The Effect of Opioids and Benzodiazepines on Exacerbation Rate and Overall Survival in Patients with Chronic Obstructive Pulmonary Disease on Long-Term Non-Invasive Ventilation †
Abstract
1. Introduction
2. Materials and Methods
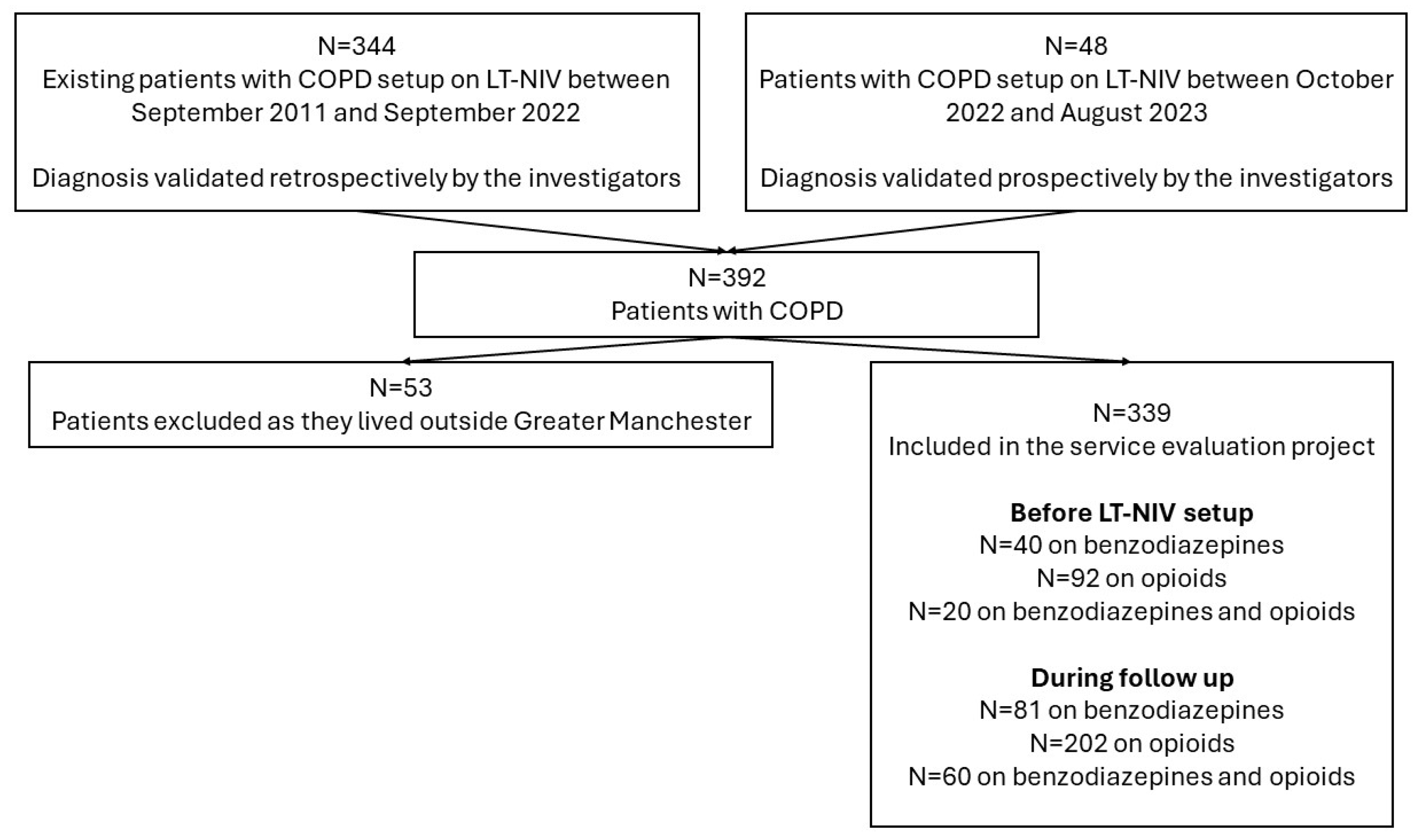
2.1. Project Design and Subjects
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Follow-Up Data
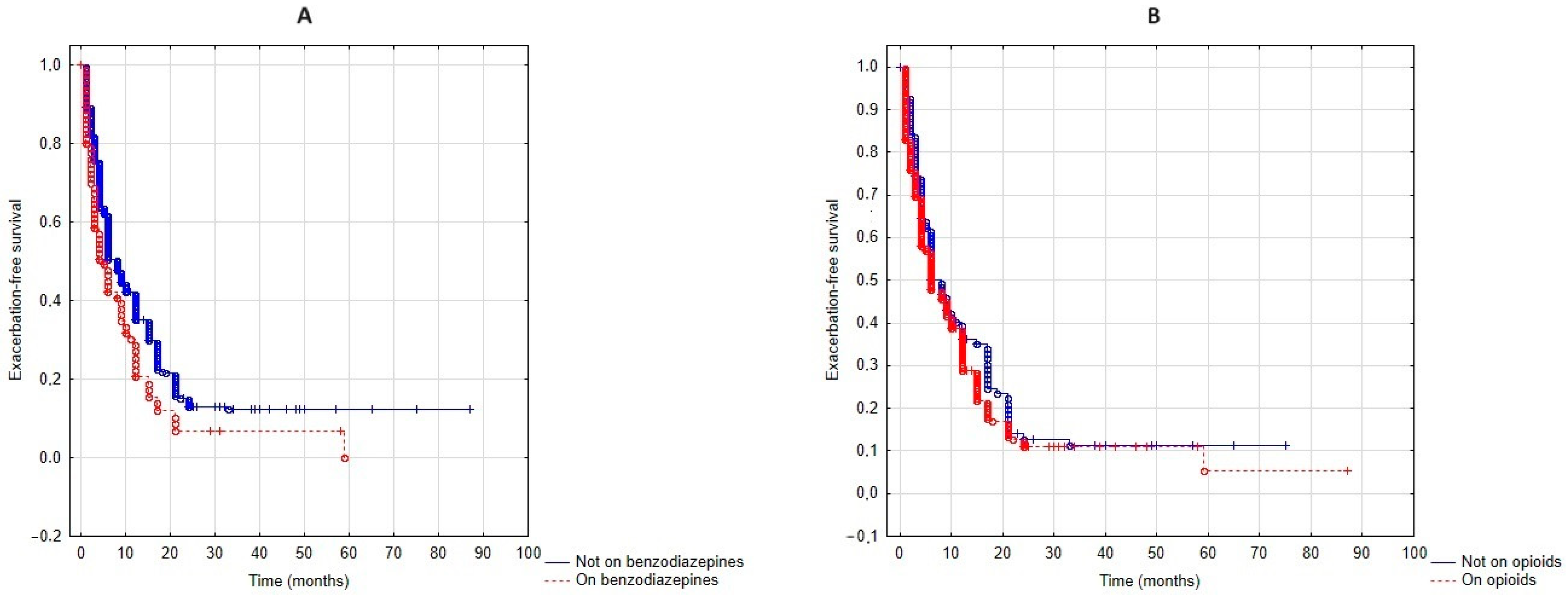
3.3. Exacerbation-Free Survival
3.4. Overall Survival
3.5. The Effect of Combined Benzodiazepine and Opioid Use on Exacerbation-Free Survival and Overall Survival
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet. Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Hoyert, D.L.; Xu, J. Deaths: Preliminary Data for 2011. National Vital Statistics Reports: From the Centers for Disease Control and Prevention. Natl. Cent. Health Stat. Natl. Vital Stat. Syst. 2012, 61, 1–51. [Google Scholar]
- O’Donnell, D.E.; Milne, K.M.; James, M.D.; de Torres, J.P.; Neder, J.A. Dyspnea in COPD: New Mechanistic Insights and Management Implications. Adv. Ther. 2020, 37, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Anzueto, A.; Miravitlles, M. Pathophysiology of dyspnea in COPD. Postgrad. Med. 2017, 129, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Jakeways, N.; McKeever, T.; Lewis, S.A.; Weiss, S.T.; Britton, J. Relationship between FEV1 reduction and respiratory symptoms in the general population. Eur. Respir. J. 2003, 21, 658–663. [Google Scholar] [CrossRef]
- Miravitlles, M.; Ribera, A. Understanding the impact of symptoms on the burden of COPD. Respir. Res. 2017, 18, 67. [Google Scholar] [CrossRef]
- Nishimura, K.; Izumi, T.; Tsukino, M.; Oga, T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002, 121, 1434–1440. [Google Scholar] [CrossRef]
- Edmonds, P.; Karlsen, S.; Khan, S.; Addington-Hall, J. A comparison of the palliative care needs of patients dying from chronic respiratory diseases and lung cancer. Palliat. Med. 2001, 15, 287–295. [Google Scholar] [CrossRef]
