Predelivery Haemostatic Biomarkers in Women with Non-Severe Postpartum Haemorrhage
Abstract
1. Introduction
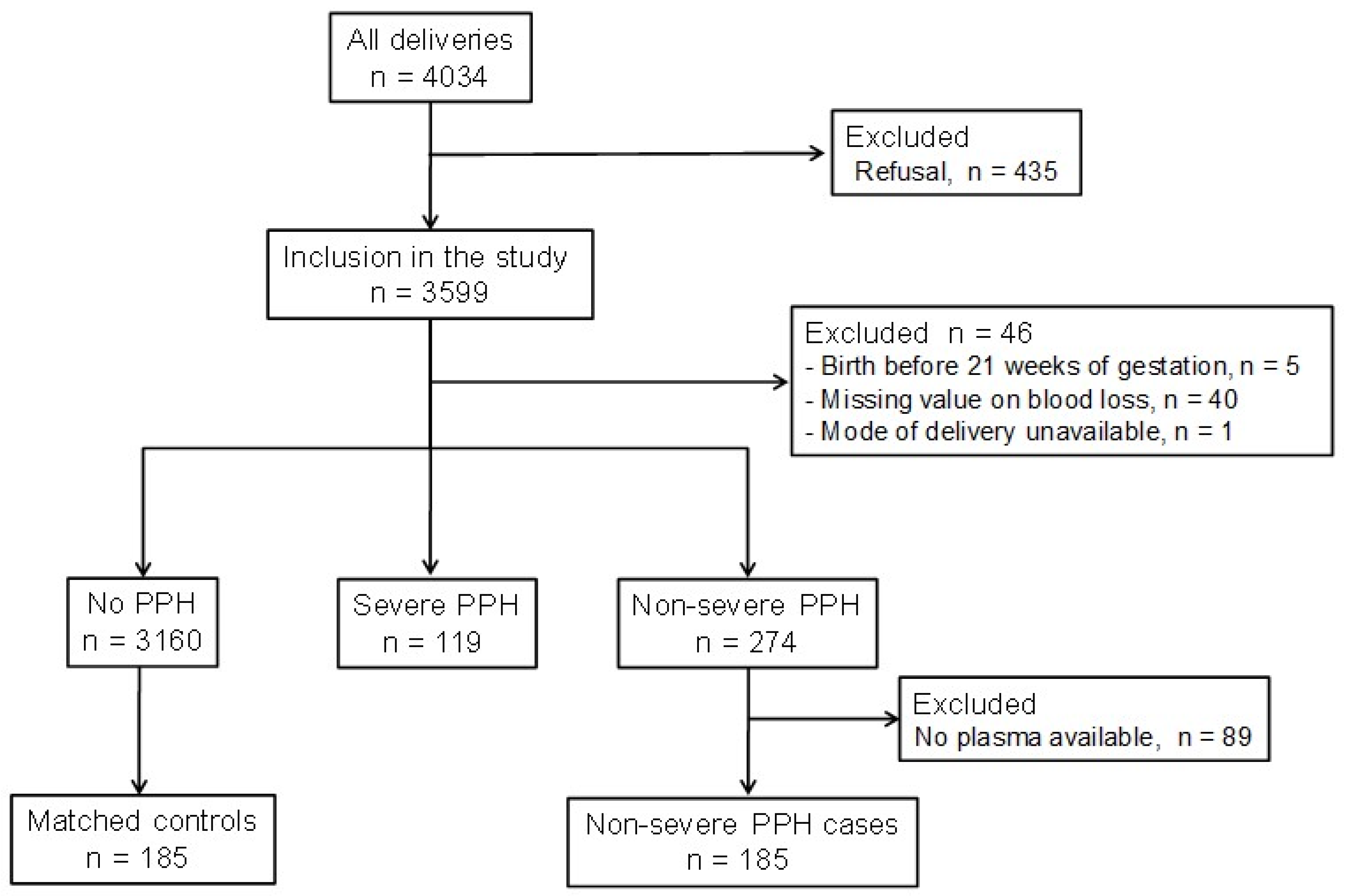
2. Materials and Methods
2.1. Recruitment of Participants, Blood and Data Collection
2.2. Definition of Non-Severe PPH
2.3. Selection of Cases and Controls
2.4. Thrombin Generation Assay (TGA)
2.5. Plasmin Generation Assay (PGA)
2.6. Statistical Analysis
2.7. Ethics Approval
3. Results
3.1. Characteristics of the Pregnant Women
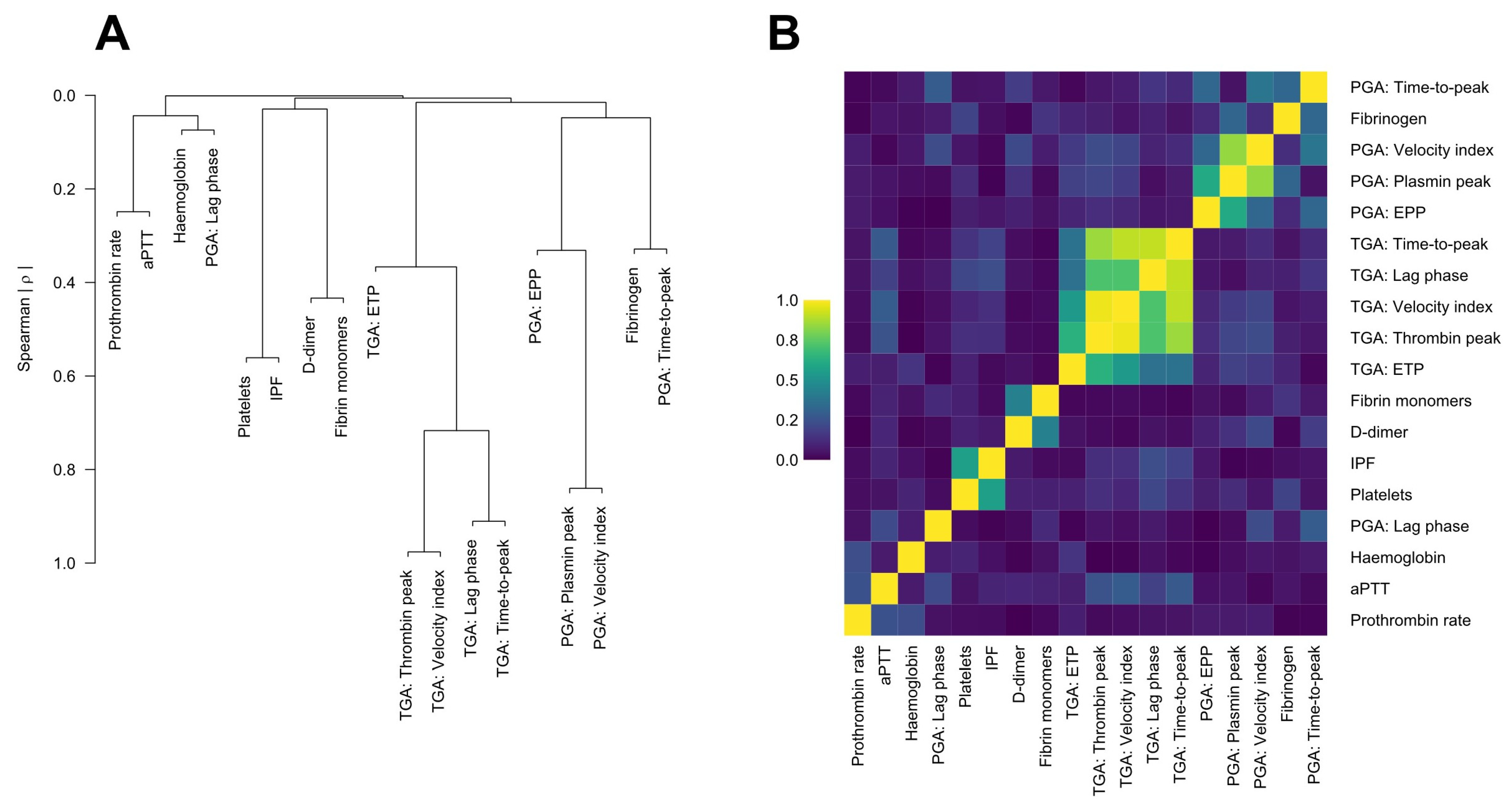
3.2. Predelivery Haemostatic Biomarkers and Their Association with Non-Severe PPH
3.3. Thrombin Generation Assay (TGA)
3.4. Plasmin Generation Assay (PGA)
3.5. Logistic Regression Analysis for Parameters Associated with Non-Severe PPH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patek, K.; Friedman, P. Postpartum Hemorrhage—Epidemiology, Risk Factors, and Causes. Clin. Obstet. Gynecol. 2023, 66, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Oyelese, Y.; Ananth, C.V. Postpartum Hemorrhage: Epidemiology, Risk Factors, and Causes. Clin. Obstet. Gynecol. 2010, 53, 147–156. [Google Scholar] [CrossRef] [PubMed]
- McLintock, C. Prevention and treatment of postpartum hemorrhage: Focus on hematological aspects of management. Hematology 2020, 2020, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Calvert, C.; Thomas, S.L.; Ronsmans, C.; Wagner, K.S.; Adler, A.J.; Filippi, V. Identifying Regional Variation in the Prevalence of Postpartum Haemorrhage: A Systematic Review and Meta-Analysis. PLoS ONE 2012, 7, e41114. [Google Scholar] [CrossRef] [PubMed]
- Dahlke, J.D.; Mendez-Figueroa, H.; Maggio, L.; Hauspurg, A.K.; Sperling, J.D.; Chauhan, S.P.; Rouse, D.J. Prevention and management of postpartum hemorrhage: A comparison of 4 national guidelines. Am. J. Obstet. Gynecol. 2015, 213, 76.e1–76.e10. [Google Scholar] [CrossRef] [PubMed]
