Isolated Intracranial Hypertensions as Onset of Myelin Oligodendrocyte Glycoprotein Antibody Disease
Abstract
1. Introduction
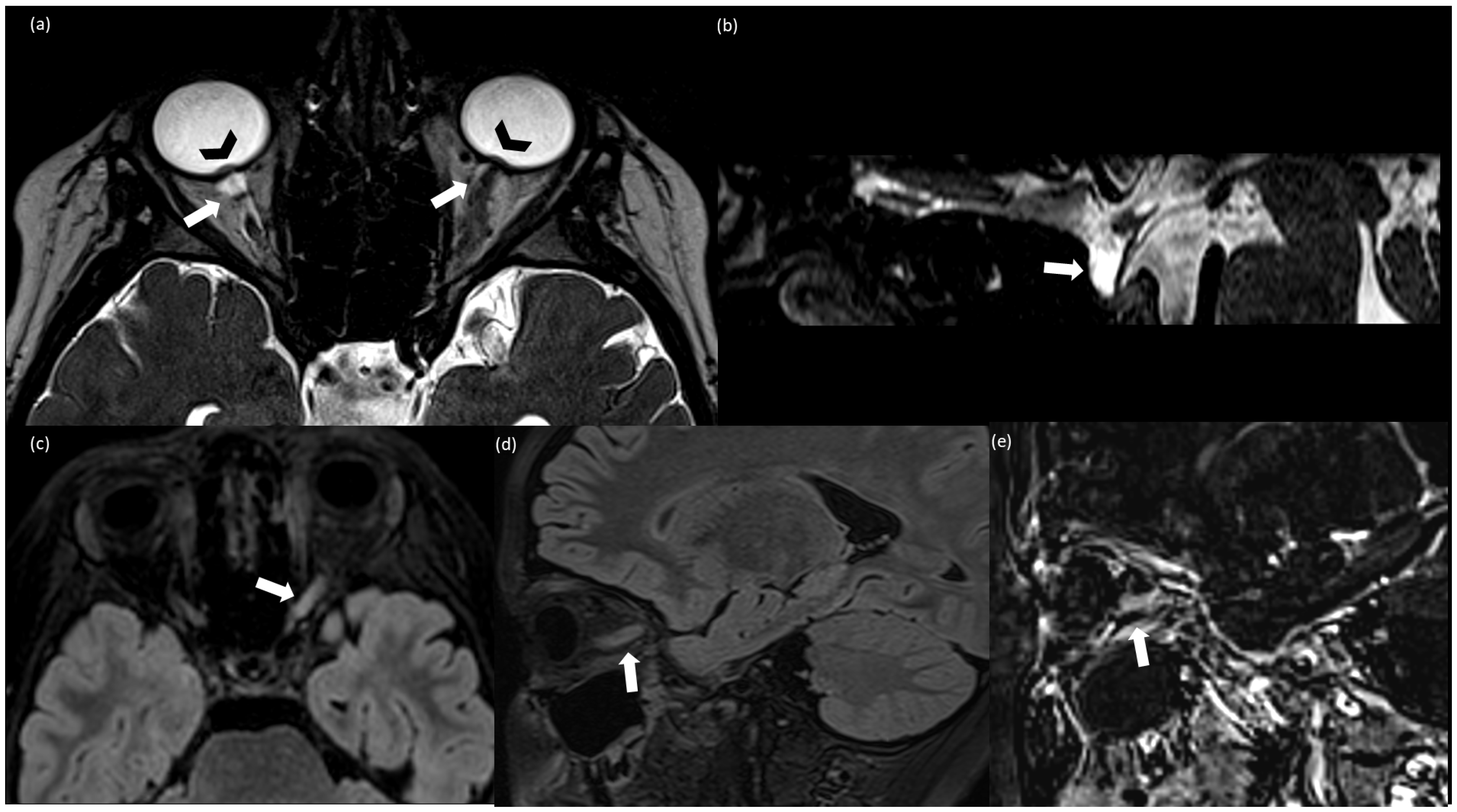
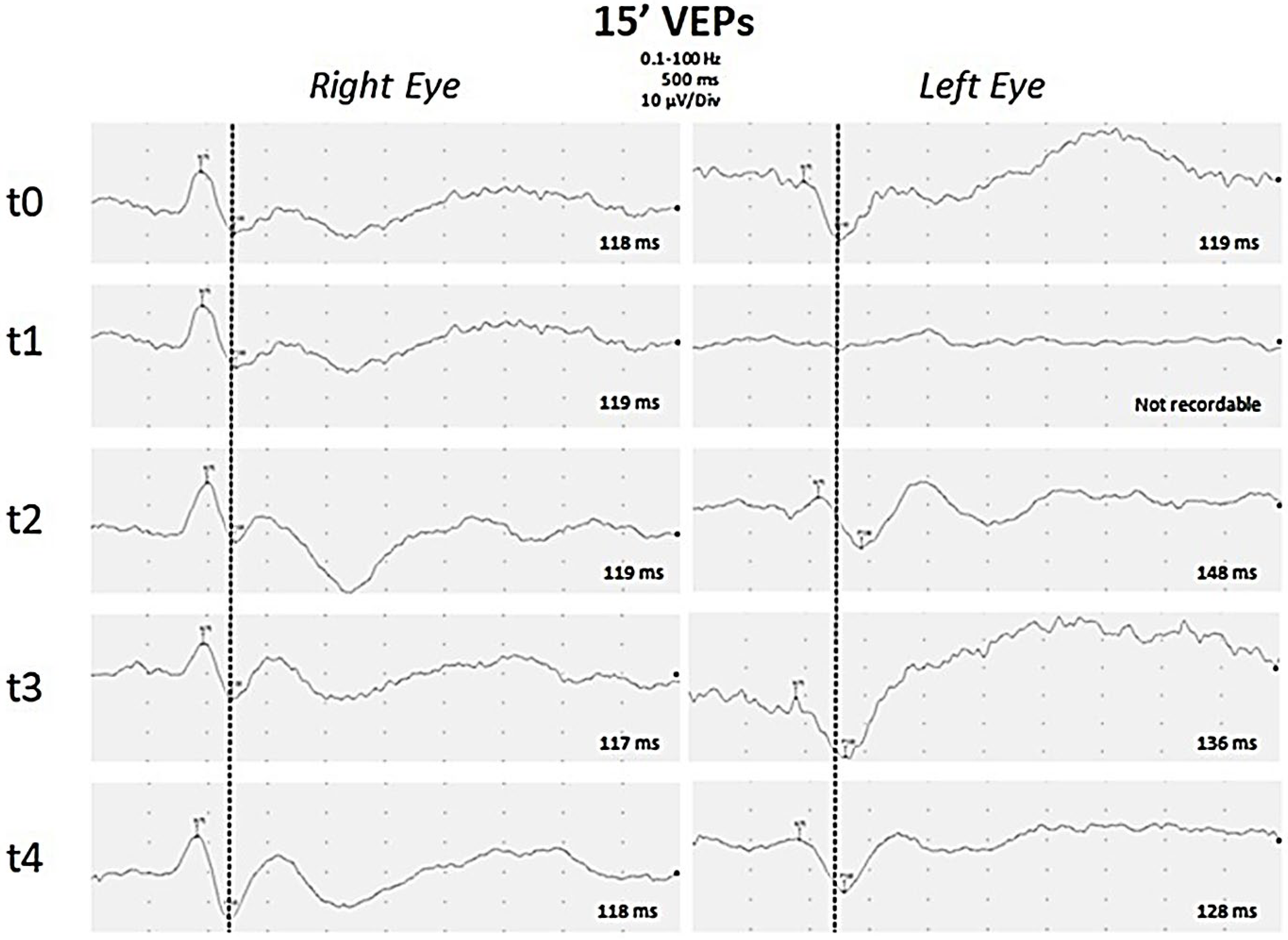
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banwell, B.; Bennett, J.L.; Marignier, R.; Kim, H.J.; Brilot, F.; Flanagan, E.P. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 2023, 22, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Yang, L.; Kessi, M.; He, F.; Zhang, C.; Wu, L.; Yin, F.; Peng, J. Myelin Oligodendrocyte Glycoprotein (MOG) Antibody Diseases in Children in Central South China: Clinical Features, Treatments, Influencing Factors, and Outcomes. Front. Neurol. 2019, 10, 868. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.N.; Wang, C.; Greenberg, B.M. Acute Disseminated Encephalomyelitis (ADEM) and Increased Intracranial Pressure Associated with Anti-Myelin Oligodendrocyte Glycoprotein Antibodies. Pediatr. Neurol. 2019, 99, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Mollan, S.P.; Aguiar, M.; Evison, F.; Frew, E.; Sinclair, A.J. The expanding burden of idiopathic intracranial hypertension. Eye 2019, 33, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef]
- Zhou, D.J.; Powers, A.M.; Cave, C.A. Perplexing Initial Presentations of MOGAD in Two Children: Intracranial Hypertension and New-Onset Seizure. Neurohospitalist 2023, 13, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, Y.; Oshi, M.; Kamal, N.M.; Aljabri, M.; Abosabie, S.; Elhaj, W.M.; Abosabie, S.A. Pediatric myelin oligodendrocyte glycoprotein antibody associated diseaseasymmetric papilledema and elevated ICP are two of the chameleons: A case report. Medicine 2023, 102, e32986. [Google Scholar] [CrossRef] [PubMed]
- Valdrighi, A.; Russ, J.; Waubant, E.; Rasool, N.; Francisco, C. Atypical myelin oligodendrocyte glycoprotein antibody disease presenting with isolated elevated intracranial pressure. Neuroimmunol. Rep. 2021, 1, 100028. [Google Scholar] [CrossRef]
- Maran, J.J.; Sharpe, C.; Carroll, S. Paediatric MOG-antibody disease presenting with intracranial hypertension and unilateral vision loss without radiological evidence of optic neuritis. J. Neuroimmunol. 2023, 15, 578083. [Google Scholar] [CrossRef]
