Abstract
Background/Objectives: Human cytomegalovirus (HCMV) is the most frequent cause of congenital infections. The HCMV-specific T-cell response in primary infection may help define reliable correlates of immune protection in pregnancy. In this study, the antigen-specific T-cell response against different HCMV proteins (IE-1, pp65, gB, gHgLpUL128L) was investigated in pregnant women with primary infection and in control subjects with remote infection to identify possible components of a vaccine. Methods: Blood samples from 35 pregnant women with HCMV primary infection and 30 HCMV-seropositive healthy adult subjects with remote infection were tested. The antigen-specific T-cell response was measured using cytokine intracellular staining after stimulation with IE-1, pp65, gB and gHgLpUL128L peptides pool. Results: The pp65-specific CD4+ T-cell response was higher in pregnant women with HCMV primary infection at the late time point and in control subjects with remote infection, while the pregnant women at the early time point showed a higher gB-specific CD8+ T-cell response. Regarding the CD4+ and CD8+ T-cell phenotypes, we observed that HCMV-specific CD4+ and CD8+ T cells expressing CD45RA+ remained constant in pregnant women with primary infection at the early and late time points and in subjects with remote infection, while HCMV-specific CD4+ and CD8+ T cells expressing IL-7R+ or producing IL-2 were higher in control subjects with remote infection than in pregnant women with HCMV primary infection. Conclusions: The T-cell response was higher against gB in the early phase of infection and against pp65 in the late phase. Therefore, these proteins should be taken into consideration as candidates for a vaccine.
1. Introduction
Human cytomegalovirus (HCMV) is the most frequent cause of congenital infection, occurring in approximately 0.5–2% of pregnancies [1,2] and resulting in long-term sequelae in 20% of cases, including 13% of infants who are symptomatic at birth and 7% of infants who are asymptomatic at birth [3,4,5]. Among the most common long-term effects is sensorineural hearing loss (SNHL) [6,7,8,9,10,11]. According to observations, 7–21% of asymptomatic neonates with congenital infection have SNHL at 3–4 years old, and some children start showing symptoms at 7 years old or beyond [12].Congenital infection illness arising from primary maternal infection is frequently linked to more neurological damage and more severe SNHL, even if the incidence of SNHL in infants delivered to women with primary or non-primary infection is comparable [13,14,15,16]. The risk of congenital cytomegalovirus infection is around 40% after primary infection and much lower after non-primary infection [17,18,19,20]. The intrauterine transmission is associated with delayed development of the T and B cell response [21,22]; for example, the reduction in re-expression of CD45RA [23] on the surface of HCMV-specific effector memory T cells correlates with HCMV transmission to the fetus . Instead, a reduced risk of HCMV transmission to the fetus appears to be associated with the rapid development of HCMV-specific CD4+ T cells with a long-term memory phenotype, suggesting that IL-7R expression may be a predictive marker of protection [22]. In a previous study, we found that many years are required for the development of a long-term memory (LTM) response comparable to that of remote infection [24]. A HCMV vaccine should be preventing HCMV primary infection and repeated episodes of reactivation/reinfection and directly or indirectly protecting the two high-risk populations of pregnant women and immunocompromised patients [25]. In this way, the circulation of different HCMV strains may be reduced among pregnant women and immunocompromised patients [25].
In this study, we investigated the antigen-specific T-cell response against the non-structural protein IE-1 (produced in the immediate-early phase), the structural protein pp65 (internal tegument protein) and the envelope glycoprotein complexes, including the pentamer gHgLpUL128L and gB, in pregnant women at 2 and 12 months after HCMV primary infection and in control subjects with remote infection. We also investigated the correlation between the antigen-specific T-cell response and the protection against virus transmission to the fetus.
2. Methods
2.1. Subjects
Thirty-five pregnant women with HCMV primary infection were enrolled within three months after the onset of infection at Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. Diagnosis of primary HCMV infection was based on two or more of the following parameters: presence of IgM, IgG seroconversion, low IgG avidity index, and detection of HCMV DNA in blood [26].
This retrospective study was performed according to guidelines and approved by the Ethics Committee and Fondazione IRCCS Policlinico San Matteo Institutional Review Board (Procedure number 20180075214). Participation in the study was discussed with the patient during the first clinical visit. All patients signed an informed consent. Data and clinical samples were coded to prevent the patient’s identity from being traced back to them and handled by doctors and investigators. Coded clinical samples were stored until the end of the study.
The participants were divided into two groups, those with HCMV primary infection and those with HCMV remote infection. Blood samples from 35 pregnant women with HCMV primary infection were collected and tested at the early time point (median time: 60; IQR 49–65 days after onset infection); 15 of them were also analyzed at a later time point (median time: 360; IQR 356–412 days after onset infection). In addition, 30 HCMV-seropositive healthy adult subjects (23 females and 7 males) with remote HCMV infection were enrolled as controls.
