Longitudinal Analysis of Bone Metabolic Markers and Bone Mechanical Properties in STZ-Induced Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Experimental Groups
2.4. Validation of Diabetes
2.5. Serum Assays
2.6. Bone Collection
2.7. Statistical Analysis
3. Results
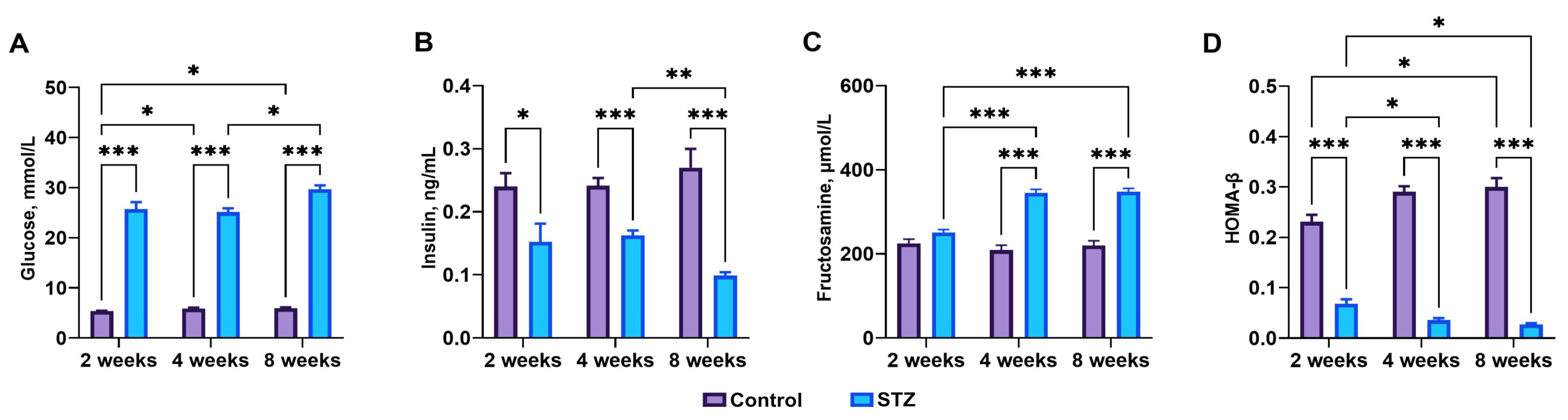
3.1. Streptozotocin Effect, Basal Blood Glucose, Plasma Insulin, and Fructosamine Levels
3.2. Indices of Insulin Sensitivity
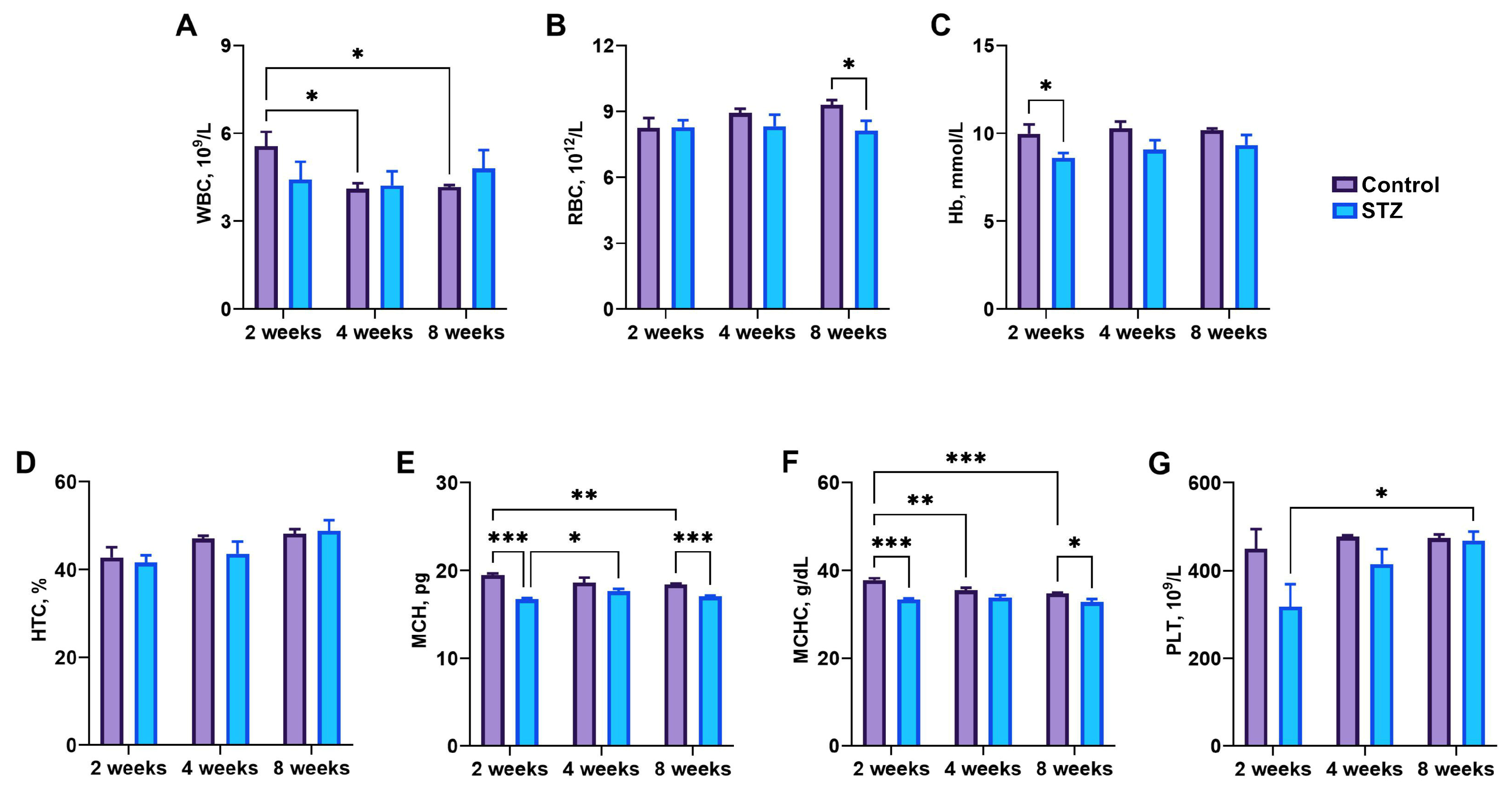
3.3. Blood Basal Parameters
