92.5 (86–96) | 0.40 |
| T24 | 96 (94.25–97) | 96 (92.75–98.5) | 0.81 | |
| T48 | 95 (94–98) | 96 (90–99) | 0.54 | |
| FiO2 (%) † | T0 | 0.21 (0.21–0.55) | 0.6 (0.21–0.87) | 0.24 |
| T24 | 0.21 (21–40) | 0.45 (40–71) | 0.001 | |
| T48 | 0.21 (21–40) | 0.40 (35–60) | <0.001 | |
| PaO2/FiO2 † | T0 | 390.70 (315.87–459.75) | 267.55 (109.28–429.82) | 0.11 |
| T24 | 371 (291.25–433.25) | 176 (102.14–241.25) | <0.001 | |
| T48 | 333 (225–423) | 195 (108–288) | <0.001 | |
| Temperature (°C) † | T0 | 37.65 (36.32–38.8) | 37.40 (36.6–38.15) | 0.22 |
| T24 | 37 (36.42–37.87) | 37 (36.15–37.2) | 0.79 | |
| T48 | 36 (36–37.5) | 36.5 (36–37) | 0.67 | |
| Scores | ||||
| SOFA † | T0 | 5 (2.25–9.75) | 9.5 (5.50–12.75) | 0.06 |
| T24 | 6 (3–9) | 11 (8–14.25) | 0.001 | |
| T48 | 5 (2–7) | 10 (7.25–14) | <0.001 | |
| APACHE II †† | T0 | 18.8 ± 5.6 | 26 ± 7.7 | 0.01 |
| T24 | 14.6 ± 5.1 | 24.9 ± 7.1 | <0.001 | |
| T48 | 13.1 ± 6.8 | 24.4 ± 8 | <0.001 | |
| SAPS II † | T0 | 46 (39.25–55.50) | 59.5 (56–78) | 0.001 |
| T24 | 44 (35.5–49) | 63.5 (48–79) | 0.002 | |
| T48 | 39 (33–51) | 62 (41–84) | 0.01 | |
| Comorbidities, n (%) | ||||
| Cardiovascular disease | T0 | 24 (82.8) | 15 (88.2) | 1.00 |
| Diabetes | T0 | 18 (62.1) | 9 (52.9) | 0.76 |
| Chronic kidney disease | T0 | 7 (24.1) | 3 (17.6) | 0.88 |
| Chronic lung disease | T0 | 9 (31) | 4 (23.5) | 0.83 |
| Obesity | T0 | 13 (44.8) | 13 (76.5) | 0.25 |
| Neuropsychiatry | T0 | 11 (37.9) | 11 (64.7) | 0.14 |
| Biomarker (Plasma Levels) | Time | Survival Group (n = 29) | Non-Survival Group (n = 17) | p |
|---|---|---|---|---|
| IL-6 (pg/mL) † | T0 | 406.50 (91.22–535.07) | 441.60 (304.90–791.05) | 0.11 |
| T24 | 129.85 (67.70–369.82) | 402.10 (245.95–669.60) | 0.003 | |
| T48 | 75.60 (40.87–213.12) | 238.70 (117.95–531.55) | 0.001 | |
| suPAR (ng/mL) †† | T0 | 7343.8 ± 1971.1 | 8512.1 ± 1848.4 | 0.04 |
| T24 | 6556.3 ± 1809 | 8641.8 ± 1765.3 | <0.001 | |
| T48 | 6405.4 ± 2020.7 | 8318.9 ± 2449.1 | 0.005 | |
| PCT (pg/mL) † | T0 | 13.85 (2.87–31.17) | 23.10 (7.95–58.15) | 0.13 |
| T24 | 9.95 (3.67–38.82) | 21.7 (8.30–81.35) | 0.11 | |
| T48 | 6 (1.75–19.72) | 15.6 (6.45–71.05) | 0.01 | |
| hsCRP (pg/mL) † | T0 | 26.05 (15.20–29.67) | 18.40 (16.50–22.40) | 0.11 |