- Bikov, A.; Lange, P.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.A.; Celli, B.R.; Cowans, N.J.; Crim, C.; Dixon, I.J.; Martinez, F.J.; et al. FEV(1) is a stronger mortality predictor than FVC in patients with moderate COPD and with an increased risk for cardiovascular disease. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 1135–1142. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, J.; He, X.; Hao, Y.; Wang, K.; Gibson, P.G. Sputum inflammatory cell-based classification of patients with acute exacerbation of chronic obstructive pulmonary disease. PLoS ONE 2013, 8, e57678. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, A.P.; Currow, D.C.; Frith, P.; Fazekas, B.S.; McHugh, A.; Bui, C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 2003, 327, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.T.; Higginson, I.J.; Booth, S.; Harding, R.; Weingärtner, V.; Bausewein, C. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst. Rev. 2016, 10, Cd007354. [Google Scholar] [CrossRef] [PubMed]
- Solano, J.P.; Gomes, B.; Higginson, I.J. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J. Pain Symptom Manag. 2006, 31, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Montandon, G.; Qin, W.; Liu, H.; Ren, J.; Greer, J.J.; Horner, R.L. PreBotzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 1292–1301. [Google Scholar] [CrossRef]
- Forster, A.; Juge, O.; Morel, D. Effects of midazolam on cerebral blood flow in human volunteers. Anesthesiology 1982, 56, 453–455. [Google Scholar] [CrossRef]
- Adcock, J.J.; Schneider, C.; Smith, T.W. Effects of codeine, morphine and a novel opioid pentapeptide BW443C, on cough, nociception and ventilation in the unanaesthetized guinea-pig. Br. J. Pharmacol. 1988, 93, 93–100. [Google Scholar] [CrossRef]
- Johnston, M.; Watts, S.; Drake-Lee, A. In vitro effects of diazepam on human ciliary function. Acta Oto-Laryngol. 1997, 117, 856–859. [Google Scholar] [CrossRef]
- Vozoris, N.T.; Wang, X.; Fischer, H.D.; Bell, C.M.; O’Donnell, D.E.; Austin, P.C.; Stephenson, A.L.; Gill, S.S.; Rochon, P.A. Incident opioid drug use and adverse respiratory outcomes among older adults with COPD. Eur. Respir. J. 2016, 48, 683–693. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Bornefalk-Hermansson, A.; Franklin, K.A.; Midgren, B.; Ekström, M.P. Hypo- and hypercapnia predict mortality in oxygen-dependent chronic obstructive pulmonary disease: A population-based prospective study. Respir. Res. 2014, 15, 30. [Google Scholar] [CrossRef]