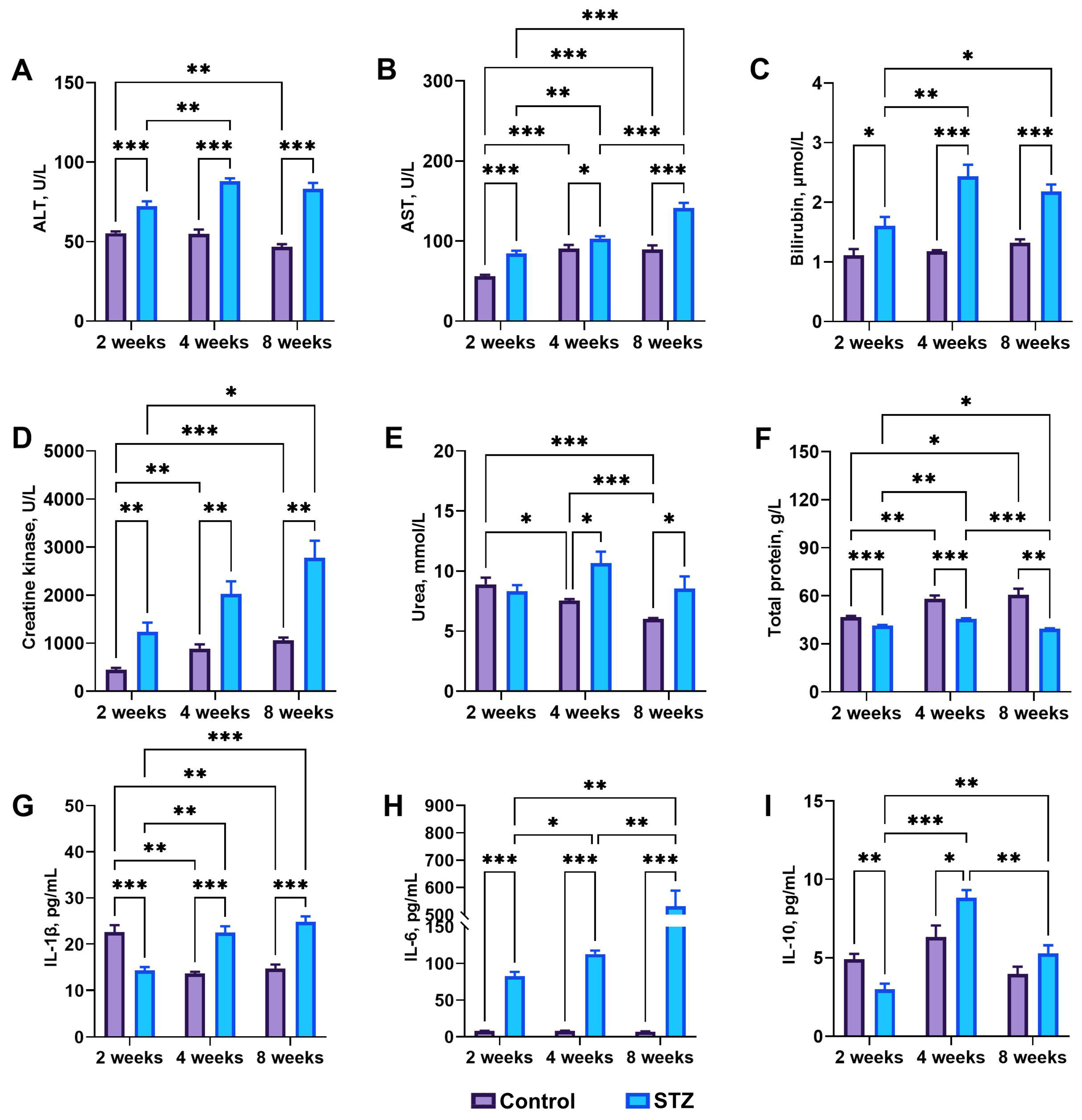
3.4. Basal Biochemical Analysis: IL-1β, IL-6, and IL-10
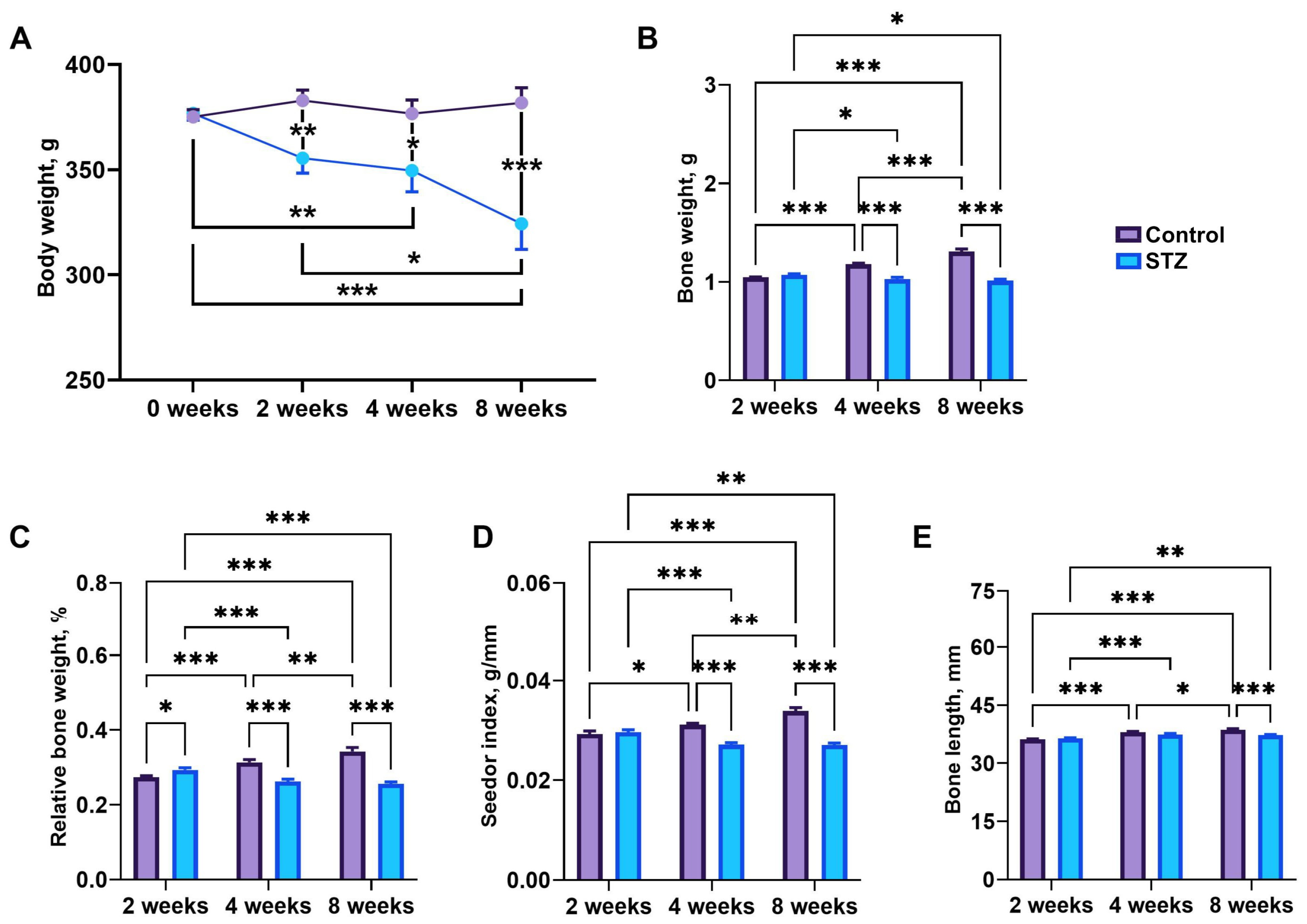
3.5. Body Weight and Bone Morphology
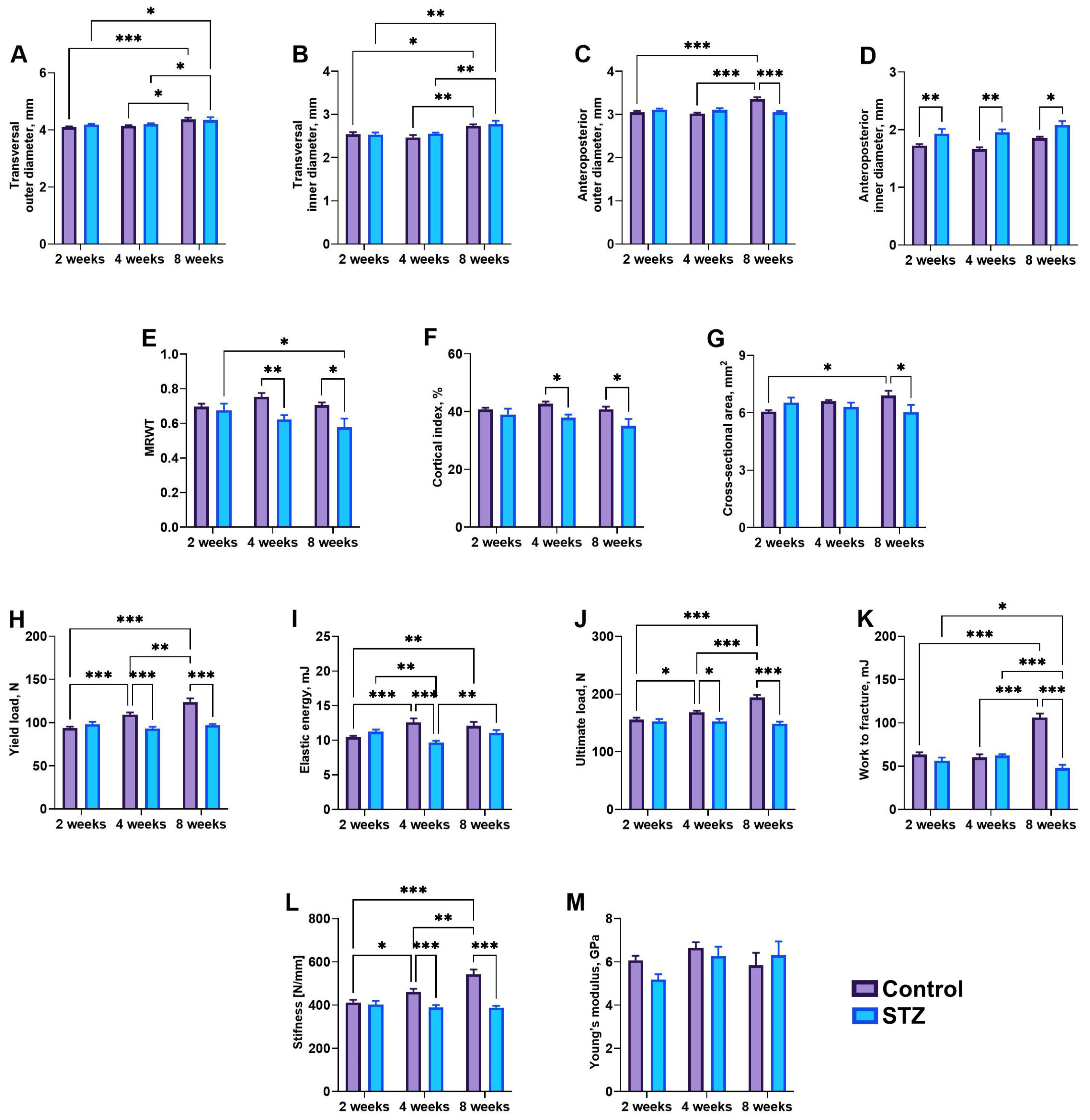
3.6. Bone Geometry and Mechanical Parameters