- Neary, C.; Naheed, S.; McLernon, D.J.; Blac, M. Predicting risk of postpartum haemorrhage: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Charbit, B.; Mandelbrot, L.; Samain, E.; Baron, G.; Haddaoui, B.; Keita, H.; Sibony, O.; Mahieu-Caputo, D.; Hurtaud-Roux, M.F.; Huisse, M.G.; et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J. Thromb. Haemost. 2007, 5, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Cortet, M.; Deneux-Tharaux, C.; Dupont, C.; Colin, C.; Rudigoz, R.-C.; Bouvier-Colle, M.-H.; Huissoud, C. Association between fibrinogen level and severity of postpartum haemorrhage: Secondary analysis of a prospective trial. Br. J. Anaesth. 2012, 108, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Huissoud, C.; Carrabin, N.; Audibert, F.; Levrat, A.; Massignon, D.; Berland, M.; Rudigoz, R. Bedside assessment of fibrinogen level in postpartum haemorrhage by thrombelastometry. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 1097–1102. [Google Scholar] [CrossRef]
- Collins, P.; Cannings-John, R.; Bruynseels, D.; Mallaiah, S.; Dick, J.; Elton, C.; Weeks, A.; Sanders, J.; Aawar, N.; Townson, J.; et al. Viscoelastometric-guided early fibrinogen concentrate replacement during postpartum haemorrhage: OBS2, a double-blind randomized controlled trial. Br. J. Anaesth. 2017, 119, 411–421. [Google Scholar] [CrossRef]
- De Moreuil, C.; Dargaud, Y.; Nougier, C.; Dupré, P.-F.; Trémouilhac, C.; Le Joliff, D.; Rosec, S.; Lucier, S.; Pabinger, I.; Ay, C.; et al. Women with severe postpartum hemorrhage have a decreased endogenous thrombin potential before delivery. J. Thromb. Haemost. 