Behavioral Alterations of Spatial Cognition and Role of the Apolipoprotein E-ε4 in Patients with MCI Due to Alzheimer’s Disease: Results from the BDSC-MCI Project
Abstract
1. Introduction
Aims of the Study
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.2.1. Clinical Assessment
2.2.2. ApoE Genotyping
2.2.3. Gait Assessment
2.3. Statistics
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Colombo, D.; Serino, S.; Tuena, C.; Pedroli, E.; Dakanalis, A.; Cipresso, P.; Riva, G. Egocentric and Allocentric Spatial Reference Frames in Aging: A Systematic Review. Neurosci. Biobehav. Rev. 2017, 80, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Burgess, N. Spatial Memory: How Egocentric and Allocentric Combine. Trends Cogn. Sci. 2006, 10, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Tolman, E.C. Cognitive Maps in Rats and Men. Psychol. Rev. 1948, 55, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Tuena, C.; Mancuso, V.; Stramba-Badiale, C.; Pedroli, E.; Stramba-Badiale, M.; Riva, G.; Repetto, C. Egocentric and Allocentric Spatial Memory in Mild Cognitive Impairment with Real-World and Virtual Navigation Tasks: A Systematic Review. J. Alzheimer’s Dis. 2021, 79, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Burgess, N. Spatial Cognition and the Brain. Ann. N. Y. Acad. Sci. 2008, 1124, 77–97. [Google Scholar] [CrossRef]
- Doeller, C.F.; Burgess, N. Distinct error-correcting and incidental learning of location relative to landmarks and boundaries. Proc. Natl. Acad. Sci. USA 2008, 105, 5909–5914. [Google Scholar] [CrossRef]
- Coughlan, G.; Laczó, J.; Hort, J.; Minihane, A.M.; Hornberger, M. Spatial navigation deficits—Overlooked cognitive marker for preclinical Alzheimer disease? Nat. Rev. Neurol. 2018, 14, 496–506. [Google Scholar] [CrossRef]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Tiraboschi, P.; Hansen, L.A.; Masliah, E.; Alford, M.; Thal, L.J.; Corey-Bloom, J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology 2004, 62, 1977–1983. [Google Scholar] [CrossRef]
- Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein e and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Berlau, D.J.; Corrada, M.M.; Head, E.; Kawas, C.H. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology 2009, 72, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.E.; Huey, E.D.; Devanand, D.P. Association of APOE e2 genotype with Alzheimer’s and non-Alzheimer’s neurodegenerative pathologies. Nat. Commun. 2020, 11, 4727. [Google Scholar] [CrossRef] [PubMed]
- Cammisuli, D.M.; Tuena, C.; Riva, G.; Repetto, C.; Axmacher, N.; Chandreswaran, V.; Isella, V.; Pomati, S.; Zago, S.; Difonzo, T.; et al. Exploring the Remediation of Behavioral Disturbances of Spatial Cognition in Community-Dwelling Senior Citizens with Mild Cognitive Impairment via Innovative Technological Apparatus (BDSC-MCI Project): Protocol for a Prospective, Multi-Center Observational Study. J. Pers. Med. 2024, 14, 192. [Google Scholar] [CrossRef]
- Montero-Odasso, M.M.; Barnes, B.; Speechley, M.; Muir Hunter, S.W.; Doherty, T.J.; Duque, G.; Gopaul, K.; Sposato, L.A.; Casas-Herrero, A.; Borrie, M.J.; et al. Disentangling Cognitive-Frailty: Results from the Gait and Brain Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1476–1482. [Google Scholar] [CrossRef]
- Dumurgier, J.; Artaud, F.; Touraine, C.; Rouaud, O.; Tavernier, B.; Dufouil, C.; Singh-Manoux, A.; Tzourio, C.; Elbaz, A. Gait speed and decline in gait speed as predictors of incident dementia. J. Gerontol. Ser. A 2017, 72, 655–661. [Google Scholar] [CrossRef]
- Sakurai, R.; Watanabe, Y.; Osuka, Y.; Taniguchi, Y.; Kawai, H.; Kim, H.; Kitamura, A.; Inagaki, H.; Montero-Odasso, M.; Awata, S.; et al. Overlap Between Apolipoprotein Eε4 Allele and Slowing Gait Results in Cognitive Impairment. Front. Aging. Neurosci. 2019, 11, 247. [Google Scholar] [CrossRef]
- Sakurai, R.; Montero-Odasso, M. Apolipoprotein E4 Allele and Gait Performance in Mild Cognitive Impairment: Results from the Gait and Brain Study. J. Gerontol. Ser. A. 2017, 72, 1676–1682. [Google Scholar] [CrossRef]
- Ghosh, A.; Puthusseryppady, V.; Chan, D.; Mascolo, C.; Hornberger, M. Machine learning detects altered spatial navigation features in outdoor behaviour of Alzheimer’s disease patients. Sci. Rep. 2022, 12, 3160. [Google Scholar] [CrossRef]