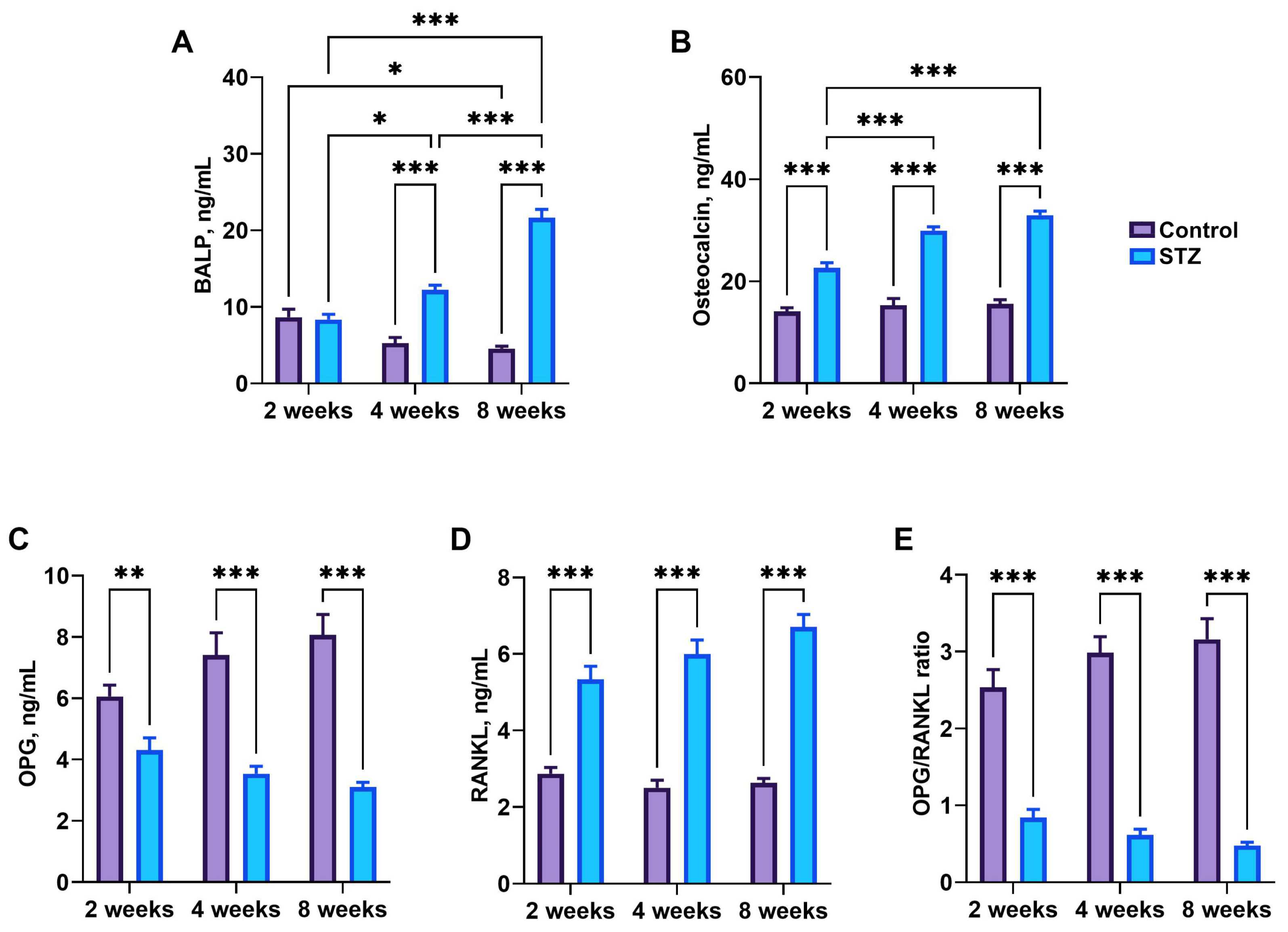
3.7. Bone Metabolism Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. U.S. Centers for Disease Control and Prevetion National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 21 August 2024).