- Vozoris, N.T.; Fischer, H.D.; Wang, X.; Stephenson, A.L.; Gershon, A.S.; Gruneir, A.; Austin, P.C.; Anderson, G.M.; Bell, C.M.; Gill, S.S.; et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with COPD. Eur. Respir. J. 2014, 44, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Au, D.H.; Udris, E.M.; Fihn, S.D.; McDonell, M.B.; Curtis, J.R. Differences in health care utilization at the end of life among patients with chronic obstructive pulmonary disease and patients with lung cancer. Arch. Intern. Med. 2006, 166, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Csoma, B.; Vulpi, M.R.; Dragonieri, S.; Bentley, A.; Felton, T.; Lázár, Z.; Bikov, A. Hypercapnia in COPD: Causes, Consequences, and Therapy. J. Clin. Med. 2022, 11, 3180. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.B.; Rehal, S.; Arbane, G.; Bourke, S.; Calverley, P.M.A.; Crook, A.M.; Dowson, L.; Duffy, N.; Gibson, G.J.; Hughes, P.D.; et al. Effect of Home Noninvasive Ventilation with Oxygen Therapy vs Oxygen Therapy Alone on Hospital Readmission or Death After an Acute COPD Exacerbation: A Randomized Clinical Trial. JAMA 2017, 317, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Köhnlein, T.; Windisch, W.; Köhler, D.; Drabik, A.; Geiseler, J.; Hartl, S.; Karg, O.; Laier-Groeneveld, G.; Nava, S.; Schönhofer, B.; et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: A prospective, multicentre, randomised, controlled clinical trial. Lancet. Respir. Med. 2014, 2, 698–705. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Pierce, R.J.; Hillman, D.; Esterman, A.; Ellis, E.E.; Catcheside, P.G.; O’Donoghue, F.J.; Barnes, D.J.; Grunstein, R.R. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: A randomised controlled trial. Thorax 2009, 64, 561–566. [Google Scholar] [CrossRef]
- Cherian, M.; Adam, V.; Ross, B.; Bourbeau, J.; Kaminska, M. Mortality in individuals with COPD on long-term home non-invasive ventilation. Respir. Med. 2023, 218, 107378. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Chai, A.; Csoma, B.; Bentley, A.; Bikov, A. Benzodiazepines and Opiates Shorten Exacerbation-Free Time in COPD Patients on Long-Term Non-Invasive Ventilation. In Proceedings of the Sleep 2024, Houston, TX, USA, 1–5 June 2024; p. A201. [Google Scholar]
- Ekström, M.P.; Bornefalk-Hermansson, A.; Abernethy, A.P.; Currow, D.C. Safety of benzodiazepines and opioids in very severe respiratory disease: National prospective study. BMJ 2014, 348, g445. [Google Scholar] [CrossRef]