- Lotan, I.; Brody, J.; Hellmann, M.A.; Bialer, O.; Ganelin-Cohen, E.; Michaeli, N.; Marignier, R.; Stiebel-Kalish, H. Myelin oligodendrocyte glycoprotein-positive optic neuritis masquerading as pseudotumor cerebri at presentation. J. Neurol. 2018, 265, 1985–1988. [Google Scholar] [CrossRef]
- Narula, S.; Liu, G.T.; Avery, R.A.; Banwell, B.; Waldman, A.T. Elevated cerebrospinal fluid opening pressure in a pediatric demyelinating disease cohort. Pediatr. Neurol. 2015, 52, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Jeantin, L.; Hesters, A.; Fournier, D.; Lebrun-Vignes, B.; Méneret, A.; Papeix, C.; Touitou, V.; Maillart, E. Anti-MOG associated disease with intracranial hypertension after COVID-19 vaccination. J. Neurol. 2022, 269, 5647–5650. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.N.; Wang, C.; Sguigna, P.; Husari, K.; Greenberg, B. Atypical Anti-MOG syndrome with aseptic meningoencephalitis and pseudotumor cerebri-like presentations. Mult. Scler. Relat. Disord. 2019, 27, 30–33. [Google Scholar] [CrossRef]
- Chaudhuri, J.R.; Bagul, J.J.; Swathi, A.; Singhal, B.S.; Reddy, N.C.; Vallam, K.K. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease Presenting as Intracranial Hypertension: A Case Report. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e200020. [Google Scholar] [CrossRef] [PubMed]
- Papetti, L.; Panella, E.; Monte, G.; Ferilli, M.A.N.; Tarantino, S.; Checchi, M.P.; Valeriani, M. Pediatric Onset Multiple Sclerosis and Obesity: Defining the Silhouette of Disease Features in Overweight Patients. Nutrients 2023, 15, 4880. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Panthangi, V.; Kurupp, A.R.; Raju, A.; Luthra, G.; Shahbaz, M.; Almatooq, H.; Foucambert, P.; Esbrand, F.D.; Khan, S. A Systematic Review on Whether an Association Exists between Adolescent Obesity and Idiopathic Intracranial Hypertension. Cureus 2022, 8, e28071. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, P. Commentary: The role of inflammation in idiopathic intracranial hypertension. Indian J. Ophthalmol. 