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and Incidence of Type 1 Diabetes in the World: A Systematic Review and Meta-Analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2019, 43, S14–S31. [CrossRef]
- PAHO, Pan American Health Organization. Burden of Disease from Diabetes. Available online: https://www.paho.org/en/enlace/burden-disease-diabetes (accessed on 21 August 2024).
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Kemink, S.A.; Hermus, A.R.; Swinkels, L.M.; Lutterman, J.A.; Smals, A.G. Osteopenia in Insulin-Dependent Diabetes Mellitus; Prevalence and Aspects of Pathophysiology. J. Endocrinol. Investig. 2000, 23, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Inzerillo, A.M.; Epstein, S. Osteoporosis and Diabetes Mellitus. Rev. Endocr. Metab. Disord. 2004, 5, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.A.; Alcolado, J.C. Animal Models of Diabetes Mellitus. Diabet. Med. 2005, 22, 359–370. [Google Scholar] [CrossRef]
- Motyl, K.; McCabe, L.R. Streptozotocin, Type I Diabetes Severity and Bone. Biol. Proced. Online 2009, 11, 296–315. [Google Scholar] [CrossRef]
- Lenzen, S. The Mechanisms of Alloxan- and Streptozotocin-Induced Diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef]
- Reusser, F. Mode of Action of Streptozotocin. J. Bacteriol. 1971, 105, 580–588. [Google Scholar] [CrossRef]
- Bolzán, A.D.; Bianchi, M.S. Genotoxicity of Streptozotocin. Mutat. Res. 2002, 512, 121–134. [Google Scholar] [CrossRef]
- Ferrer, J.; Benito, C.; Gomis, R. Pancreatic Islet GLUT2 Glucose Transporter mRNA and Protein Expression in Humans with and without NIDDM. Diabetes 1995, 44, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Sobrevilla, J.; Boone-Villa, V.D.; Aguilar, C.N.; Román-Ramos, R.; Vega-Avila, E.; Campos-Sepúlveda, E.; Alarcón-Aguilar, F. Effect of Varying Dose and Administration of Streptozotocin on Blood Sugar in Male CD1 Mice. Proc. West. Pharmacol. Soc. 2011, 54, 5–9. [Google Scholar]
- Szkudelski, T. The Mechanism of Alloxan and Streptozotocin Action in B Cells of the Rat Pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Valentovic, M.A.; Alejandro, N.; Betts Carpenter, A.; Brown, P.I.; Ramos, K. Streptozotocin (STZ) Diabetes Enhances Benzo(Alpha)Pyrene Induced Renal Injury in Sprague Dawley Rats. Toxicol. Lett. 2006, 164, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the Mechanism of Cell Death Resulting from Streptozotocin Challenge in Experimental Animals, Its Practical Use and Potential Risk to Humans. J. Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef]
- Epstein, S.; LeRoith, D. Diabetes and Fragility Fractures—A Burgeoning Epidemic? Bone 2008, 43, 3–6. [Google Scholar] [CrossRef]
- Moeckel, B.; Huo, M.H.; Salvati, E.A.; Pellicci, P.M. Total Hip Arthroplasty in Patients with Diabetes Mellitus. J. Arthoplasty 1993, 8, 279–284. [Google Scholar] [CrossRef]
- Thompson, R.C.; Clohisy, D.R. Deformity Following Fracture in Diabetic Neuropathic Osteoarthropathy. Operative Management of Adults Who Have Type-I Diabetes. J. Bone Jt. Surg. Am. 1993, 75, 1765–1773. [Google Scholar] [CrossRef]
- Gilbert, M.P.; Pratley, R.E. The Impact of Diabetes and Diabetes Medications on Bone Health. Endocr. Rev. 2015, 36, 194–213. [Google Scholar] [CrossRef]
- Reddy, G.K.; Stehno-Bittel, L.; Hamade, S.; Enwemeka, C.S. The Biomechanical Integrity of Bone in Experimental Diabetes. Diabetes Res. Clin. Pract. 2001, 54, 1–8. [Google Scholar] [CrossRef]