- Bikov, A.; Frent, S.; Deleanu, O.; Meszaros, M.; Birza, M.R.; Popa, A.M.; Manzur, A.R.; Gligor, L.; Mihaicuta, S. Time Spent with Saturation below 80% versus 90% in Patients with Obstructive Sleep Apnoea. J. Clin. Med. 2023, 12, 4205. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Bernelid, E.; Currow, D.C.; Ekström, M. Prescription of opioids for breathlessness in end-stage COPD: A national population-based study. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2651–2657. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.; Lai, C.Y.; Lin, C.L.; Kao, C.H. Adverse Respiratory Events Associated with Hypnotics Use in Patients of Chronic Obstructive Pulmonary Disease: A Population-Based Case-Control Study. Medicine 2015, 94, e1110. [Google Scholar] [CrossRef]
- Meeraus, W.H.; Mullerova, H.; El Baou, C.; Fahey, M.; Hessel, E.M.; Fahy, W.A. Predicting Re-Exacerbation Timing and Understanding Prolonged Exacerbations: An Analysis of Patients with COPD in the ECLIPSE Cohort. Int. J. Chronic Obstr. Pulm. Dis. 2021, 16, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Csoma, B.; Bikov, A.; Tóth, F.; Losonczy, G.; Müller, V.; Lázár, Z. Blood eosinophils on hospital admission for COPD exacerbation do not predict the recurrence of moderate and severe relapses. ERJ Open Res. 2021, 7, 00543. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; McCasland, C.R.; Light, R.W. The effect of triazolam on the arousal response to airway occlusion during sleep in normal subjects. Am. Rev. Respir. Dis. 1992, 146, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Chiner, E.; Llombart, M.; Valls, J.; Pastor, E.; Sancho-Chust, J.N.; Andreu, A.L.; Sánchez-de-la-Torre, M.; Barbé, F. Association between Obstructive Sleep Apnea and Community-Acquired Pneumonia. PLoS ONE 2016, 11, e0152749. [Google Scholar] [CrossRef]
- Hasani, A.; Spiteri, M.A.; Pavia, D.; Lopez-Vidriero, M.T.; Agnew, J.E.; Clarke, S.W. Effect of temazepam on tracheobronchial mucus clearance. Thorax 1992, 47, 298–300. [Google Scholar] [CrossRef][Green Version]
- Jones, C.M.; McAninch, J.K. Emergency Department Visits and Overdose Deaths From Combined Use of Opioids and Benzodiazepines. Am. J. Prev. Med. 2015, 49, 493–501. [Google Scholar] [CrossRef]
