Real-World Safety Profile of Biologic Drugs for Severe Uncontrolled Asthma: A Descriptive Analysis from the Spanish Pharmacovigilance Database
Abstract
1. Introduction
1.1. Background
1.2. Overview of Biological Agents for Asthma
1.3. Post-Approval Pharmacovigilance of Biological Drug in Asthma
1.4. Justification and Aim of the Study
2. Materials and Methods
2.1. Biological Medicines under Study
2.2. Data Source and Analysis of Cases Registered in the FEDRA Database
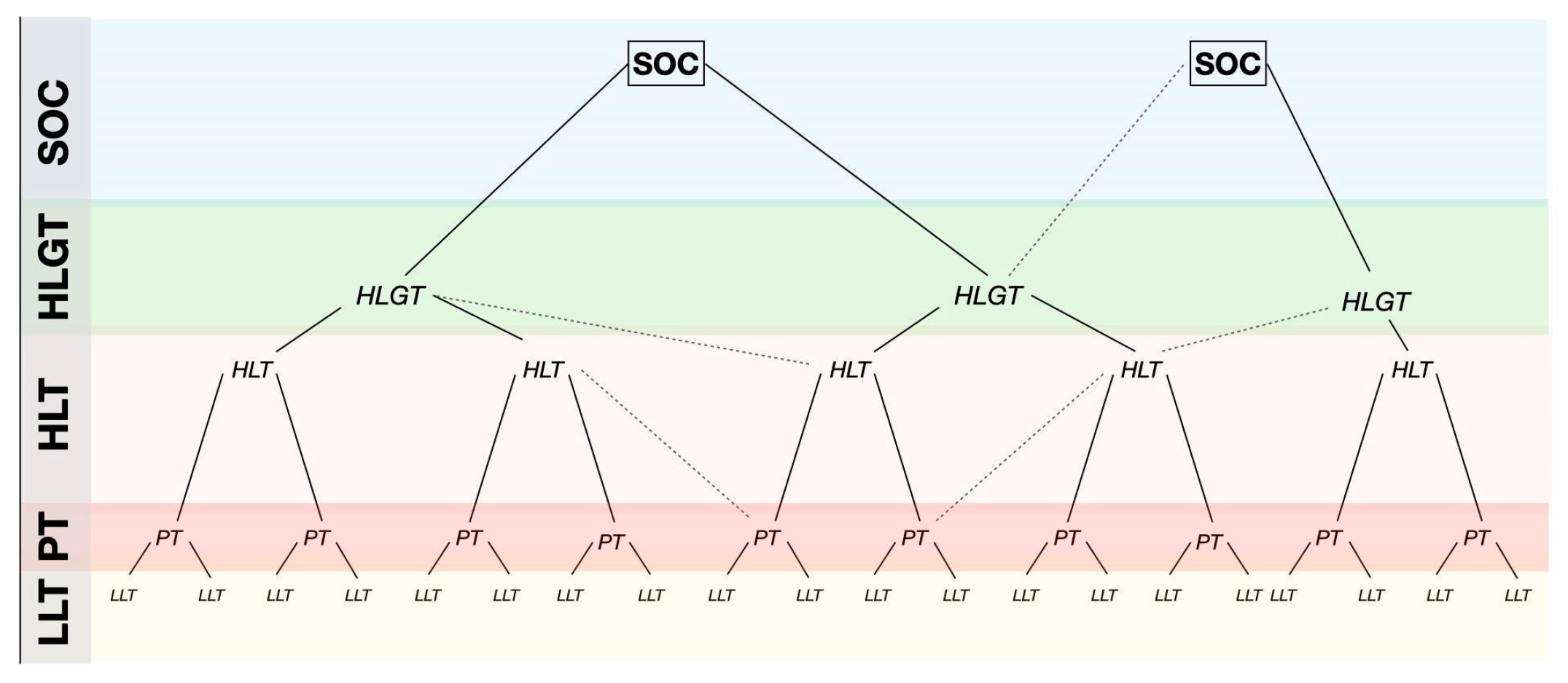
2.3. Medical Dictionary for Regulatory Activities (MedDRA)
2.4. Institutional Review Board Statement
3. Results
3.1. Global Findings from the Analysis of Overall Data
3.2. Active Ingredient
3.2.1. Omalizumab
3.2.2. Mepolizumab
- ▪ A total of 15 cases of paresthesia, hypoesthesia, or dysesthesia, with 5 cases deemed severe.
- ▪ A total of 6 cases of muscle weakness and atrophy, 3 of which were severe.
- ▪ A total of 5 cases of tremor, 3 of which were severe.
- ▪ In total, 4 cases of central nervous system (CNS) vascular disorders, all serious.