2.2. Diagnosis of HCMV
LIASON® CMVIgMII (cat: 310755, Diasorin, Saluggia, Italy) assay was used to determine HCMV-specific IgM and values above 22 U/mL were considered positive; LIASON®IgGII (cat: 310745, Diasorin) assay was used to determine IgG antibody, the values above 14 U/mL were considered positive; LIASON®IgGAvII (cat: 310765, Diasorin) assay was used to determine IgG AI: AI < 0.15 was considered low, between 0.15 and 0.25 intermediate, and >0.25 high [26]. These kits detected antibodies specific for HCMV proteins pp150, pp52 and pp28.
Congenital HCMV infection was detected in two ways: either in the first trimester, 20–21 weeks of pregnancy, by HCMV DNA detection and virus isolation in amniotic fluid, and after delivery, by viral DNA detection in urine within two weeks after birth [26].
DNA was isolated from 200 µL of whole blood using QIAsymphony RGQ System and the QIAsymphony DNA Mini Kit (cat: 937236, QIAGEN, Hamburg, Germany). HCMV DNA amplification was performed with an in-house developed method targeting a region in the US8 gene [27]. Results were given as copies/mL (limit of detection 90 copies/mL).
2.3. Protein Peptides Pool
To evaluate the antigen-specific T-cell response, peptides pool representative of IE-1 (cat: P13202, JPT, Peptide Technologies, Berlin, Germany), pp65, gB, and gHgLpUL128L peptides pool (15 mers, overlapping by 10 amino acids; cat: na, all from A&A Labs LLC, San Diego, CA, USA), were used. A peptide pool of human actin (15 mers, overlapping by 10 amino acids; cat: na, Pepscan, Le-lystad, The Netherlands) was used as a negative control.
2.4. PBMC Isolation
Peripheral blood mononuclear cell (PBMC) were isolated from heparin blood, by standard density gradient centrifugation using Lymphoprep (cat: 1114547, Sentinel Diagnostics, Milano, Italy). Isolated PBMC were cryopreserved in RPMI-1640 (cat: ECB90062, Euroclone, Milano, Italy) supplemented with 10% dimethyl sulfoxide (DMSO) (cat: A3672,0100, PanReac AppliChem ITW reagents, Monza, Italy) and 25% human albumin (cat: na, Kedrion Biopharma, Lucca, Italy).
2.5. Stimulation with HCMV–Specific Peptides Pool and Cytokine Flow Cytometry Analysis
PBMC were stimulated for 16–18 h with peptide pools from IE-1, pp65, gB, gHgLpUL128L, and human actin [1 µg/mL] in the presence of 0.5 µg/mL of co-stimulator molecules, CD28 (clone CD28.2; cat: 555726, BD Biosciences, San Jose, CA, USA) and CD49d (clone 9F10; cat: 555502, BD Biosciences), and brefeldin A (cat: B7651-SGM, Sigma-Aldrich-Merck, Darmstadt, Germany) at a final concentration of 10 µg/mL. Cells were seeded in 96-well round bottom plates at a density of 0.5–1 × 106 cells/200 µL culture medium per well. The culture medium was RPMI 1640 (Euroclone, Milan, Italy) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin (cat: ECB3001D, Euroclone), 2 mM L-glutamine (cat: ECB3000D, Euroclone), and 10% heat-inactivated FBS (cat: ECS1104L, Euroclone). PBMC were then incubated overnight at 37 °C with 5% CO2. MAb CD127 (IL-7R) PE (clone hIL-7R-M21; cat: 557938, BD Biosciences), which was added during the overnight incubation. Then, PBMC were washed with PBS (cat: ECB4004L, Euroclone) 2 mM EDTA (cat: 139-33-3, Sigma-Adrich-Merck) and stained with CD8 V500 (clone RPA-T8 ; cat: 560774, BD Biosciences) and CD197 (CCR7) BV421 (clone G043H7; cat: 353208, Biolegend, San Diego, CA, USA) in PBS 5% FBS for 30 min at 4 °C. Cells were then washed with PBS 5% FBS, fixed and permeabilized using Cytofix/Cytoperm (cat: 554722, BD Biosciences) for 20 min at 4 °C. Final staining with CD3 PerCP Cy 5.5 (clone UCHT1; cat: 560835), CD4 APC Cy7 (clone RPA-T4; cat: 557871), IFN-γ PECy7 (clone B27; cat: 557643), IL-2 APC (clone MQ1-17H12; cat: 554567) and CD45RA FITC (clone HI100; cat: 555488) (all from BD Biosciences) antibodies in Perm/Wash buffer 1× (cat: 554723, BD Biosciences) for 45 min at room temperature . Finally, cells were washed with Perm/Wash 1X and resuspended in PBS 1% paraformaldehyde (cat: 1.04002.1000, Sigma-Aldrich-Merck). Analysis was performed with FACS Canto II and FACS Lyric flow cytometer using FACSDiva™ v6.1.3 and BD FACSuite v.1.5 software (all from BD Biosciences). After the identification of memory T cells by exclusion of naïve T cells (CD45RA+ CCR7+), the percentage of IFN-γ producing CD4+ and CD8+ T cells was determined by subtracting the percentage of PBMCs incubated with human actin peptides from the percentage of PBMCs incubated with each HCMV proteins peptide pools, and among them the percentages of CD45RA+ or IL-7R+ or IL-2+ cells were calculated. Regarding IFN-γ CD4+ and CD8+ T cell response, a value < 0.05% antigen-specific T-cell was considered negative while a value ≥ 0.05% was considered positive.