| T24 | 17.90 (14.6 –23.7) | 21.5 (15.61–26.72) | 0.52 | |
| T48 | 22.80 (20.32–28.85) | 17 (14.10–21.75) | 0.01 | |
| sTREM-1 (pg/mL) † | T0 | 264.75 (89.80–741.50) | 224.50 (119.6–813.90) | 0.50 |
| T24 | 229.75 (110.47–474.72) | 341.60 (77.90–555.70) | 0.57 | |
| T48 | 175.95 (63.72–467.10) | 184.70 (65.90–551.60) | 0.69 | |
| AZU1 (ng/mL) † | T0 | 8.30 (7.55–9.07) | 7.30 (7.00–8.60) | 0.09 |
| T24 | 7.80 (6.82–8.57) | 7.60 (7.10–9.50) | 0.82 | |
| T48 | 7.95 (6.95–9.12) | 7.60 (6.90–9.15) | 0.63 |
| Time | AUC (95% CI) | Cutoff Values | Se (95% CI) | Sp (95% CI) | p | |
|---|---|---|---|---|---|---|
| Biomarkers | ||||||
| IL-6 (pg/mL) | T0 | 0.630 (0.476–0.766) | >246.6 | 80 (56.3–94.3) | 48.15 (28.7–68.1) | 0.11 |
| T24 | 0.698 (0.547–0.823) | >109 | 90 (68.3–98.8) | 44.44 (25.5–64.7) | 0.009 | |
| T48 | 0.720 (0.570–0.841) | >96.6 | 80 (56.3–94.3) | 62.96 (42.4–80.6) | 0.004 | |
| suPAR (ng/mL) | T0 | 0.695 (0.544–0.821) | >7434 | 85 (62.1–96.8) | 59.26 (38.8–77.6) | 0.01 |
| T24 | 0.813 (0.672–0.912) | >8168 | 75 (50.9–91.3) | 81.48 (61.9–93.7) | <0.001 | |
| T48 | 0.731 (0.581–0.849) | >8465 | 50 (27.2–72.8) | 88.89 (70.8–97.6) | 0.002 | |
| PCT (pg/mL) | T0 | 0.595 (0.442–0.736) | >19.8 | 50 (27.2–72.8) | 70.37 (49.8–86.2) | 0.26 |
| T24 | 0.662 (0.509–0.793) | >10 | 75 (50.9–91.3) | 59.26 (38.8–77.6) | 0.04 | |
| T48 | 0.706 (0.556–0.830) | >2.4 | 95 (75.1–99.9) | 40.74 (22.4–61.2) | 0.006 | |
| hsCRP (pg/mL) | T0 | 0.591 (0.438–0.732) | >24.9 | 80 (56.3–94.3) | 48.15 (28.7–68.1) | 0.59 |
| T24 | 0.551 (0.399–0.696) | >18 | 30 (11.9–54.3) | 92.59 (75.7–99.1) | 0.55 | |
| T48 | 0.551 (0.517–0.800) | >18.1 | 60 (36.1–80.9) | 81.48 (61.9–93.7) | 0.04 | |
| sTREM-1 (pg/mL) | T0 | 0.554 (0.402–0.699) | >189 | 70 (45.7–88.1) | 51.85 (31.9–71.3) | 0.53 |
| T24 | 0.509 (0.359–0.658) | >429.8 | 40 (19.1–63.9) | 74.07 (53.7–88.9) | 0.91 | |
| T48 | 0.504 (0.354–0.653) | >70.7 | 35 (15.4–59.2) | 77.78 (57.7–91.4) | 0.96 | |
| AZU1 (ng/mL) | T0 | 0.608 (0.455–0.747) | >7.3 | 45 (23.1–68.5) | 81.48 (61.9–93.7) | 0.20 |
| T24 | 0.507 (0.358–0.656) | >9 | 35 (15.4–59.2) | 88.89 (70.8–97.6) | 0.93 | |
| T48 | 0.520 (0.368–0.670) | >7.8 | 60 (36.1–80.9) | 53.85 (33.4–73.4) | 0.82 | |