- Puthusseryppady, V.; Morrissey, S.; Spiers, H.; Patel, M.; Hornberger, M. Predicting real world spatial disorientation in Alzheimer’s disease patients using virtual reality navigation tests. Sci. Rep. 2022, 12, 13397. [Google Scholar] [CrossRef]
- Mc Ardle, R.; Morris, R.; Hickey, A.; Del Din, S.; Koychev, I.; Gunn, R.N.; Lawson, J.; Zamboni, G.; Ridha, B.; Sahakian, B.J.; et al. Gait in Mild Alzheimer’s Disease: Feasibility of Multi-Center Measurement in the Clinic and Home with Body-Worn Sensors: A Pilot Study. J. Alzheimer’s Dis. 2018, 63, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Saykin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Prichep, L.; Mosconi, L.; John, E.R.; Glodzik-Sobanska, L.; Boksay, I.; Monteiro, I.; Torossian, C.; Vedvyas, A.; Ashraf, N.; et al. The pre–mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimer’s Dement. 2008, 4, S98–S108. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Gauthier, S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int. Psychogeriatr. 2008, 20, 1–16. [Google Scholar] [CrossRef]
- Cerman, J.; Andel, R.; Laczo, J.; Vyhnalek, M.; Nedelska, Z.; Mokrisova, I.; Sheardova, K.; Hort, J. Subjective Spatial Navigation Complaints—A Frequent Symptom Reported by Patients with Subjective Cognitive Decline, Mild Cognitive Impairment and Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 15, 219–228. [Google Scholar] [CrossRef]
- Rabin, L.A.; Smart, C.M.; Amariglio, R.E. Subjective Cognitive Decline in Preclinical Alzheimer’s Disease. Annu. Rev. Clin. Psychol. 2017, 13, 369–396. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, L.; Blennow, K.; Dekosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Measso, G.; Cavarzeran, F.; Zappalà, G.; Lebowitz, B.D.; Crook, T.H.; Pirozzolo, F.J.; Amaducci, L.A.; Massari, D.; Grigoletto, F. The mini-mental state examination: Normative study of an Italian random sample. Dev. Neuropsychol. 1993, 9, 77–85. [Google Scholar] [CrossRef]
- Magni, E.; Binetti, G.; Bianchetti, A.; Rozzini, R.; Trabucchi, M. Mini-mental state examination: A normative study in Italian elderly population. Eur. J. Neurol. 1996, 3, 198–202. [Google Scholar] [CrossRef]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef]