2.6. Statistical Analysis
Statistical analyses were performed with GraphPad Prism 8.3.0 (GraphPad Software Inc., La Jolla, CA, USA). Comparison between three groups was performed using the Kruskal–Wallis test and Dunn’s post-test with correction for multiple comparisons, and two groups were compared using the Friedman test. The Mann–Whitney U-test was applied for unpaired comparison, while the Chi-square test was used to compare the difference in frequencies.
3. Results
3.1. HCMV-Specific T-Cell Response
The percentage of IFN-γ producing CD4+ and CD8+ T cells was calculated after stimulation with IE-1, pp65, gB, and gHgLpUL128L peptide pools in pregnant women with HCMV primary infection at an early and a late time point and in control subjects with HCMV remote infection. In particular, control subjects with remote infection and pregnant women with primary infection at the late time point developed higher levels of pp65-specific CD4+ T-cell response than pregnant women with primary infection at the early time point (p < 0.001) (Figure 1A). However, pregnant women with primary infection at the early time point showed higher gB-specific CD8+ T-cell response than control subjects with remote infection (p < 0.001) (Figure 1B). On the other hand, no difference was observed in the three groups for the HCMV-specific CD4+ and CD8+ T-cell responses detected by the other stimuli (Figure 1A,B).
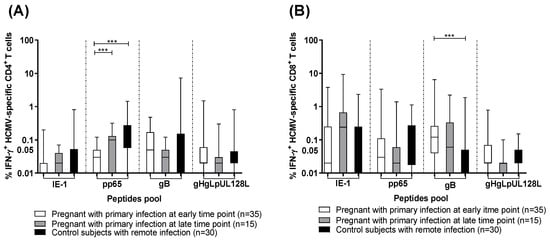
Figure 1.
Percentage of HCMV-specific T cells producing IFN-γ after stimulation with IE-1, pp65, gB, gHgLpUL128L in pregnant women with HCMV primary infection at the early and the late time points, and in control subjects with HCMV remote infection. (A) Antigen-specific IFN-γ+ CD4+ T cells and (B) IFN-γ+ CD8+ T cells. Early time point: median: 60; (IQR49-65) days after onset infection. Late time point: median: 360; (IQR 356-412) days after onset infection. *** p < 0.001. Dash lines divide the different peptides pool.
3.2. Frequencies of Responders to Different HCMV Peptides Pool
We calculate the number of pregnant women and controls responding to different stimuli, considering the IFN-γ CD4+ and CD8+ T-cell response negative with a p value < 0.05% and positive with a p value ≥ 0.05%. At the early time point, eight pregnant women (23%) showed a HCMV-specific CD4+ T-cell response against one or two peptide pools, seven (20%) showed a HCMV-specific CD4+ T-cell response against three peptides pool and two (5%) showed a HCMV-specific CD4+ T-cell response against four peptides pool, while ten (29%) showed no HCMV-specific CD4+ T-cell response (Figure 2A). Regarding the HCMV-specific CD8+ T-cell response, ten (29%) pregnant women showed a HCMV-specific CD8+ T-cell response against one peptide pool, 6 (17%) against two peptides pool, seven (20%) and 5 (14%) against three and four peptide pools, respectively, while seven (20%) showed no HCMV-specific CD8+ T-cell response (Figure 2B).
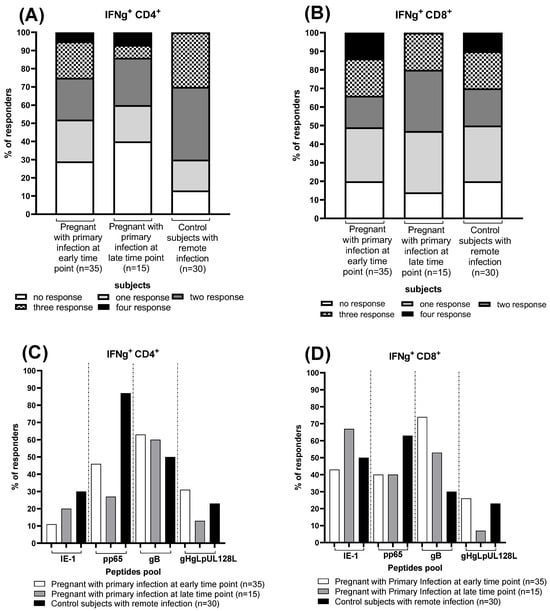
Figure 2.