- Dixit, P.K.; Ekstrom, R.A. Decreased Breaking Strength of Diabetic Rat Bone and Its Improvement by Insulin Treatment. Calcif. Tissue Int. 1980, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Boskey, A.L.; Gundberg, C.M.; Vigorita, V.J.; Devlin, V.J.; Beyer, M.M. The Mineral and Mechanical Properties of Bone in Chronic Experimental Diabetes. J. Orthop. Res. 1988, 6, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghe, J.; Suiker, A.M.; Einhorn, T.A.; Geusens, P.; Visser, W.J.; Van Herck, E.; Van Bree, R.; Magitsky, S.; Bouillon, R. Brittle Bones in Spontaneously Diabetic Female Rats Cannot Be Predicted by Bone Mineral Measurements: Studies in Diabetic and Ovariectomized Rats. J. Bone Miner. Res. 1994, 9, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.U.; Ullah, K.; Rasool, A.; Manzoor, R.; Yuan, Y.; Tareen, A.M.; Kaleem, I.; Riaz, N.; Hameed, S.; Bashir, S. Comparative Impact of Streptozotocin on Altering Normal Glucose Homeostasis in Diabetic Rats Compared to Normoglycemic Rats. Sci. Rep. 2023, 13, 7921. [Google Scholar] [CrossRef] [PubMed]
- Leslie, R.D.; Evans-Molina, C.; Freund-Brown, J.; Buzzetti, R.; Dabelea, D.; Gillespie, K.M.; Goland, R.; Jones, A.G.; Kacher, M.; Phillips, L.S.; et al. Adult-Onset Type 1 Diabetes: Current Understanding and Challenges. Diabetes Care 2021, 44, 2449–2456. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.M.; Kim, C.; Banerjee, T.; Lee, J.M. Fluctuations in the Incidence of Type 1 Diabetes in the United States from 2001 to 2015: A Longitudinal Study. BMC Med. 2017, 15, 199. [Google Scholar] [CrossRef]
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group; Magliano, D.J.; Maniam, J.; et al. Global Incidence, Prevalence, and Mortality of Type 1 Diabetes in 2021 with Projection to 2040: A Modelling Study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Antunes, L.C.; Elkfury, J.L.; Jornada, M.N.; Foletto, K.C.; Bertoluci, M.C. Validation of HOMA-IR in a Model of Insulin-Resistance Induced by a High-Fat Diet in Wistar Rats. Arch. Endocrinol. Metab. 2016, 60, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Knopp, J.L.; Holder-Pearson, L.; Chase, J.G. Insulin Units and Conversion Factors: A Story of Truth, Boots, and Faster Half-Truths. J. Diabetes Sci. Technol. 2019, 13, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Okoduwa, S.I.R.; Umar, I.A.; James, D.B.; Inuwa, H.M. Appropriate Insulin Level in Selecting Fortified Diet-Fed, Streptozotocin-Treated Rat Model of Type 2 Diabetes for Anti-Diabetic Studies. PLoS ONE 2017, 12, e0170971. [Google Scholar] [CrossRef]
- Ferretti, J.L.; Capozza, R.F.; Mondelo, N.; Zanchetta, J.R. Interrelationships between Densitometric, Geometric, and Mechanical Properties of Rat Femora: Inferences Concerning Mechanical Regulation of Bone Modeling. J. Bone Miner. Res. 1993, 8, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Osiak-Wicha, C.; Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Andres, K.; Schwarz, T.; Świetlicki, M.; Mielnik-Błaszczak, M.; Arciszewski, M.B. Developmental Changes in Tibia and Humerus of Goose: Morphometric, Densitometric, and Mechanical Analysis. Animal 2023, 17, 100960. [Google Scholar] [CrossRef] [PubMed]
- Muszyński, S.; Kwiecień, M.; Tomaszewska, E.; Świetlicka, I.; Dobrowolski, P.; Kasperek, K.; Jeżewska-Witkowska, G. Effect of Caponization on Performance and Quality Characteristics of Long Bones in Polbar Chickens. Poult. Sci. 2017, 96, 491–500. [Google Scholar] [CrossRef]
- Ar’Rajab, A.; Ahrén, B. Long-Term Diabetogenic Effect of Streptozotocin in Rats. Pancreas 1993, 8, 50–57. [Google Scholar] [CrossRef]
- McNeill, J.H. (Ed.) Experimental Models of Diabetes, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Kaikini, A.A.; Dhodi, D.; Muke, S.; Peshattiwar, V.; Bagle, S.; Korde, A.; Sarnaik, J.; Kadwad, V.; Sachdev, S.; Sathaye, S. Standardization of Type 1 and Type 2 Diabetic Nephropathy Models in Rats: Assessment and Characterization of Metabolic Features and Renal Injury. J. Pharm. Bioallied. Sci. 2020, 12, 295–307. [Google Scholar] [CrossRef]
- Nansseu, J.R.N.; Fokom-Domgue, J.; Noubiap, J.J.N.; Balti, E.V.; Sobngwi, E.; Kengne, A.P. Fructosamine Measurement for Diabetes Mellitus Diagnosis and Monitoring: A Systematic Review and Meta-Analysis Protocol. BMJ Open 2015, 5, e007689. [Google Scholar] [CrossRef]