- Le, T.T.; Park, S.; Choi, M.; Wijesinha, M.; Khokhar, B.; Simoni-Wastila, L. Respiratory events associated with concomitant opioid and sedative use among Medicare beneficiaries with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2020, 7, e000483. [Google Scholar] [CrossRef]
- Afzal, A.; Kiyatkin, E.A. Interactions of benzodiazepines with heroin: Respiratory depression, temperature effects, and behavior. Neuropharmacology 2019, 158, 107677. [Google Scholar] [CrossRef]
- Budweiser, S.; Jörres, R.A.; Riedl, T.; Heinemann, F.; Hitzl, A.P.; Windisch, W.; Pfeifer, M. Predictors of survival in COPD patients with chronic hypercapnic respiratory failure receiving noninvasive home ventilation. Chest 2007, 131, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Soriano, J.B.; Carrizo, S.J.; Boldova, A.; Celli, B.R. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: The overlap syndrome. Am. J. Respir. Crit. Care Med. 2010, 182, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; Montes de Oca, M.; Mendez, R.A.; Pinto Plata, V.; Cabral, H.J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Soler-Cataluña, J.J.; Martínez-García, M.A.; Román Sánchez, P.; Salcedo, E.; Navarro, M.; Ochando, R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005, 60, 925–931. [Google Scholar] [CrossRef]


| Patients on Benzodiazepines (n = 40) | Patients Not on Benzodiazepines (n = 299) | p-Value 1 | Patients on Opioids (n = 92) | Patients Not on Opioids (n = 247) | p-Value 2 | Patients on Both Benzodiazepines and Opioids (n = 20) | p-Value 3 | p-Value 4 | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 65/60–71/ | 65/59–71/ | 0.97 | 62/56–70/ | 66/60–72/ | <0.01 | 67/62–71/ | 0.89 | 0.17 |
| Sex (% females) | 63 | 58 | 0.58 | 59 | 58 | 0.95 | 65 | 0.74 | 0.52 |
| BMI (kg/m2) | 27.8/22.0–33.8/ | 32.8/25.1–39.7/ | <0.01 | 30.2/23.7–41.3/ | 32.7/24.9–38.1/ | 0.79 | 26.7/23.9–30.0/ | 0.82 | 0.04 |
| Number of exacerbations in the year pre–NIV setup | 3.5/2.8–7.0/ | 3.0/2.0–5.0/ | 0.16 | 3.0/2.0–7.0/ | 3.0/2.0–7.0/ | 0.23 | 4.0/3.0–7.0/ | 0.25 | 0.34 |
| CCI | 1.0/1.0–2.0/ | 2.0/1.0–3.0/ | 0.04 | 2.0/1.0–2.0/ | 2.0/1.0–3.0/ | 0.10 | 1.0/1.0–2.0/ | 0.26 | 0.14 |
| Anxiety (%) | 43 | 18 | <0.01 | 33 | 16 | <0.01 | 60 | 0.03 | <0.01 |
| mMRC | 3.89 ± 0.32 | 3.70 ± 0.69 | 0.25 | 3.91 ± 0.38 | 3.65 ± 0.73 | <0.01 | 3.94 ± 0.25 | 0.40 | 0.75 |