2021, 6, 1506–1507. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Walker, E.A.; Burdon, M.A.; van Beek, A.P.; Kema, I.P.; Hughes, B.A.; Murray, P.I.; Nightingale, P.G.; Stewart, P.M.; Rauz, S.; et al. Cerebrospinal fluid corticosteroid levels and cortisol metabolism in patients with idiopathic intracranial hypertension: A link between 11beta-HSD1 and intracranial pressure regulation? J. Clin. Endocrinol. Metab. 2010, 95, 5348–5356. [Google Scholar] [CrossRef]
- Verma, M.; Kipari, T.M.; Zhang, Z.; Man, T.Y.; Forster, T.; Homer, N.Z.; Seckl, J.R.; Holmes, M.C.; Chapman, K.E. 11beta-hydroxysteroid dehydrogenase-1 deficiency alters brain energy metabolism in acute systemic inflammation. Brain Behav. Immun. 2018, 69, 223–234. [Google Scholar] [CrossRef]
- Edwards, L.J.; Sharrack, B.; Ismail, A.; Tench, C.R.; Gran, B.; Dhungana, S.; Brettschneider, J.; Tumani, H.; Constantinescu, C.S. Increased levels of interleukins 2 and 17 in the cerebrospinal fluid of patients with idiopathic intracranial hypertension. Am. J. Clin. Exp. Immunol. 2013, 2, 234–244. [Google Scholar]
- Dhungana, S.; Sharrack, B.; Woodroofe, N. Cytokines and chemokines in idiopathic intracranial hypertension. Headache 2009, 49, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Altıokka-Uzun, G.; Tüzün, E.; Ekizoğlu, E.; Ulusoy, C.; Yentür, S.; Kürtüncü, M.; Saruhan-Direskeneli, G.; Baykan, B. Oligoclonal bands and increased cytokine levels in idiopathic intracranial hypertension. Cephalalgia 2015, 35, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Stiebel-Kalish, H.; Rubarth, K.; Shouchane-Blum, K.; Tiosano, A.; Lotan, I.; Hellmann, M.A.; Wilf-Yarkoni, A.; Bialer, O.; Flanagan, E.P.; Pittock, S.J.; et al. Obesity is associated with myelin oligodendrocyte glycoprotein antibody-associated disease in acute optic neuritis. Sci. Rep. 2022, 1, 21312. [Google Scholar] [CrossRef] [PubMed]
| Case/Reference | Age/Sex | Weight or BMI (kg/m2) | CSF Opening Pressure (cmH2O) | CSF Protein (mg/dL) | CSF White Blood Cell (count/mm3) | BOC | Associated MRI Findings to IHH | Clinical Findings | VEP/OCT | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| Adults Subjects | ||||||||||