Frequency of CD4+ and CD8+ T-cell responders to HCMV peptide pools (IE-1, pp65, gB and gHgLpUL128L) detected in pregnant women with HCMV primary at the early and the late time point and in control subjects with HCMV remote infection. Percentage of responders to different numbers HCMV peptides pool (A,B) and frequencies of responders to IE-1, pp65, gB and gHgLpUL128L peptides pool (C,D), are reported. Early time point: median: 60; (IQR 49–65) days after onset infection. Late time point: median: 360; (IQR 356–412) days after onset infection. Dash lines divide the different peptides pool.
Among the fifteen pregnant women with HCMV primary infection at the late time point, we observed that three (20%) had the HCMV-specific CD4+ T-cell response against one peptide pool, four (26%) against two peptide pools and 1 (7%) against three and four peptide pools, while six (40%) showed no HCMV-specific CD4+ T-cell response (Figure 2A). For HCMV-specific CD8+ T-cell response, five (33%) pregnant women showed HCMV-specific CD8+ T-cell response against one and two peptide pools, 3 (20%) against the three peptides pool and nobody against four peptides pool, while 2 (14%) showed no HCMV-specific CD8+ T-cell response (Figure 2B).
Regarding the thirty control subjects with HCMV remote infection, six (17%) showed a HCMV-specific CD4+ T-cell response against one peptide pool, 12 (40%) and nine (30%) against two and three peptides pool, respectively, and nobody against four peptide pools, while three (13%) showed no HCMV-specific CD4+ T-cell response (Figure 2A). For HCMV-specific CD8+ T-cell response, nine (30%) control subjects showed a HCMV-specific CD8+ T-cell response against one peptide pool, 6 (20%) against two and three peptides pool and three (10%) against four peptides pool, while six (20%) showed no HCMV-specific CD8+ T-cell response (Figure 2B).
Regarding the different HCMV peptide pools, the percentage of responders for each group have been reported. About 70 percent of pregnant women at the early time point, have an HCMV-specific T-cell response for gB in both CD4+ and CD8+ T cells. The pp65 CD4+ and CD8+ T-cell response is present in about 40% of them, and the same percentage of women show a CD8+ T-cell response for IE-1. Most women with primary infection at the late time point show a CD8+ T-cell response for IE-1, present in about 70%, and for gB (~50%). The CD4+ T-cell response to gB is also present in a large part of them (~60%). Finally, analyzing the control group, it emerged that almost all of them (90%) develop a pp65 CD4+ T-cell response, while pp65 CD8+ T-cell response is found in nearly 70% of them. gHgLpUL128L-specific CD4+ and CD8+ T-cell response is the one found the less in all groups of subjects (Figure 2C,D).
3.3. CD45RA+ Effector Memory, IL-7R+ Long-Term Memory and IL2+ Producing CD4+ and CD8+ T Cells
In addition to the HCMV-specific IFN-γ+ T-cell response, we analyzed the different CD4+ and CD8+ T-cell phenotypes. The percentage of IFN-γ producing CD4+ (Figure 3A) and CD8+ (Figure 3B) T cells showing a terminally differentiated effector memory phenotype (CD45RA+) remained constant in pregnant women with primary infection at the early and the late time points and in subjects with remote infection. The long-term memory phenotype of pp65 and gB-specific CD4+ T cells according to IL-7R expression was more represented in control subjects with remote infection than in pregnant women with primary infection at early and late time points (Figure 3C). Similarly, the percentage of IE-1, pp65, and gB-specific CD8+ T cells expressing IL-7R+ was higher in control subjects with remote infection than in pregnant women with HCMV primary infection at the early and late time points (Figure 3D).
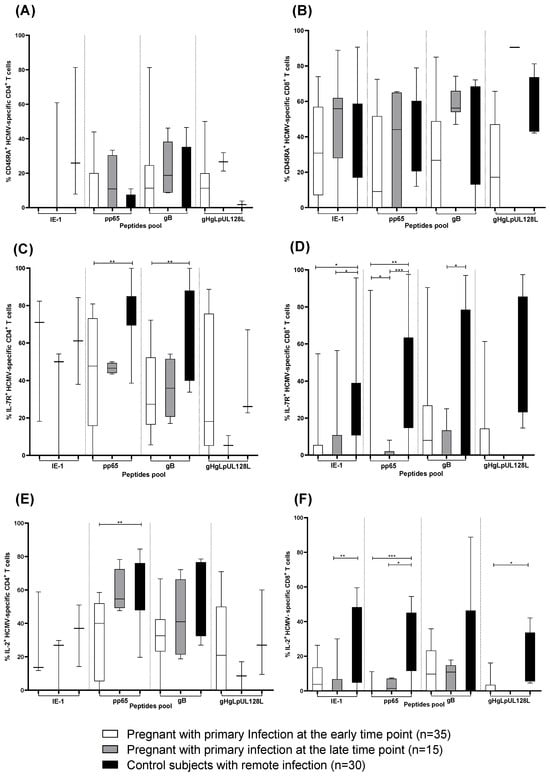
Figure 3.