2023, 21, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Salomon, C.; De Moreuil, C.; Hannigsberg, J.; Trémouilhac, C.; Drugmanne, G.; Gatineau, F.; Nowak, E.; Anouilh, F.; Briend, D.; Le Moigne, E.; et al. Haematological parameters associated with postpartum haemorrhage after vaginal delivery: Results from a French cohort study. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102168. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy: Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- World Health Organisation. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. 2012. Available online: http://apps.who.int/iris/bitstream/handle/10665/75411/9789241548502_eng.pdf;jsessionid=8F11A00EB1C7BD4C058948030FBD1D77?sequence=1 (accessed on 14 January 2024).
- Solomon, C.; Collis, R.; Collins, P. Haemostatic monitoring during postpartum haemorrhage and implications for management. Br. J. Anaesth. 2012, 109, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.; Ay, C.; Rejtö, J.; Wolberg, A.S.; Haslacher, H.; Koder, S.; Pabinger, I.; Gebhart, J. Thrombin-generating potential, plasma clot formation, and clot lysis are impaired in patients with bleeding of unknown cause. J. Thromb. Haemost. 2019, 17, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Miszta, A.; Ahmadzia, H.K.; Luban, N.L.C.; Li, S.; Guo, D.; Holle, L.A.; Berger, J.S.; James, A.H.; Gobburu, J.V.S.; Anker, J.v.D.; et al. Application of a plasmin generation assay to define pharmacodynamic effects of tranexamic acid in women undergoing cesarean delivery. J. Thromb. Haemost. 2021, 19, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Statistically Efficient Ways to Quantify Added Predictive Value of New Measurements. Available online: https://www.fharrell.com/post/addvalue/ (accessed on 14 January 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 14 January 2024).