- Brossaud, J.; Bosch-Bouju, C.; Marissal-Arvy, N.; Campas-Lebecque, M.-N.; Helbling, J.-C.; Webster, S.P.; Walker, B.R.; Fioramonti, X.; Ferreira, G.; Barat, P.; et al. Memory Deficits in a Juvenile Rat Model of Type 1 Diabetes Are Due to Excess 11β-HSD1 Activity, Which Is Upregulated by High Glucose Concentrations Rather than Insulin Deficiency. Diabetologia 2023, 66, 1735–1747. [Google Scholar] [CrossRef]
- Alamri, B.N.; Bahabri, A.; Aldereihim, A.A.; Alabduljabbar, M.; Alsubaie, M.M.; Alnaqeb, D.; Almogbel, E.; Metias, N.S.; Alotaibi, O.A.; Al-Rubeaan, K. Hyperglycemia Effect on Red Blood Cells Indices. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, P.; Yan, Z.; Liu, Z.; Ma, Q.; Zhang, Z.; Wang, Y.; Su, Y. The Relationship between Erythrocytes and Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 6656062. [Google Scholar] [CrossRef] [PubMed]
- Jevrić-Causević, A.; Malenica, M.; Dujić, T. Creatine Kinase Activity in Patients with Diabetes Mellitus Type I and Type II. Bosn. J. Basic Med. Sci. 2006, 6, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bowe, B.; Li, T.; Xian, H.; Yan, Y.; Al-Aly, Z. Higher Blood Urea Nitrogen Is Associated with Increased Risk of Incident Diabetes Mellitus. Kidney Int. 2018, 93, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Leeds, J.S.; Forman, E.M.; Morley, S.; Scott, A.R.; Tesfaye, S.; Sanders, D.S. Abnormal Liver Function Tests in Patients with Type 1 Diabetes Mellitus: Prevalence, Clinical Correlations and Underlying Pathologies. Diabet. Med. 2009, 26, 1235–1241. [Google Scholar] [CrossRef]
- Stadler, M.; Bollow, E.; Fritsch, M.; Kerner, W.; Schuetz-Fuhrmann, I.; Krakow, D.; Merger, S.; Riedl, M.; Jehle, P.; Holl, R.W.; et al. Prevalence of Elevated Liver Enzymes in Adults with Type 1 Diabetes: A Multicentre Analysis of the German/Austrian DPV Database. Diabetes Obes. Metab. 2017, 19, 1171–1178. [Google Scholar] [CrossRef]
- Yazdi, H.B.; Hojati, V.; Shiravi, A.; Hosseinian, S.; Vaezi, G.; Hadjzadeh, M.-A.-R. Liver Dysfunction and Oxidative Stress in Streptozotocin-Induced Diabetic Rats: Protective Role of Artemisia Turanica. J. Pharmacopuncture 2019, 22, 109–114. [Google Scholar] [CrossRef]
- Udo, N.V.; Effiong, O.O.; Otu, O.V.; Olusola, A.E.; Oleba, O.E. Comparative Effects of Aloe Vera Gel and Aqueous Leaf Extract of Viscum Album on Bilirubin Excretion in Streptozotocin—Induced Diabetic Rats. Int. J. Biochem. Res. Rev. 2014, 4, 99–115. [Google Scholar] [CrossRef]
- Hull, T.D.; Agarwal, A. Bilirubin: A Potential Biomarker and Therapeutic Target for Diabetic Nephropathy. Diabetes 2014, 63, 2613–2616. [Google Scholar] [CrossRef]
- Cheriyath, P.; Gorrepati, V.S.; Peters, I.; Nookala, V.; Murphy, M.E.; Srouji, N.; Fischman, D. High Total Bilirubin as a Protective Factor for Diabetes Mellitus: An Analysis of NHANES Sata from 1999–2006. J. Clin. Med. Res. 2010, 2, 201–206. [Google Scholar] [CrossRef]
- Scridon, A.; Perian, M.; Marginean, A.; Fisca, C.; Vantu, A.; Ghertescu, D.; Chevalier, P.; Serban, R.C. Wistar rats with long-term streptozotocin-induced type 1 diabetes mellitus replicate the most relevant clinical, biochemical, and hematologic features of human diabetes. Rev. Romana Med. Lab. 2015, 23, 263–274. [Google Scholar] [CrossRef]
- Amirshahrokhi, K.; Zohouri, A. Carvedilol Prevents Pancreatic β-Cell Damage and the Development of Type 1 Diabetes in Mice by the Inhibition of Proinflammatory Cytokines, NF-κB, COX-2, iNOS and Oxidative Stress. Cytokine 2021, 138, 155394. [Google Scholar] [CrossRef] [PubMed]