- Aiello, E.N.; Gramegna, C.; Esposito, A.; Gazzaniga, V.; Zago, S.; Difonzo, T.; Maddaluno, O.; Appollonio, I.; Bolognini, N. The Montreal Cognitive Assessment (MoCA): Updated norms and psychometric insights into adaptive testing from healthy individuals in Northern Italy. Aging Clin. Exp. Res. 2022, 34, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A. Geriatric depression scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar] [PubMed]
- Kim, H.Y. Statistical notes for clinical researchers: Assessing normal distribution (2) using skewness and kurtosis. Restor. Dent. Endod. 2013, 38, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Green, J.A. Too many zeros and/or highly skewed? A tutorial on modelling health behaviour as count data with Poisson and negative binomial regression. Health. Psychol. Behav. Med. 2021, 9, 436–455. [Google Scholar] [CrossRef]
- Cammisuli, D.M.; Isella, V.; Verde, F.; Silani, V.; Ticozzi, N.; Pomati, S.; Bellocchio, V.; Granese, V.; Vignati, B.; Marchesi, G.; et al. Behavioral Disorders of Spatial Cognition in Patients with Mild Cognitive Impairment due to Alzheimer’s Disease: Preliminary Findings from the BDSC-MCI Project. J. Clin. Med. 2024, 13, 1178. [Google Scholar] [CrossRef]
- Jessen, F.; Han, Y.; Jiang, J. Editorial: Exploring reliable markers and prediction indexes for the progression from subjective cognitive decline to cognitive impairment, volume II. Front. Aging Neurosci. 2023, 15, 1208478. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, F.; Long, C.; Zhu, Y.; Jiang, Y.; Zhu, Z.; Lu, J.; Zhang, X.; Nedelska, Z.; Hort, J.; et al. Spatial navigation is associated with subcortical alterations and progression risk in subjective cognitive decline. Alzheimer’s Res. Ther. 2023, 15, 86. [Google Scholar] [CrossRef]
- Patai, E.Z.; Spiers, H.J. The Versatile Wayfinder: Prefrontal Contributions to Spatial Navigation. Trends. Cogn. Sci. 2021, 25, 520–533. [Google Scholar] [CrossRef]
- Laczó, J.; Andel, R.; Vyhnalek, M.; Vlcek, K.; Nedelska, Z.; Matoska, V.; Gazova, I.; Mokrisova, I.; Sheardova, K.; Hort, J. APOE and spatial navigation in amnestic MCI: Results from a computer-based test. Neuropsychology 2014, 28, 676–684. [Google Scholar] [CrossRef]