3.2.3. Benralizumab
3.2.4. Dupilumab
3.2.5. Reslizumab
3.2.6. Tezepelumab
4. Discussion
4.1. SmPC and Risk
4.2. ADRs and Underlying Disease
4.3. ADRs and Eosinophilic Granulomatosis with Polyangiitis (EGPA)
4.4. Neoplastic Disorders and Biological Therapy
4.5. ADRs and Vascular Disorders
4.6. Specific Comments on ADRS for Active Ingredients in Biological Agents Primarily Indicated for Severe Refractory Asthma
4.6.1. Omalizumab
4.6.2. Mepolizumab
4.6.3. Benralizumab
4.6.4. Dupilumab
5. Limitations
6. Conclusions and Future Research Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
FEDRA Database of the Spanish System of Pharmacovigilance of Human Medicines (SEFV-H) Disclaimer
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2023. Available online: https://ginasthma.org/ (accessed on 9 April 2024).
- Ferkol, T.; Schraufnagel, D. The Global Burden of Respiratory Disease. Ann. Am. Thorac. Soc. 2014, 11, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Fletcher, M.; van der Molen, T. Asthma control and management in 8000 European patients: The REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim. Care Respir. Med. 2014, 24, 14009. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.L. The national review of asthma deaths: What did we learn and what needs to change? Breathe Sheff. Engl. 2005, 11, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Sadatsafavi, M.; Rousseau, R.; Chen, W.; Zhang, W.; Lynd, L.; FitzGerald, J.M. The preventable burden of productivity loss due to suboptimal asthma control: A population-based study. Chest 2014, 145, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Rogliani, P.; Calzetta, L.; Matera, M.G.; Laitano, R.; Ritondo, B.L.; Hanania, N.A.; Cazzola, M. Severe Asthma and Biological Therapy: When, Which, and for Whom. Pulm. Ther. 2020, 6, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; Pasha, M.A.; Tang, D.D. Current Understanding of Asthma Pathogenesis and Biomarkers. Cells 2022, 11, 2764. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.P. Endotyping asthma: New insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet 2008, 372, 1107–1119. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Camiolo, M.; Fitzpatrick, A.; Gauthier, M.; Wenzel, S.E. Are We Meeting the Promise of Endotypes and Precision Medicine in Asthma? Physiol. Rev. 2020, 100, 983–1017. [Google Scholar] [CrossRef]
- McDowell, P.J.; Heaney, L.G. Different endotypes and phenotypes drive the heterogeneity in severe asthma. Allergy 2020, 75, 302–310. [Google Scholar] [CrossRef]
- Espuela-Ortiz, A.; Martin-Gonzalez, E.; Poza-Guedes, P.; González-Pérez, R.; Herrera-Luis, E. Genomics of Treatable Traits in Asthma. Genes 2023, 14, 1824. [Google Scholar] [CrossRef] [PubMed]
- Sze, E.; Bhalla, A.; Nair, P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy 2020, 75, 311–325. [Google Scholar] [CrossRef]
- Kim, S.R. Next-Generation Therapeutic Approaches for Uncontrolled Asthma: Insights into the Heterogeneity of Non-Type 2 Inflammation. Allergy Asthma Immunol. Res. 2024, 16, 1–5. [Google Scholar] [CrossRef]
- Xie, Y.; Abel, P.W.; Casale, T.B.; Tu, Y. TH17 cells and corticosteroid insensitivity in severe asthma. J. Allergy Clin. Immunol. 2022, 149, 467–479. [Google Scholar] [CrossRef]
- Carr, T.F.; Peters, M.C. Novel potential treatable traits in asthma: Where is the research taking us? J. Allergy Clin. Immunol. Glob. 2022, 1, 27–36. [Google Scholar] [CrossRef]
- Stokes, J.R.; Casale, T.B. Characterization of asthma endotypes: Implications for therapy. Ann. Allergy Asthma Immunol. 2016, 117, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Y.; Zhang, X.; Wang, J.; Wang, G.; Oliver, B.G.; Zhang, H.P.; Kang, D.Y.; Wang, L.; Qiu, Z.X.; Li, W.M. Multidimensional Assessment of Asthma Identifies Clinically Relevant Phenotype Overlap: A Cross-Sectional Study. J. Allergy Clin. Immunol. Pract. 2021, 9, 349–362.e18. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Guida, G.; Bertolini, F.; Di Stefano, A.; Carriero, V. Phenotype overlap in the natural history of asthma. Eur. Respir. Rev. 2023, 32, 220201. [Google Scholar] [CrossRef] [PubMed]
- Fouka, E.; Domvri, K.; Gkakou, F.; Alevizaki, M.; Steiropoulos, P.; Papakosta, D.; Porpodis, K. Recent insights in the role of biomarkers in severe asthma management. Front. Med. 2022, 9, 992565. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- EMA Medical Terms Simplifier. 2023. Available online: https://www.ema.europa.eu/en/documents/other/ema-medical-terms-simplifier_en.pdf (accessed on 9 April 2024).