- Gennaro, G.; Claudino, M.; Cestari, T.M.; Ceolin, D.; Germino, P.; Garlet, G.P.; de Assis, G.F. Green Tea Modulates Cytokine Expression in the Periodontium and Attenuates Alveolar Bone Resorption in Type 1 Diabetic Rats. PLoS ONE 2015, 10, e0134784. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.X.; Chen, D.J.; Zu, Y.X.; Wang, E.Z.; Qi, S.S. Chondroitin Sulfate Prevents STZ Induced Diabetic Osteoporosis through Decreasing Blood Glucose, Antioxidative Stress, Anti-Inflammation and OPG/RANKL Expression Regulation. Int. J. Mol. Sci. 2020, 21, 5303. [Google Scholar] [CrossRef] [PubMed]
- Gülen, S.; Dinçer, S. Effects of Leptin on Oxidative Stress in Healthy and Streptozotocin-Induced Diabetic Rats. Mol. Cell. Biochem. 2007, 302, 59–65. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Brueck, C.C.; Singh, S.K.; Dobnig, H. Osteoporosis in Patients with Diabetes Mellitus. J. Bone Miner. Res. 2007, 22, 1317–1328. [Google Scholar] [CrossRef]
- Alexopoulou, O.; Jamart, J.; Devogelaer, J.P.; Brichard, S.; de Nayer, P.; Buysschaert, M. Bone Density and Markers of Bone Remodeling in Type 1 Male Diabetic Patients. Diabetes Metab. 2006, 32, 453–458. [Google Scholar] [CrossRef]
- Verhaeghe, J.; Van Herck, E.; van Bree, R.; Moermans, K.; Bouillon, R. Decreased Osteoblast Activity in Spontaneously Diabetic Rats. In Vivo Studies on the Pathogenesis. Endocrine 1997, 7, 165–175. [Google Scholar] [CrossRef]
- Waud, C.E.; Marks, S.C.; Lew, R.; Baran, D.T. Bone Mineral Density in the Femur and Lumbar Vertebrae Decreases after Twelve Weeks of Diabetes in Spontaneously Diabetic-Prone BB/Worcester Rats. Calcif. Tissue Int. 1994, 54, 237–240. [Google Scholar] [CrossRef]
- Horcajada-Molteni, M.N.; Chanteranne, B.; Lebecque, P.; Davicco, M.J.; Coxam, V.; Young, A.; Barlet, J.P. Amylin and Bone Metabolism in Streptozotocin-Induced Diabetic Rats. J. Bone Miner. Res. 2001, 16, 958–965. [Google Scholar] [CrossRef]
- Erdal, N.; Gürgül, S.; Demirel, C.; Yildiz, A. The Effect of Insulin Therapy on Biomechanical Deterioration of Bone in Streptozotocin (STZ)-Induced Type 1 Diabetes Mellitus in Rats. Diabetes Res. Clin. Pract. 2012, 97, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Demirel, C.; Korkmaz, H.; Gurgul, S.; Yildiz, A.; Akarsu, E.; Erdal, N. The Effect of Glucovance Therapy on Biomechanical Deterioration of Bone in Streptozotocin-Induced Diabetic Rats. Acta Endocrinol. 2019, 15, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.T.; Sievänen, H.; Salo, M.K.; Tulokas, S.; Saha, H.H. Bone Mass and Structure in Adolescents with Type 1 Diabetes Compared to Healthy Peers. Osteoporos Int. 2009, 20, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszewska, E.; Dobrowolski, P.; Muszyński, S.; Donaldson, J.; Gołyński, M.; Zwolska, J.; Szadkowski, M.; Osęka, M.; Mielnik-Błaszczak, M.; Balicki, I. Longitudinal Analysis of Bone Metabolic Markers and Bone Mechanical Properties in STZ-Induced Diabetic Rats. J. Clin. Med. 2024, 13, 5595. https://doi.org/10.3390/jcm13185595
Tomaszewska E, Dobrowolski P, Muszyński S, Donaldson J, Gołyński M, Zwolska J, Szadkowski M, Osęka M, Mielnik-Błaszczak M, Balicki I. Longitudinal Analysis of Bone Metabolic Markers and Bone Mechanical Properties in STZ-Induced Diabetic Rats. Journal of Clinical Medicine. 2024; 13(18):5595. https://doi.org/10.3390/jcm13185595
Chicago/Turabian StyleTomaszewska, Ewa, Piotr Dobrowolski, Siemowit Muszyński, Janine Donaldson, Marcin Gołyński, Jowita Zwolska, Mateusz Szadkowski, Maciej Osęka, Maria Mielnik-Błaszczak, and Ireneusz Balicki. 2024. "Longitudinal Analysis of Bone Metabolic Markers and Bone Mechanical Properties in STZ-Induced Diabetic Rats" Journal of Clinical Medicine 13, no. 18: 5595. https://doi.org/10.3390/jcm13185595
APA StyleTomaszewska, E., Dobrowolski, P., Muszyński, S., Donaldson, J., Gołyński, M., Zwolska, J., Szadkowski, M., Osęka, M., Mielnik-Błaszczak, M., & Balicki, I. (2024). Longitudinal Analysis of Bone Metabolic Markers and Bone Mechanical Properties in STZ-Induced Diabetic Rats. Journal of Clinical Medicine, 13(18), 5595. https://doi.org/10.3390/jcm13185595