| 1/ Chaudhuri et al., 2022 [14] | 19/F | NA | 35 | Normal | 20 (lymphocytes) | No | Leptomeningeal enhancement | Blurred vision, severe headache, vomiting | NA | Methylprednisolone, mycophenolate, rituximab |
| 2/ Jeantin et al., 2022 [12] | 35/F | NA | 31 | NA | 189 (lymphocytes) | Yes | Unilateral ON, brain and spinal lesions | Headaches, nausea, abdominal pain, confusion | NA | Acetazolamide, methylprednisolone, azathioprine |
| 3/ Lotan et al., 2018 [10] | 40/M | 32 | 33 | Normal | Normal | NA | Bilateral ON | Blurred vision, headache | NA | Acetazolamide, methylprednisolone |
| Pediatric Subjects | ||||||||||
| 4/ Maran et al., 2023 [9] | 13/M | 44.8 | 65 | NA | NA | NA | Intrasellar arachnoid herniation; T2 hyperintense subcortical lesions in the brain | Headache, nausea, vomiting, right eye sudden vision loss | OCT: progressive thinning in temporal and inferotemporal sectors and bilateral papilledema | Acetazolamide Methylprednisolone Neurosurgical treatment of IH. |
| 5/ Alqahtani et al., 2023 [7] | 12/M | 28.8 | 35 | Normal | 75 (89% L, 4% N, 3% M, 1% E) | No | flattening of the posterior globes, prominent both Meckel cave, optic nerves tortuosity | Headache Bilateral reduced visual acuity | asymmetric bilateral papilledema and abnormal visual acuity and visual field. | Acetazolamide, methylprednisolone 30 mg/kg/day was administered for 5 days followed by low doses acetazolamide 10 mg/kg/day (duration treatment 3 months) |
| 6/ Valdrighi et al., 2021 [8] | 12/M | BMI not specified Obese | 52 | Normal | 157 (68% L, 11% N, 17% M, 4% E) | No | a partially empty sella, intraocular protrusion of the optic nerves, flattening of the posterior globes, narrowing of the venous sinuses, and tortuous optic nerves multifocal leptomeningeal enhancement. After 4 months from the onset: T2 hyperintense cerebellar and pontis lesions | Blurred vision, nausea, vomiting, headaches, cranial nerve VI palsies. | Bilateral papilledema Normal visual acuity and visual field. | Acetazolamide, methylprednisolone. After radiological relapse: monthly IV immunoglobulin |