IE1, pp65, gB and gHgLpUL128L-specific CD4+ and CD8+ T cells expressing (A,B) CD45RA+, (C,D) IL-7R+ and (E,F) producing IL-2 in pregnant women with HCMV primary infection at the early and late time point and in subjects with remote infection. Early time point: median: 60; (IQR 49–65) days after onset infection. Late time point: median: 360; (IQR 356–412) days after onset infection. * p < 0.05, ** p < 0.01, *** p < 0.001. Dash lines divide the different peptides pool.
The percentage of CD4+ T cells producing IL-2 was higher after stimulation with pp65 peptide pool in control subjects with remote infection than in pregnant women with HCMV primary infection at the early and late time points (Figure 3E). Instead, the percentage of CD8+ T cells producing IL-2 was higher after stimulation with IE-1, pp65, and gHgLpUL12L peptide pools in control subjects with remote infection than in pregnant women with HCMV primary infection at the early and late time points (Figure 3F).
3.4. HCMV-Specific T-Cell Response after Primary Infection and Virus Transmission to the Fetus
To verify whether a particular antigen-specific CD4+ or CD8+ T-cell response is associated with a lower risk of virus transmission to the fetus, the percentage HCMV-specific IFN-γ+ CD4+and CD8+ T cells after stimulation with IE-1, pp65, gB and gHgLpUL128L peptide pools was compared between 24 non-transmitting and 11 transmitting pregnant women at the early time point (Figure 4). No difference was observed for any antigen-specific CD4+ or CD8+ T-cell response between non-transmitting and transmitting pregnant women (Figure 4A,B).
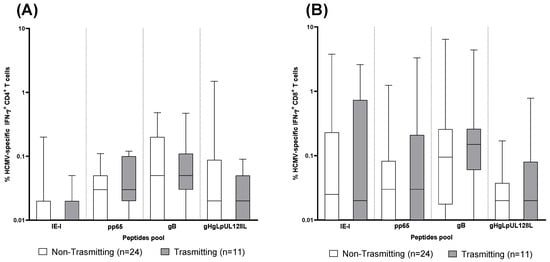
Figure 4.
CD4+ and CD8+ T-cell response to human cytomegalovirus (HCMV) peptides pool proteins (IE-1, pp65, gB and gHgLpUL128L) detected in 24 non-transmitting and 11 transmitting pregnant women at the early time point. (A) IFNγ+ CD4+ T cells. (B) IFNγ+ CD8+ T cells. Early time point: median: 60; (IQR 49–65) days after onset infection.
4. Discussion
In this study, we evaluated the antigen-specific T-cell response against different HCMV peptide pools (IE-1, pp65, gB, and gHgLpUL128L) to identify the ideal components of a vaccine. We analyzed pregnant women with HCMV primary infection at the early and late time points and control subjects with remote infection. We also investigated the correlation between the antigen-specific T-cell response and the virus transmission to the fetus.
In general, we observed that the antigen-specific CD4+ and CD8+ T-cell response was detected after IE-1, pp65, and gB stimulation. In particular, pp65 was the immunodominant target of CD4+ T cells in control subjects with remote infection, whereas gB was the immunodominant target of CD8+ T cells in pregnant women with primary infection at the early time point. Regarding the CD4+ and CD8+ T-cell phenotypes, we observed that HCMV-specific CD4+ and CD8+ T cells with a terminally differentiated effector memory phenotype (CD45RA+) remained constant in pregnant women with primary infection at the early and late time points and in subjects with remote infection, whereas HCMV-specific CD4+ and CD8+ T cells expressing IL-7R+ or producing IL-2 were higher in control subjects with remote infection than in pregnant women with HCMV primary infection. Regarding the risk of virus transmission to the fetus, no difference in HCMV-specific CD4+ and CD8+ T-cell response was observed between non-transmitting and transmitting pregnant women after stimulation with IE-1, pp65, gB, and gHgLpUL128L peptide pools.