- Laczó, J.; Andel, R.; Vlček, K.; Mat’oška, V.; Vyhnálek, M.; Tolar, M.; Bojar, M.; Hort, J. Spatial navigation and APOE in amnestic mild cognitive impairment. Neurodegener. Dis. 2011, 8, 169–177. [Google Scholar] [CrossRef]
- Laczó, J.; Cechova, K.; Parizkova, M.; Matoska, V.; Kaplan, V.; Matuskova, V.; Markova, H.; Vyhnalek, M.; Hort, J. Spatial navigation and verbal memory are influenced by the combined effects of APOE and BDNF Val66Met polymorphisms in mild cognitive impairment. Alzheimer’s Dement. 2020, 16, e044911. [Google Scholar] [CrossRef]
- Ward, A.; Crean, S.; Mercaldi, C.J.; Collins, J.M.; Boyd, D.; Cook, M.N.; Arrighi, H.M. Prevalence of Apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: A systematic review and meta-analysis. Neuroepidemiology 2012, 38, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Abondio, P.; Sazzini, M.; Garagnani, P.; Boattini, A.; Monti, D.; Franceschi, C.; Luiselli, D.; Giuliani, C. The genetic variability of APOE in different human populations and its implications for longevity. Genes 2019, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Corbo, R.M.; Scacchi, R.; Mureddu, L.; Mulas, G.; Alfano, G. Apolipoprotein E polymorphism in Italy investigated in native plasma by a simple polyacrylamide gel isoelectric focusing technique. Comparison with frequency data of other European populations. Ann. Hum. Genet. 1995, 59, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Scacchi, R.; De Bernardini, L.; Mantuano, E.; Donini, L.M.; Vilardo, T.; Corbo, R.M. Apolipoprotein E (APOE) allele frequencies in late-onset sporadic Alzheimer’s disease (AD), mixed dementia and vascular dementia: Lack of association of ϵ4 allele with AD in Italian octogenarian patients. Neurosci. Lett. 1995, 201, 231–234. [Google Scholar] [CrossRef]
- Benedetti, M.D.; Salviati, A.; Filipponi, S.; Manfredi, M.; De Togni, L.; Gomez Lira, M.; Stenta, G.; Fincati, E.; Pampanin, M.; Rizzuto, N.; et al. Prevalence of dementia and apolipoprotein e genotype distribution in the elderly of Buttapietra, Verona province, Italy. Neuroepidemiology 2002, 21, 74–80. [Google Scholar] [CrossRef]