| 7/ Narayan et al., 2019 [13] | 18/F | NA | 29 | 48 | 57–82 (84–97% L) | No | bilateral ON and multiple spinal cord lesions | Bilateral blurred vision, headache, photophobia | Bilateral papilledema and poor visual acuity | Five cycles of plasma exchange Acetazolamide and Methylprednisolone (duration 1 month) |
| 8/ Lotan et al., 2018 [10] | 6/F | 18 | 35 | Normal | 8 | NA | Brain and spinal lesions | Right eye reduced vision, headache | Bilateral papilledema | Acetazolamide, methylprednisolone |
| 9/Zhou et al., 2023 [6] | 12/M | NA | >36 | 158 | 252 | Multifocal cortical/subcortical T2 hyperintensities, bilateral ON head elevation, and enlarged, T2 hyperintense intraorbital optic nerves | Bilateral blurry vision, facial droop, slurred speech, and ataxia | Poor visual acuity papilledema | Methylprednisolone iv, | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papetti, L.; Moltoni, G.; Longo, D.; Monte, G.; Dellepiane, F.; Pro, S.; Bracaglia, G.; Ruscitto, C.; Verrotti, A.; Valeriani, M. Isolated Intracranial Hypertensions as Onset of Myelin Oligodendrocyte Glycoprotein Antibody Disease. J. Clin. Med. 2024, 13, 4468. https://doi.org/10.3390/jcm13154468
Papetti L, Moltoni G, Longo D, Monte G, Dellepiane F, Pro S, Bracaglia G, Ruscitto C, Verrotti A, Valeriani M. Isolated Intracranial Hypertensions as Onset of Myelin Oligodendrocyte Glycoprotein Antibody Disease. Journal of Clinical Medicine. 2024; 13(15):4468. https://doi.org/10.3390/jcm13154468
Chicago/Turabian StylePapetti, Laura, Giulia Moltoni, Daniela Longo, Gabriele Monte, Francesco Dellepiane, Stefano Pro, Giorgia Bracaglia, Claudia Ruscitto, Alberto Verrotti, and Massimiliano Valeriani. 2024. "Isolated Intracranial Hypertensions as Onset of Myelin Oligodendrocyte Glycoprotein Antibody Disease" Journal of Clinical Medicine 13, no. 15: 4468. https://doi.org/10.3390/jcm13154468
APA StylePapetti, L., Moltoni, G., Longo, D., Monte, G., Dellepiane, F., Pro, S., Bracaglia, G., Ruscitto, C., Verrotti, A., & Valeriani, M. (2024). Isolated Intracranial Hypertensions as Onset of Myelin Oligodendrocyte Glycoprotein Antibody Disease. Journal of Clinical Medicine, 13(15), 4468. https://doi.org/10.3390/jcm13154468