| CFS | 5.0/5.0–6.0/ | 5.0/4.0–6.0/ | 0.10 | 5.0/5.0–6.0/ | 5.0/4.0–6.0/ | 0.08 | 6.0/5.0–6.3/ | 0.16 | 0.10 |
| FEV1 (% pred) | 34/24–42/ | 39/27–50/ | 0.10 | 36/25–48/ | 39/27–51/ | 0.39 | 35/24–45/ | 0.79 | 0.45 |
| FVC (% pred) | 78/66–82/ | 69/58–85/ | 0.54 | 72/58–85/ | 69/59–85/ | 0.84 | 78/76–82/ | 0.30 | 0.28 |
| Pre–NIV setup pH | 7.41/7.38–7.44/ | 7.41/7.35–7.45/ | 0.56 | 7.41/7.37–7.44/ | 7.41/7.35–7.45/ | 0.93 | 7.41/7.38–7.43/ | 0.63 | 0.79 |
| Pre–NIV setup pO2 (kPa) | 7.50/6.85–8.30/ | 7.80/7.20–8.50/ | 0.33 | 7.80/7.10–8.50/ | 7.80/7.20–8.40/ | 0.99 | 7.65/7.00–8.53/ | 0.39 | 0.66 |
| Pre–NIV setup pCO2 (kPa) | 7.60/6.85–8.25/ | 7.80/6.90–8.95/ | 0.36 | 7.65/6.98–8.80/ | 7.75/6.80–8.88/ | 0.76 | 7.40/6.88–7.83/ | 0.80 | 0.79 |
| ODI (1/h) | 6.0/3.7–11.6/ | 11.5/7.2–27.0/ | <0.01 | 9.6/4.2–21.2/ | 11.7/6.5–23.7/ | 0.16 | 3.8/2.5–6.7/ | 0.12 | 0.09 |
| T90% (%) | 96.1/72.5–99.7/ | 89.6/62.0–99.1/ | 0.39 | 94.3/69.4–99.2/ | 89.2/62.3–99.3/ | 0.58 | 98.7/95.3–99.9/ | 0.04 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, A.; Csoma, B.; Lazar, Z.; Bentley, A.; Bikov, A. The Effect of Opioids and Benzodiazepines on Exacerbation Rate and Overall Survival in Patients with Chronic Obstructive Pulmonary Disease on Long-Term Non-Invasive Ventilation. J. Clin. Med. 2024, 13, 5624. https://doi.org/10.3390/jcm13185624
Chai A, Csoma B, Lazar Z, Bentley A, Bikov A. The Effect of Opioids and Benzodiazepines on Exacerbation Rate and Overall Survival in Patients with Chronic Obstructive Pulmonary Disease on Long-Term Non-Invasive Ventilation. Journal of Clinical Medicine. 2024; 13(18):5624. https://doi.org/10.3390/jcm13185624
Chicago/Turabian StyleChai, Andrew, Balazs Csoma, Zsofia Lazar, Andrew Bentley, and Andras Bikov. 2024. "The Effect of Opioids and Benzodiazepines on Exacerbation Rate and Overall Survival in Patients with Chronic Obstructive Pulmonary Disease on Long-Term Non-Invasive Ventilation" Journal of Clinical Medicine 13, no. 18: 5624. https://doi.org/10.3390/jcm13185624
APA StyleChai, A., Csoma, B., Lazar, Z., Bentley, A., & Bikov, A. (2024). The Effect of Opioids and Benzodiazepines on Exacerbation Rate and Overall Survival in Patients with Chronic Obstructive Pulmonary Disease on Long-Term Non-Invasive Ventilation. Journal of Clinical Medicine, 13(18), 5624. https://doi.org/10.3390/jcm13185624