- Sorbi, S.; Nacmias, B.; Forleo, P.; Piacentini, S.; Amaducci, L. Alzheimer’s Disease and Apolipoprotein E in Italy. Ann. N. Y. Acad. Sci. 1996, 777, 260–265. [Google Scholar] [CrossRef]
- Sorbi, S.; Nacmias, B.; Forleo, P.; Latorraca, S.; Gobbini, I.; Bracco, L.; Piacentini, S.; Amaducci, L. ApoE allele frequencies in Italian sporadic and familial Alzheimer’s disease. Neurosci Lett. 1994, 177, 100–102. [Google Scholar] [CrossRef]
- Di Battista, M.A.; Heinsinger, N.M.; Rebeck, G.W. Alzheimer’s disease genetic risk factor APOE-ε4 also affects normal brain function. Curr. Alzheimer Res. 2016, 13, 1200–1207. [Google Scholar] [CrossRef]
- Wang, L.; Jiao, Y.; Zhao, A.; Xu, X.; Ye, G.; Zhang, Y.; Wang, Y.; Deng, Y.; Xu, W.; Liu, J. Analysis of Genetic Association Between ABCA7 Polymorphism and Alzheimer’s Disease Risk in the Southern Chinese Population. Front. Aging Neurosci. 2022, 14, 819499. [Google Scholar] [CrossRef]

| HC | SCD | MCI | P | |
|---|---|---|---|---|
| N | 15 | 23 | 20 | - |
| Sex (male/female) | 7/8 | 9/14 | 12/8 | n.s a |
| Age (years) | 74.3 ± 7.7 (64–85) | 70.2 ± 7.4 (56–80) | 74.0 ± 4.8 (62–82) | n.s b |
| Education (years) | 12.2 ± 2.7 (8–18) | 12.0 ± 4.0 (8–18) | 10.7 ± 4.6 (5–18) | n.s b |
| ApoE genotype | 0.024 a | |||
| ε2/ε3 ε2/ε4 ε3/ε3 ε3/ε4 ε4/ε4 | 0 1 12 1 1 | 1 1 17 4 0 | 1 1 5 11 2 | - - - - - |
| Genetic risk (low/high) | 12/3 | 18/5 | 6/14 | <0.001 a |
| GDS | 5.0 ± 4.2 (0–13) | 9.3 ± 5.4 (1–21) | 9.6 ± 7.5 (0–21) | n.s. b |
| MoCA (adjusted scores) c | 24.7 ± 2.3 (21.6–29.7) | 27.8 ± 3.0 (18.6–30.0) | 20.7 ± 5.0 (10.8–27.6) | SCD > HCs > MCI b |
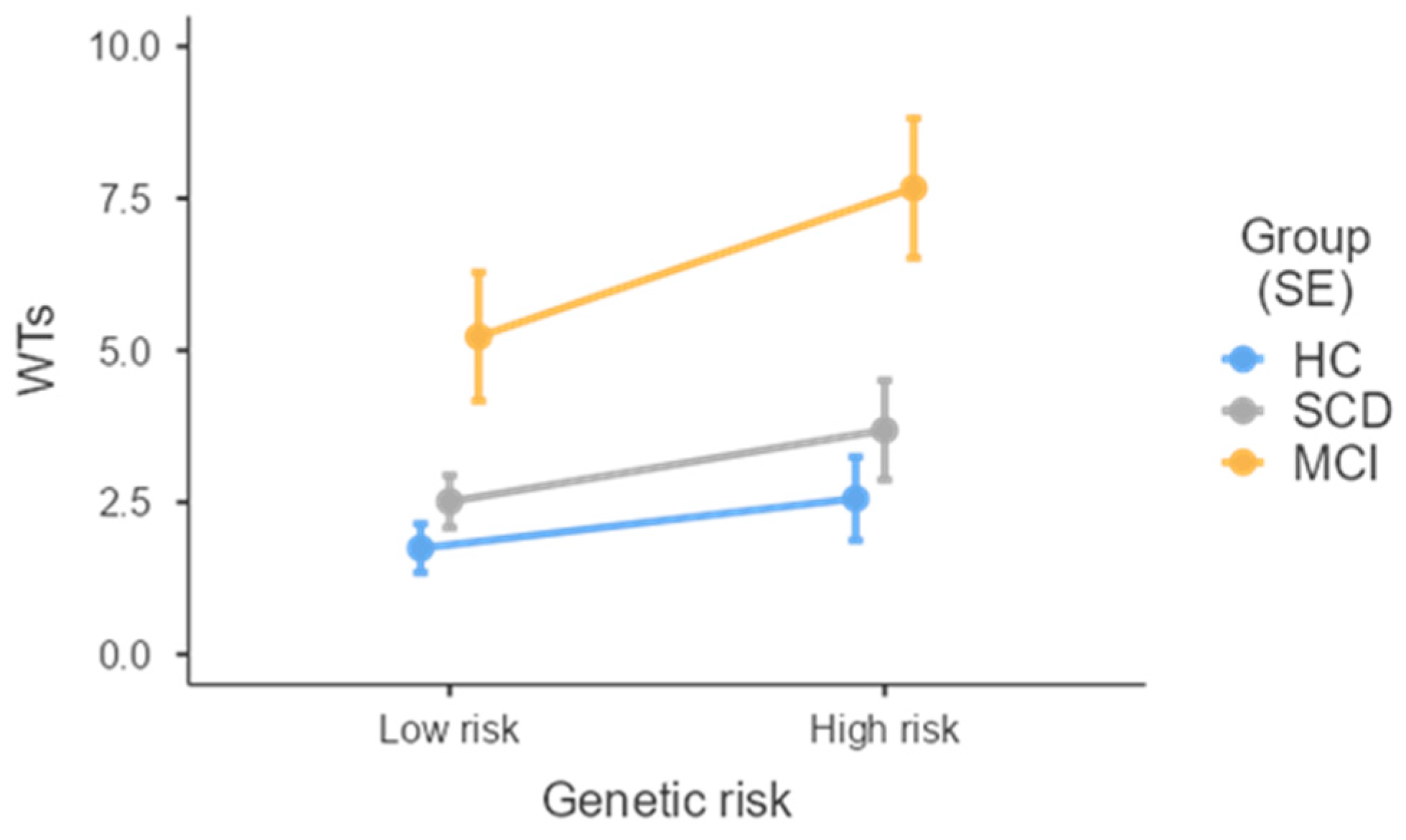
| WTs | 1.9 ± 2.1 (0–7) | 2.8 ± 2.5 (0–10) | 7.4 ± 4.2 (2–15) | MCI > HCs & SCD d |
| MsH | 13.0 ± 21.2 (0–78) | 26.6 ± 45.1 (0–170) | 105.7 ± 84.7 (0–342) | MCI > HCs d |
| TRA | 2.4 ± 0.6 (1.6–4.0) | 3.1 ± 0.9 (2.0–5.5) | 4.5 ± 1.6 (2.2–8.0) | MCI > SCD > HCs d |
| SRA | 0.6 ± 0.2 (0.3–0.9) | 0.5 ± 0.1 (0.3–0.8) | 0.4 ± 0.2 (0.2–0.8) | HC > MCI & SCD d |
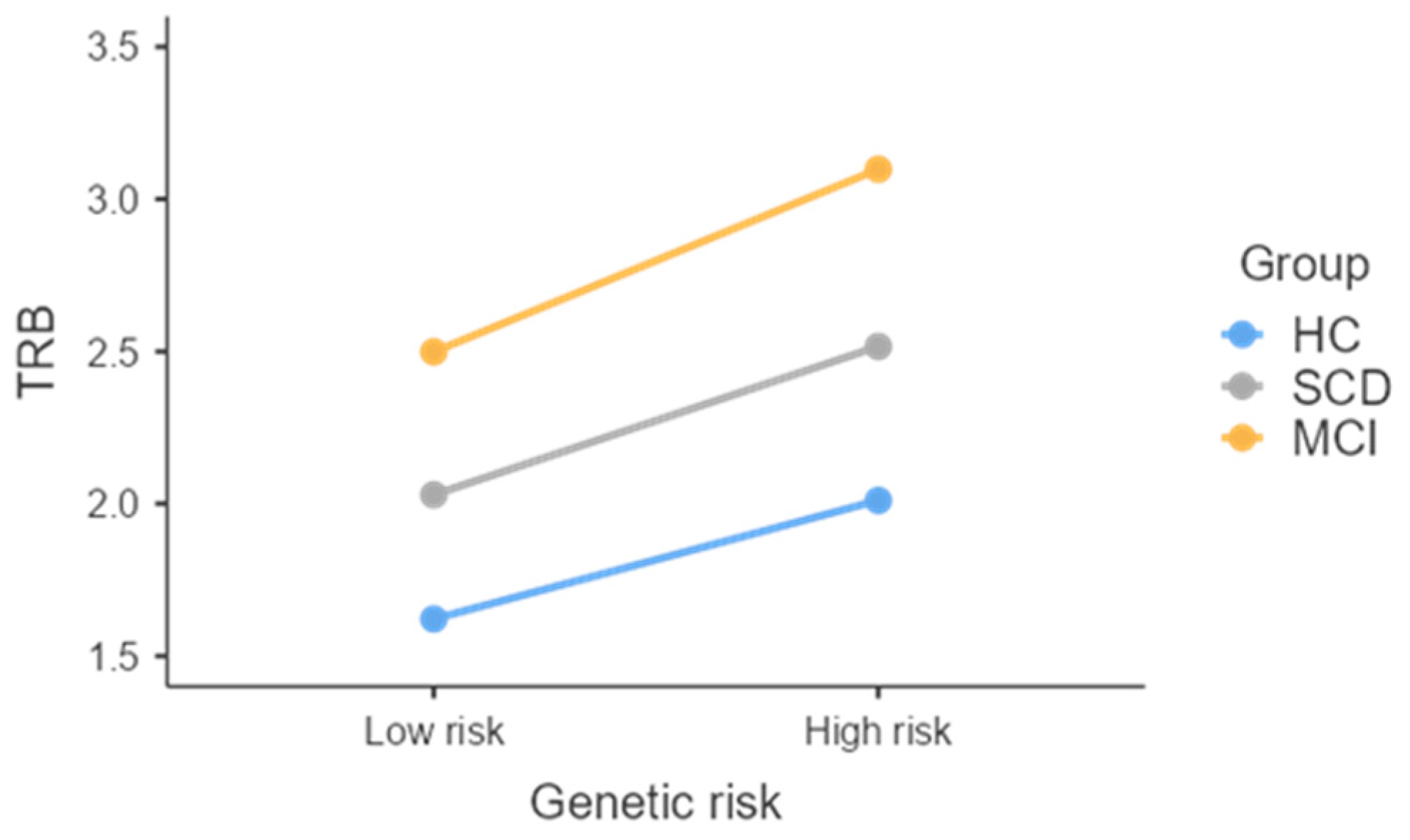
| TRB | 1.7 ± 0.38 (1.3–2.6) | 2.1 ± 0.8 (1.3–4.3) | 3.1 ± 1.7 (1.4–7.3) | MCI > HCs d |
| SRB | 0.7 ± 0.2 (0.3–1.0) | 0.5 ± 0.1 (0.3–0.6) | 0.5 ± 0.2 (0.2–0.9) | HC > MCI & SCD d |
| χ2 | p | ||
|---|---|---|---|
| WTs | |||