- Harrell, F.E., Jr. rms: Regression Modeling Strategies. R Package Version 6.7-1. 2023. Available online: https://CRAN.R-project.org/package=rms (accessed on 14 January 2024).
- De Moreuil, C.; Mehic, D.; Nopp, S.; Kraemmer, D.; Gebhart, J.; Schramm, T.; Couturaud, F.; Ay, C.; Pabinger, I. Hemostatic biomarkers associated with postpartum hemorrhage: A systematic review and meta-analysis. Blood Adv. 2023, 7, 5954–5967. [Google Scholar] [CrossRef] [PubMed]
- Eckerdal, P.; Kollia, N.; Löfblad, J.; Hellgren, C.; Karlsson, L.; Högberg, U.; Wikström, A.-K.; Skalkidou, A. Delineating the Association between Heavy Postpartum Haemorrhage and Postpartum Depression. PLoS ONE 2016, 11, e0144274. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, T.; Mokhtari, N.; Huang, J.C.; Landy, H.J. Evaluation of Risk-Assessment Tools for Severe Postpartum Hemorrhage in Women Undergoing Cesarean Delivery. Obstet. Gynecol. 2019, 134, 1308–1316. [Google Scholar] [CrossRef]
- Collège National des Gynécologues et Obstétriciens Français (CNGOF). Guidelines for postpartum hemorrhage. J. Gynecol. Obstet. Biol. Reprod. 2004, 33, 4S130–134S136. [Google Scholar]
- Bienstock, J.L.; Eke, A.C.; Hueppchen, N.A. Postpartum Hemorrhage. N. Engl. J. Med. 2021, 384, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Reommendations: Uterotonics for the Prevention of Postpartum Haemorrhage. Available online: https://www.who.int/publications/i/item/9789241550420 (accessed on 13 June 2024).
- Escobar, M.F.; Nassar, A.H.; Theron, G.; Barnea, E.R.; Nicholson, W.; Ramasauskaite, D.; Lloyd, I.; Chandraharan, E.; Miller, S.; Burke, T.; et al. FIGO recommendations on the management of postpartum hemorrhage 2022. Int. J. Gynecol. Obstet. 2022, 157 (Suppl. S1), 3–50. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, S.E.; Barsoum, J.R.; Hansen, K.C.; Sassin, A.M.; Dzieciatkowska, M.; James, A.H.; Aagaard, K.M.; Ahmadzia, H.K.; Wolberg, A.S. Agnostic identification of plasma biomarkers for postpartum hemorrhage risk. Am. J. Obstet. Gynecol. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Non-Severe PPH (n = 185) | Controls (n = 185) | p |
|---|---|---|---|
| Women’s characteristics | |||
| Age *, years, median (IQR) | 30 (26–34) | 30 (26–33) | 0.54 |
| BMI *, kg/m2, median (IQR) | 23 (21–27) | 23 (21–28) | 0.77 |
| 25–29.9 kg/m2, n (%) | 44 (23.8%) | 44 (23.8%) | |
| ≥30 kg/m2, n (%) | 24 (13.0%) | 24 (13.0%) | |
| Smokers, n (%) | 51 (27.6%) | 58 (31.4%) | 0.42 |
| Blood group | |||
| O group, n (%) | 82 (44.3%) | 86 (46.5%) | 0.68 |
| Nulliparous, n (%) | 97 (52.4%) | 89 (48.1%) | 0.41 |
| Pregnancy characteristics | |||
| Conception by ART, n (%) | 21 (11.4%) | 15 (8.1%) | 0.29 |
| Gemellar pregnancy, n (%) | 17 (9.2%) | 9 (4.9%) | 0.10 |
| Gestational diabetes, n (%) | 20 (10.8%) | 19 (10.3%) | 1.0 |
| Gestational weight gain, kg, median (IQR) | 13 (8–18) | 13 (9–17) | 0.85 |