In a previous study, the frequencies of antigen-specific T cell, in pregnant women with primary HCMV infection were determined using T cell libraries [22]. We showed that pp65 and gB proteins were recognized by CD4+ T cells, and IE-1 and pp65 proteins were recognized by CD8+ T cells; the pattern of T cell reactivity was the same in the early and late phases of infection [22]. Also, our results showed that pp65 was the immunodominant target for CD4+ in remote infection, whereas gB was the immunodominant target for CD8+ in primary infection. In another study, Fornara et al. used the cultured EliSpot to identify HCMV-specific T cells with proliferative capacity after stimulation with IE-1, IE-2, and pp65 peptides pools in pregnant women with primary HCMV infection [28]. Pregnant women tested in the second month after the onset of infection had a significantly lower response to the IE-1, IE-2, and pp65 than those with remote infection; however, pp65 was the immunodominant target of CD4+ T cells during primary infection [28]. The antigen-specific T-cell response after stimulation with IE-1 and pp65 peptide pools was also assessed in another study using the standard EliSpot assay [29]. The antigen-specific T-cell response to IE-1 and pp65 peptide pools was comparable at early and late time points was comparable [29]. It is also worth noting that the EliSpot assay targets both CD4+ and CD8+ T cells but does not discriminate between them. However, in some cases, it is important to distinguish between CD4+ and CD8+ T-cell responses. For example, in transplant patients, the long-term protection from HCMV infection is achieved when the CD4+ T-cell response is restored [30]; moreover, CD8+ T cells do not appear to be protective in the absence of the CD4+ T-cell counterpart [30].
The HCMV-specific CD4+ T cells expressing CD45RA+, measured by a flow-cytometry-based assay using HCMV-infected DC (CFC-iDC) as stimulus, showed a more rapid increase during the first month after primary infection [24], whereas the increase in CD8+ T cells expressing CD45RA+ was less evident [24]. In our results, the HCMV-specific CD4+ and CD8+T cells expressing CD45RA+, measured after stimulation with IE-1, pp65, gB, and gHgLpUL128L peptides pool, remained constant over time. This could be due to the small number of patients analyzed.
However, we observed that the HCMV-specific CD4+ and CD8+ T cells expressing IL-7R+ [31,32,33] or producing IL-2 after stimulation with IE-1, pp65, gB, and gHgLpUL128L peptide pools, showed an increase over time. The same results were observed in the other studies using HCMV-infected DC as stimulus [20,21,22]. Regarding the association between antigen-specific T cells and transmission of HCMV to the fetus, two previous studies showed that the frequencies of CD4+ and CD8+ T cells specific for IE-1, pp65, gB, and gHgLpUL128L were not different between transmitting and non-transmitting women [17].Similar results were found in our study. On the other hand, using cultured EliSpot, a significantly higher proliferative T-cell response was observed with pp65 but not with IE-1 or IE-2 [28].
The limitations of our study are the small number of patients examined, the wide range of days on which samples were taken from pregnant women with primary infection and collected in the early and late phases of infection, and the presence of both male and female control subjects with remote infection. The added value was the contemporary analysis of T-cell response against different HCMV peptide pool proteins and the CD4+ and CD8+ T-cell phenotypes.
Our study confirms no association between a particular antigenic specificity of the HCMV-specific T-cell response and transmission of HCMV to the fetus. The T-cell response was higher against gB and pp65 proteins in the early and late phases of infection in pregnant women with primary infection and in seropositive subjects, respectively, therefore, these proteins should be taken into consideration as candidates for a protective vaccine. The T-cell response is important in pregnancy and in immunocompromised patients, in particular the role of CD4+ T cells has become increasingly important. Immunocompromised patients who recovered both HCMV-specific CD4+ and CD8+ T cells were able to efficiently control HCMV replication in the blood [21,30,34,35,36,37,38]. In conclusion, a theoretically optimal recombinant HCMV vaccine composition should include the pp65 and gB proteins, which induce a protective T-cell response, and the pentameric complex gHgLpUL128L for the neutralizing antibody response [17,39,40].