| Genetic risk | 3.22 | 0.073 | |
| Group | 17.97 | <0.001 | |
| MsH | |||
| Genetic risk | 2.04 | 0.153 | |
| Group | 8.76 | 0.013 | |
| TRA | |||
| Genetic risk | 5.57 | 0.018 | |
| Group | 28.40 | <0.001 | |
| SRA | |||
| Genetic risk | 0.23 | 0.635 | |
| Group | 14.93 | <0.001 | |
| TRB | |||
| Genetic risk | 3.35 | 0.067 | |
| Group | 8.80 | 0.012 | |
| SRB | |||
| Genetic risk | 0.01 | 0.926 | |
| Group | 24.56 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cammisuli, D.M.; Bellocchio, V.; Milesi, A.; Aiello, E.N.; Poletti, B.; Verde, F.; Silani, V.; Ticozzi, N.; Marchesi, G.; Granese, V.; et al. Behavioral Alterations of Spatial Cognition and Role of the Apolipoprotein E-ε4 in Patients with MCI Due to Alzheimer’s Disease: Results from the BDSC-MCI Project. J. Clin. Med. 2024, 13, 5447. https://doi.org/10.3390/jcm13185447
Cammisuli DM, Bellocchio V, Milesi A, Aiello EN, Poletti B, Verde F, Silani V, Ticozzi N, Marchesi G, Granese V, et al. Behavioral Alterations of Spatial Cognition and Role of the Apolipoprotein E-ε4 in Patients with MCI Due to Alzheimer’s Disease: Results from the BDSC-MCI Project. Journal of Clinical Medicine. 2024; 13(18):5447. https://doi.org/10.3390/jcm13185447
Chicago/Turabian StyleCammisuli, Davide Maria, Virginia Bellocchio, Alessandra Milesi, Edoardo Nicolò Aiello, Barbara Poletti, Federico Verde, Vincenzo Silani, Nicola Ticozzi, Gloria Marchesi, Valentina Granese, and et al. 2024. "Behavioral Alterations of Spatial Cognition and Role of the Apolipoprotein E-ε4 in Patients with MCI Due to Alzheimer’s Disease: Results from the BDSC-MCI Project" Journal of Clinical Medicine 13, no. 18: 5447. https://doi.org/10.3390/jcm13185447
APA StyleCammisuli, D. M., Bellocchio, V., Milesi, A., Aiello, E. N., Poletti, B., Verde, F., Silani, V., Ticozzi, N., Marchesi, G., Granese, V., Vignati, B., Isella, V., Zago, S., Difonzo, T., Pomati, S., Porta, G., Cattaldo, S., Mauro, A., & Castelnuovo, G. (2024). Behavioral Alterations of Spatial Cognition and Role of the Apolipoprotein E-ε4 in Patients with MCI Due to Alzheimer’s Disease: Results from the BDSC-MCI Project. Journal of Clinical Medicine, 13(18), 5447. https://doi.org/10.3390/jcm13185447