| Pre-eclampsia, n (%) | 12 (6.5%) | 9 (4.9%) | 0.50 |
| IUGR, n (%) | 6 (3.2%) | 3 (1.6%) | 0.51 |
| Placental abruption, n (%) | 2 (1.1%) | 1 (0.5%) | 0.50 |
| Abnormal placental insertion, n (%) | 17 (9.2%) | 4 (2.2%) | 0.003 |
| Antepartum haemorrhage, n (%) | 25 (13.5%) | 14 (7.6%) | 0.01 |
| Delivery characteristics | |||
| Term * at delivery, WG, median (IQR) | 39 (37–40) | 39 (37–40) | 0.77 |
| Induced labour, n (%) | 69 (37.3%) | 67 (36.2%) | 0.83 |
| Vaginal * delivery, n (%) | 72 (38.9%) | 72 (38.9%) | 1.0 |
| Instrumental delivery, n (%) | 27 (14.6%) | 17 (9.2%) | 0.11 |
| C-section *, n (%) | 113 (61.1%) | 113 (61.1%) | 1.0 |
| Emergency C-section, n (%) | 77 (41.6%) | 91 (49.2%) | 0.14 |
| Episiotomy, n (%) | 42 (22.7%) | 19 (10.3%) | 0.001 |
| Perineal tears, n (%) | 48 (25.9%) | 40 (21.6%) | 0.33 |
| Macrosomia, n (%) | 17 (9.2%) | 16 (8.6%) | 0.86 |
| Haemostatic Biomarkers | Non-Severe PPH (n = 185) | Controls (n = 185) | p |
|---|---|---|---|
| Blood count parameters | |||
| Haemoglobin, g/dL, median (IQR) | 12.1 (11.4–12.8) | 12.3 (11.4–12.9) | 0.35 |
| Platelets, G/L, median (IQR) | 217 (181–259) | 242 (196–280) | 0.003 |
| IPF, ratio, median (IQR) | 5.1 (3.3–7.9) | 4.9 (3.1–7.7) | 0.63 |
| Conventional haemostatic tests | |||
| Prothrombin rate, %, median (IQR) | 100 (94–100) | 99 (94–100) | 0.73 |
| aPTT, ratio, median (IQR) | 1.01 (0.95–1.06) | 0.99 (0.94–1.06) | 0.12 |
| Fibrinogen, g/L, median (IQR) | 4.85 (4.35–5.63) | 5.09 (4.46–5.56) | 0.32 |
| D-dimer, µg/mL, median (IQR) | 1.64 (1.24–2.21) | 1.54 (1.08–2.06) | 0.06 |
| Fibrin monomers, µg/mL, median (IQR) | 5.64 (4.39–9.61) | 5.32 (3.98–7.08) | 0.10 |
| Thrombin generation assay | |||
| Lag phase, min, median (IQR) | 14.1 (12.6–15.1) | 14.1 (12.1–15.6) | 0.89 |
| Thrombin peak, nmol/L, median (IQR) | 294.5 (237.5–389.8) | 314.9 (239.3–388.1) | 0.57 |
| Time to peak, min, median (IQR) | 23.6 (20.6–25.6) | 23.1 (20.6–25.6) | 0.58 |
| Velocity index, nmol/L/min, median (IQR) | 32.6 (22.9–48.3) | 35.1 (24.2–49.0) | 0.50 |
| ETP, nmol/L × min, median (IQR) | 5 419 (4 978–5 919) | 5 349 (5 038–5 876) | 0.78 |
| Plasmin generation assay | |||
| Lag phase, min, median (IQR) | 2.7 (2.6–3.0) | 2.7 (2.3–3.0) | 0.10 |
| Plasmin peak, nmol/L, median (IQR) | 66.8 (52.9–78.0) | 67.9 (56.3–78.0) | 0.46 |
| Time to peak, min, median (IQR) | 7.3 (7.0–8.0) | 7.3 (7.0–8.0) | 0.67 |
| Velocity index, nmol/L/min, median (IQR) | 14.2 (11.9–17.1) | 13.8 (11.4–17.0) | 0.65 |
| EPP, nmol/L × min, median (IQR) | 840.8 (540.6–1 111.8) | 844.4 (537.1–1 070.4) | 0.87 |
| Biomarker | 25th Percentile | 75th Percentile | Difference | OR | 95% CI | Fraction of New Information |
|---|---|---|---|---|---|---|
| Platelets (G/L) | 187.50 | 271.50 | 84.00 | 0.61 | 0.37–0.86 | 0.252 |
| Immature platelet fraction (%) | 3.25 | 7.95 | 4.70 | 0.76 | 0.51–1.05 | 0.094 |