Author Contributions
D.L. designed the study. F.Z. analyzed, interpreted the data, and drafted the manuscript. F.Z. and C.F. collected and managed the data. F.Z., P.d., P.Z. and G.C. methodology. M.F., A.A. and A.S. enrolled the participants. D.L., F.Z. and P.d. revised and edited the manuscript. A.S. and F.B. supervised the study. A.A. formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by Ministero della Salute, Ricerca Corrente (Grant no. 053618), Ministero della Salute, Ricerca Finalizzata (Grant no. PE 2016-02362470).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Ethics Committee Area Pavia protocol code P-20180075214 (approval date 3 October 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cannon, M.J.; Davis, K.F. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 2005, 5, 70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Britt, W. HCMV: Pathogenesis and disease consequences. In Human Herpesviurses: Biology, Therapy, and Immunoprophylaxis; Arvin, A.C.-F.G., Mocarski, E., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007; Volume 1. [Google Scholar]
- Dollard, S.C.; Grosse, S.D.; Ross, D.S. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 2007, 17, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.B.; Stagno, S.; Pass, R.F.; Britt, W.J.; Boll, T.J.; Alford, C.A. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N. Engl. J. Med. 1992, 326, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Townsend, C.L.; Forsgren, M.; Ahlfors, K.; Ivarsson, S.A.; Tookey, P.A.; Peckham, C.S. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin. Infect. Dis. 2013, 56, 1232–1239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mussi-Pinhata, M.M.; Yamamoto, A.Y.; Moura Brito, R.M.; de Lima Isaac, M.; de Carvalho e Oliveira, P.F.; Boppana, S.; Britt, W.J. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin. Infect. Dis. 2009, 49, 522–528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hicks, T.; Fowler, K.; Richardson, M.; Dahle, A.; Adams, L.; Pass, R. Congenital cytomegalovirus infection and neonatal auditory screening. J. Pediatr. 1993, 123, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.; Ahlfors, K.; Ivarsson, S.; Lernmark, B.; Svanberg, L. Congenital cytomegalovirus infection and sensorineural hearing loss. Ear Hear. 1984, 5, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Dahle, A.J.; Fowler, K.B.; Wright, J.D.; Boppana, S.B.; Britt, W.J.; Pass, R.F. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J. Am. Acad. Audiol. 2000, 11, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.B.; Boppana, S.B. Congenital cytomegalovirus (CMV) infection and hearing deficit. J. Clin. Virol. 2006, 35, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Dar, L.; Pati, S.K.; Patro, A.R.; Deorari, A.K.; Rai, S.; Kant, S.; Broor, S.; Fowler, K.B.; Britt, W.J.; Boppana, S.B. Congenital cytomegalovirus infection in a highly seropositive semi-urban population in India. Pediatr. Infect. Dis. J. 2008, 27, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Foulon, I.; Naessens, A.; Foulon, W.; Casteels, A.; Gordts, F. A 10-year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. J. Pediatr. 2008, 153, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.A.; Fowler, K.B.; Ashrith, G.; Stagno, S.; Britt, W.J.; Pass, R.F.; Boppana, S.B. Hearing loss in children with congenital cytomegalovirus infection born to mothers with preexisting immunity. J. Pediatr. 2006, 148, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Demmler, G.J. Infectious Diseases Society of America and Centers for Disease Control. Summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev. Infect. Dis. 1991, 13, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Britt, W.J.; Alford, C.A. Cytomegalovirus. In Fields Virology, 3rd ed.; Fields, B.N., Knipe, D.M., Howley, P.M., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1996; Volume 2, pp. 2493–2523. [Google Scholar]
- Stagno, S. Cytomegalovirus. In Infectious Diseases of the Fetus and Newborn Infant, 6th ed.; Remington, J.S., Klein, J.O., Wilson, C.B., Baker, C.J., Eds.; W. B. Saunders: Philadelphia, PA, USA, 2006; pp. 389–424. [Google Scholar]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Stagno, S.; Pass, R.F.; Cloud, G.; Britt, W.J.; Henderson, R.E.; Walton, P.D.; Veren, D.A.; Page, F.; Alford, C.A. Primary cytomegalovirus infection in pregnancy: Incidence, transmission to the fetus, and clinical outcome. JAMA 1986, 256, 1904–1908. [Google Scholar] [CrossRef]
- Stagno, S.; Pass, R.F.; Dworsky, M.E.; Henderson, R.E.; Moore, E.G.; Walton, P.D.; Alford, C.A. Congenital cytomegalovirus infection: The relative importance of primary and recurrent maternal infection. N. Engl. J. Med. 1986, 306, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Britt, W.J. Congenital Human Cytomegalovirus Infection and the Enigma of Maternal Immunity. J. Virol. 2017, 91, e02392-16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nelson, C.S.; Baraniak, I.; Lilleri, D.; Reeves, M.B.; Griffiths, P.D.; Permar, S.R. Immune Correlates of Protection Against Human Cytomegalovirus Acquisition, Replication, and Disease. J. Infect. Dis. 2020, 221 (Suppl. S1), S45–S59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mele, F.; Fornara, C.; Jarrossay, D.; Furione, M.; Arossa, A.; Spinillo, A.; Lanzavecchia, A.; Gerna, G.; Sallusto, F.; Lilleri, D. Phenotype and specificity of T cells in primary human cytomegalovirus infection during pregnancy: IL-7Rpos long-term memory phenotype is associated with protection from vertical transmission. PLoS ONE 2017, 12, e0187731. [Google Scholar] [CrossRef]