| Time | AUC (95% CI) | Cutoff Values | Se (95% CI) | Sp (95% CI) | p | |
|---|---|---|---|---|---|---|
| Biomarkers | ||||||
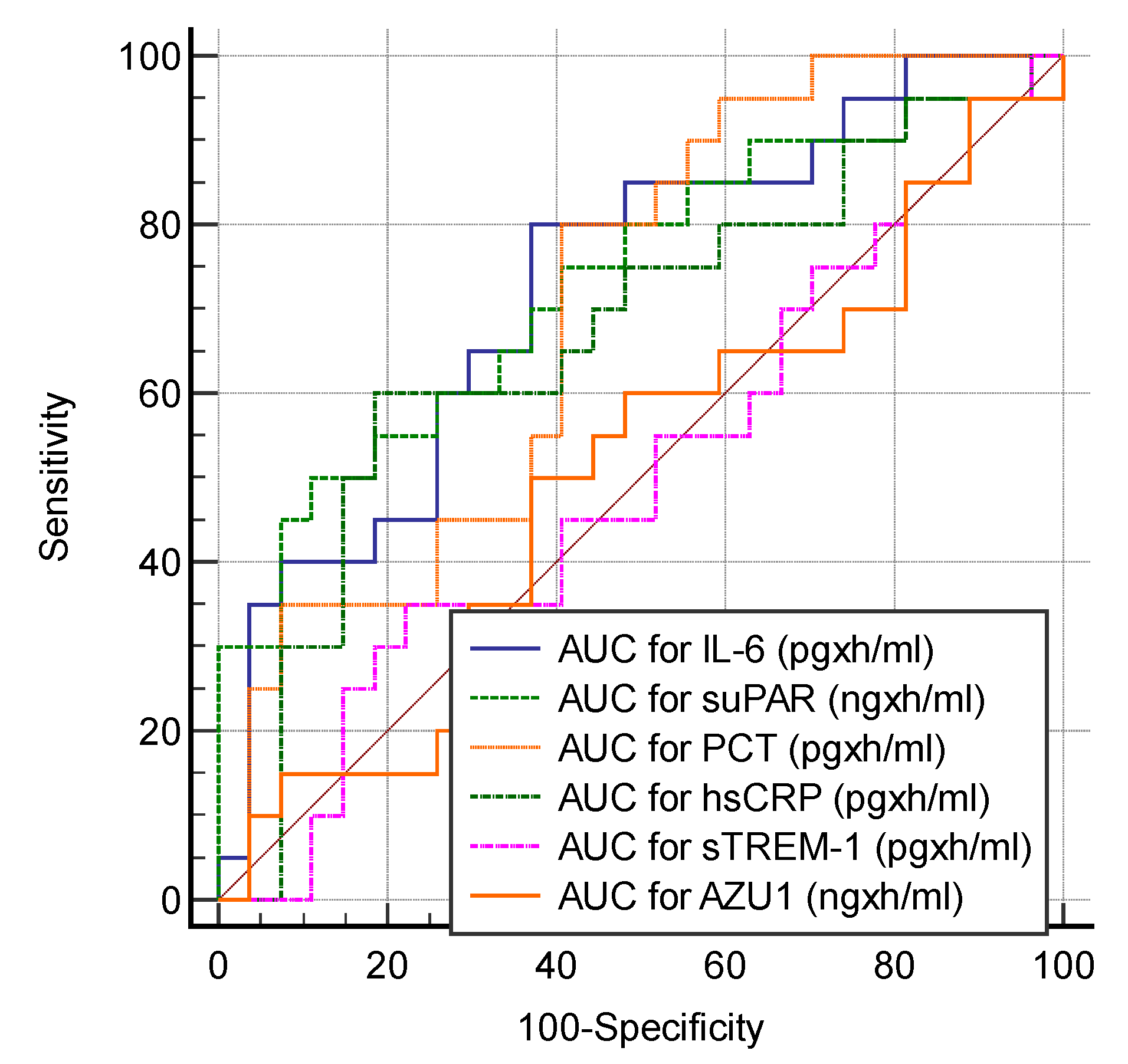
| AUC for IL-6 (pgxh/mL) | T0–T48 | 0.730 (0.580–0.849) | >180 | 80 (56.3–94.3) | 62.96 (42.4–80.6) | 0.002 |
| AUC for suPAR (ngxh/mL) | T0–T48 | 0.733 (0.584–0.852) | >13,558 | 50 (27.2–72.8) | 88.89 (70.8–97.6) | 0.002 |
| AUC for PCT (pgxh/mL) | T0–T48 | 0.700 (0.549–0.825) | >10.03 | 80 (56.3–94.3) | 59.26 (38.8–77.6) | 0.009 |
| AUC for hsCRP (pgxh/mL) | T0–T48 | 0.670 (0.518–0.800) | >30 | 60 (36.0–80.9) | 81.48 (61.9–93.7) | 0.04 |
| AUC for sTREM-1 (pgxh/mL) | T0–T48 | 0.504 (0.354–0.653) | >119.63 | 35 (15.4–59.2) | 77.78 (57.7–91.4) | 0.96 |
| AUC for AZU1 (ngxh/mL) | T0–T48 | 0.506 (0.356–0.655) | >12.23 | 50 (27.2–72.8) | 62.96 (42.4–80.6) | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luka, S.; Golea, A.; Tat, R.M.; Lupan Mureșan, E.M.; Voicescu, G.T.; Vesa, Ș.C.; Ionescu, D. Biomarkers as Predictors of Mortality in Sepsis and Septic Shock for Patients Admitted to Emergency Department: Who Is the Winner? A Prospective Study. J. Clin. Med. 2024, 13, 5678. https://doi.org/10.3390/jcm13195678
Luka S, Golea A, Tat RM, Lupan Mureșan EM, Voicescu GT, Vesa ȘC, Ionescu D. Biomarkers as Predictors of Mortality in Sepsis and Septic Shock for Patients Admitted to Emergency Department: Who Is the Winner? A Prospective Study. Journal of Clinical Medicine. 2024; 13(19):5678. https://doi.org/10.3390/jcm13195678
Chicago/Turabian StyleLuka, Sonia, Adela Golea, Raluca Mihaela Tat, Eugenia Maria Lupan Mureșan, George Teo Voicescu, Ștefan Cristian Vesa, and Daniela Ionescu. 2024. "Biomarkers as Predictors of Mortality in Sepsis and Septic Shock for Patients Admitted to Emergency Department: Who Is the Winner? A Prospective Study" Journal of Clinical Medicine 13, no. 19: 5678. https://doi.org/10.3390/jcm13195678
APA StyleLuka, S., Golea, A., Tat, R. M., Lupan Mureșan, E. M., Voicescu, G. T., Vesa, Ș. C., & Ionescu, D. (2024). Biomarkers as Predictors of Mortality in Sepsis and Septic Shock for Patients Admitted to Emergency Department: Who Is the Winner? A Prospective Study. Journal of Clinical Medicine, 13(19), 5678. https://doi.org/10.3390/jcm13195678