| aPTT ratio (%) | 0.94 | 1.06 | 0.12 | 1.31 | 0.98–2.02 | 0.093 |
| Prothrombin time (%) | 94.00 | 100.00 | 6.00 | 1.15 | 0.90–1.51 | 0.066 |
| D-dimer (µg/mL) | 1.16 | 2.12 | 0.96 | 1.10 | 0.88–1.96 | 0.024 |
| Haemoglobin (g/dL) | 11.40 | 12.90 | 1.50 | 0.92 | 0.65–1.30 | 0.009 |
| PC1 plasmin generation assay | −0.92 | 0.96 | 1.88 | 0.93 | 0.63–1.25 | 0.007 |
| Fibrinogen (g/L) | 4.41 | 5.58 | 1.17 | 0.96 | 0.65–1.38 | 0.002 |
| Fibrin monomers (µg/mL) | 4.20 | 8.23 | 4.04 | 1.00 | 0.94–1.05 | <0.001 |
| PC1 thrombin generation assay | −0.75 | 1.12 | 1.87 | 0.99 | 0.72–1.30 | <0.001 |
| Adjusted for clinical covariates: | ||||||
| Age (years)—matched | 26.00 | 33.00 | 7.00 | 0.99 | 0.78–1.24 | |
| BMI (kg/m2)—matched | 20.70 | 27.50 | 6.80 | 1.08 | 0.88–1.34 | |
| Nulliparous (yes/no) | No | Yes | 1.18 | 0.73–2.04 | ||
| Gemellar pregnancy (yes/no) | No | Yes | 2.01 | 0.62–7.56 | ||
| Abnormal placental insertion (yes/no) | No | Yes | 3.37 | 1.06–25.06 | ||
| Antepartum haemorrhage (yes/no) | No | Yes | 1.51 | 0.67–3.65 | ||
| Pre-eclampsia (yes/no) | No | Yes | 0.77 | 0.14–3.42 | ||
| Macrosomia (yes/no) | No | Yes | 1.39 | 0.58–3.58 | ||
| Induced labour (yes/no) | No | Yes | 1.09 | 0.67–1.84 | ||
| Term at delivery (WG)—matched | 37.00 | 40.00 | 3.00 | 1.04 | 0.80–1.34 | |
| Type of delivery—matched (elective/emergency C-section) (vaginal/emergency C-section) | 1.67 1.23 | 0.78–3.97 0.91–1.66 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Moreuil, C.; Pan-Petesch, B.; Mehic, D.; Kraemmer, D.; Schramm, T.; Albert, C.; Trémouilhac, C.; Lucier, S.; Galinat, H.; Le Roux, L.; et al. Predelivery Haemostatic Biomarkers in Women with Non-Severe Postpartum Haemorrhage. J. Clin. Med. 2024, 13, 4231. https://doi.org/10.3390/jcm13144231
de Moreuil C, Pan-Petesch B, Mehic D, Kraemmer D, Schramm T, Albert C, Trémouilhac C, Lucier S, Galinat H, Le Roux L, et al. Predelivery Haemostatic Biomarkers in Women with Non-Severe Postpartum Haemorrhage. Journal of Clinical Medicine. 2024; 13(14):4231. https://doi.org/10.3390/jcm13144231
Chicago/Turabian Stylede Moreuil, Claire, Brigitte Pan-Petesch, Dino Mehic, Daniel Kraemmer, Theresa Schramm, Casilda Albert, Christophe Trémouilhac, Sandy Lucier, Hubert Galinat, Liana Le Roux, and et al. 2024. "Predelivery Haemostatic Biomarkers in Women with Non-Severe Postpartum Haemorrhage" Journal of Clinical Medicine 13, no. 14: 4231. https://doi.org/10.3390/jcm13144231
APA Stylede Moreuil, C., Pan-Petesch, B., Mehic, D., Kraemmer, D., Schramm, T., Albert, C., Trémouilhac, C., Lucier, S., Galinat, H., Le Roux, L., Gebhart, J., Couturaud, F., Wolberg, A. S., Ay, C., & Pabinger, I. (2024). Predelivery Haemostatic Biomarkers in Women with Non-Severe Postpartum Haemorrhage. Journal of Clinical Medicine, 13(14), 4231. https://doi.org/10.3390/jcm13144231