- Geginat, J.; Lanzavecchia, A.; Sallusto, F. Proliferation and differentiation potential of human CD8 memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 2003, 101, 4260–4266. [Google Scholar] [CrossRef]
- Fornara, C.; Furione, M.; Zavaglio, F.; Arossa, A.; Spinillo, A.; Gerna, G.; Lilleri, D. Slow cytomegalovirus-specific CD4+ and CD8+ T-cell differentiation: 10-year follow-up of primary infection in a small number of immunocompetent hosts. Eur. J. Immunol. 2021, 51, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A.; Starr, S.E.; Friedman, H.M.; Gonczol, E.; Brayman, K. Vaccines for the prevention of human cytomegalovirus infection. Rev. Infect. Dis. 1990, 12 (Suppl. S7), S827–S838. [Google Scholar] [CrossRef] [PubMed]
- Revello, M.G.; Furione, M.; Rognoni, V.; Arossa, A.; Gerna, G. Cytomegalovirus DNAemia in pregnant women. J. Clin. Virol. 2014, 61, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Furione, M.; Rognoni, V.; Cabano, E.; Baldanti, F. Kinetics of human cytomegalovirus (HCMV) DNAemia in transplanted patients expressed in international units as determined with the Abbott RealTime CMV assay and an in-house assay. J. Clin. Virol. 2012, 55, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Fornara, C.; Cassaniti, I.; Zavattoni, M.; Furione, M.; Adzasehoun, K.M.G.; De Silvestri, A.; Comolli, G.; Baldanti, F. Human Cytomegalovirus-Specific Memory CD4+ T-Cell Response and Its Correlation with Virus Transmission to the Fetus in Pregnant Women with Primary Infection. Clin. Infect. Dis. 2017, 65, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Chiereghin, A.; Verucchi, G.; Lazzarotto, T. CMV-Specific Cell-Mediated Immunity in Immunocompetent Adults with Primary CMV Infection: A Case Series and Review of the Literature. Viruses 2021, 13, 816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sester, U.; Gärtner, B.C.; Wilkens, H.; Schwaab, B.; Wössner, R.; Kindermann, I.; Girndt, M.; Meyerhans, A.; Mueller-Lantzsch, N.; Schäferse, H.-J.; et al. Differences in CMV-specific T-cell levels and long-term susceptibility to CMV infection after kidney, heart and lung transplantation. Am. J. Transplant. 2005, 5, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Huster, K.M.; Busch, V.; Schiemann, M.; Linkemann, K.; Kerksiek, K.M.; Wagner, H.; Busch, D.H. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 2004, 101, 5610–5615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaech, S.M.; Tan, J.T.; Wherry, E.J.; Konieczny, B.T.; Surh, C.D.; Ahmed, R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003, 4, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, E.M.M.; de Bree, G.J.; Remmerswaal, E.B.M.; Yong, S.-L.; Tesselaar, K.; Berge, I.J.M.T.; van Lier, R.A.W. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood 2005, 106, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Gamadia, L.E.; Remmerswaal, E.B.; Weel, J.F.; Bemelman, F.; van Lier, R.A.; Ten Berge, I.J. Primary immune responses to human CMV: A critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood 2003, 101, 2686–2692. [Google Scholar] [CrossRef] [PubMed]
- Pourgheysari, B.; Piper, K.P.; McLarnon, A.; Arrazi, J.; Bruton, R.; Clark, F.; Cook, M.; Mahendra, P.; Craddock, C.; Moss, P.A.H. Early reconstitution of effector memory CD4+ CMV-specific T cells protects against CMV reactivation following allogeneic SCT. Bone Marrow Transplant. 2009, 43, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Widmann, T.; Sester, U.; Gärtner, B.C.; Schubert, J.; Pfreundschuh, M.; Köhler, H.; Sester, M. Levels of CMV specific CD4 T cells are dynamic and correlate with CMV viremia after allogeneic stem cell transplantation. PLoS ONE 2008, 3, e3634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meyers, J.D.; Flournoy, N.; Thomas, E.D. Cytomegalovirus infection and specific cell-mediated immunity after marrow transplant. J. Infect. Dis. 1980, 142, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Reusser, P.; Riddell, S.R.; Meyers, J.D.; Greenberg, P.D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: Pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 1991, 78, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Macagno, A.; Bernasconi, N.L.; Vanzetta, F.; Dander, E.; Sarasini, A.; Revello, M.G.; Gerna, G.; Sallusto, F.; Lanzavecchia, A. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J. Virol. 2010, 84, 1005–1013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kabanova, A.; Perez, L.; Lilleri, D.; Marcandalli, J.; Agatic, G.; Becattini, S.; Preite, S.; Fuschillo, D.; Percivalle, E.; Sallusto, F.; et al. Antibody-driven design of a human cytomegalovirus gHgLpUL128L subunit vaccine that selectively elicits potent neutralizing antibodies. Proc. Natl. Acad. Sci. USA 2014, 111, 17965–17970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).