- Lommatzsch, M.; Brusselle, G.G.; Canonica, G.W.; Jackson, D.J.; Nair, P.; Buhl, R.; Virchow, J.C. Disease-modifying anti-asthmatic drugs. Lancet 2022, 399, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Farne, H.A.; Wilson, A.; Milan, S.; Banchoff, E.; Yang, F.; Powell, C.V. Anti-IL-5 therapies for asthma. Cochrane Database Syst. Rev. 2022, 7, CD010834. [Google Scholar]
- González-Pérez, R.; Poza-Guedes, P.; Mederos-Luis, E.; Sánchez-Machín, I. Real-Life Performance of Mepolizumab in T2-High Severe Refractory Asthma with the Overlapping Eosinophilic-Allergic Phenotype. Biomedicines 2022, 10, 2635. [Google Scholar] [CrossRef]
- Papaioannou, A.I.; Fouka, E.; Bartziokas, K.; Kallieri, M.; Vontetsianos, A.; Porpodis, K.; Rovina, N.; Loukides, S.; Bakakos, P. Defining response to therapy with biologics in severe asthma: From global evaluation to super response and remission. Expert Rev. Respir. Med. 2023, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; McDonald, V.M.; Pavord, I.D.; Gibson, P.G. Asthma remission: What is it and how can it be achieved? Eur. Respir. J. 2022, 60, 2102583. [Google Scholar] [CrossRef]
- Humbert, M.; Beasley, R.; Ayres, J.; Slavin, R.; Hébert, J.; Bousquet, J.; Beeh, K.M.; Ramos, S.; Canonica, G.W.; Hedgecock, S.; et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005, 60, 309–316. [Google Scholar] [CrossRef]
- Chang, T.W. The pharmacological basis of anti-IgE therapy. Nat. Biotechnol. 2000, 18, 157–162. [Google Scholar] [CrossRef]
- BIFIMED: Buscador de la Información Sobre la Situación de Financiación de los Medicamentos—Nomenclátor de ABRIL—2024 [BIFIMED: Information Search Engine on the Financing Situation of Medicines—Nomenclátor APRIL—2024]. Available online: https://www.sanidad.gob.es/profesionales/medicamentos.do?metodo=verDetalle&cn=652563 (accessed on 9 April 2024).
- McGregor, M.C.; Krings, J.G.; Nair, P.; Castro, M. Role of Biologics in Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 433–445. [Google Scholar] [CrossRef]
- Corren, J. New Targeted Therapies for Uncontrolled Asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Nucala: EPAR—Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf (accessed on 8 April 2024).
- Cinqaero: EPAR—Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/cinqaero-epar-product-information_en.pdf (accessed on 8 April 2024).
- Fasenra: EPAR—Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/fasenra-epar-product-information_en.pdf (accessed on 8 April 2024).
- Informe de Posicionamiento Terapéutico de benralizumab (Fasenra®) Como Tratamiento Adicional en el Asma Grave No Controlada Eosinofílica. Available online: https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT-benralizumab-Fasenra-asma_EPOC.pdf (accessed on 9 April 2024).
- Tezspire: EPAR—Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/tezspire-epar-product-information_en.pdf (accessed on 1 April 2024).
- Dupixent: EPAR—Product Information. Available online: https://www.ema.europa.eu/en/search?f%5B0%5D=ema_search_entity_is_document%3Adocument&search_api_fulltext=Dupixent%3A%20EPAR%20-%20Product%20Information (accessed on 1 April 2024).
- Bavbek, S.; Pagani, M.; Alvarez-Cuesta, E.; Castells, M.; Dursun, A.B.; Hamadi, S.; Madrigal-Burgaleta, R.; Sanchez-Sanchez, S.; Vultaggio, A. Hypersensitivity reactions to biologicals: An EAACI position paper. Allergy 2022, 77, 39–54. [Google Scholar] [CrossRef]
- Sitek, A.; Chiarella, S.E.; Pongdee, T. Hypersensitivity reactions to biologics used in the treatment of allergic diseases: Clinical features, diagnosis and management. Front. Allergy 2023, 4, 1219735. [Google Scholar] [CrossRef] [PubMed]
- Real Decreto 577/2013, de 26 de Julio, por el Que se Regula la Farmacovigilancia de Medicamentos de Uso Humano. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2013-8191 (accessed on 8 April 2024).
- Bate, A.; Evans, S.J.W. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol. Drug Saf. 2009, 18, 427–436. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Good Pharmacovigilance Practices (GVP) Module IX Addendum I—Methodological Aspects of Signal Detection from Spontaneous Reports of Suspected Adverse Reactions; European Medicines Agency: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Grupo de Trabajo Guía de Señales del SEFV-H. Guía de Señales del SEFV-H. Draft Version. 2024. [Google Scholar]
- Evans, S.J.; Waller, P.C.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef] [PubMed]
- ANEXO-005 Normas para la Correcta Interpretación y Utilización de los Datos del Sistema Español de Farmacovigilancia Humana (SEFV-H) [ANNEX-005 Standards for the Correct Interpretation and Use of Data from the Spanish Human Pharmacovigilance System (SEFV-H)].
- ANEXO-008 v1-Campos que No se Pueden Liberar Desde FEDRA 3 a Personas Ajenas al SEFV-H.pdf. [ANNEX-008 v1-Fields That Cannot Be Released from FEDRA 3 to People Outside the SEFV-H.pdf.]. Available online: https://etrabajo.msc.es/eRoom/Farmacovigilancia1/GRUPODELSEFVH/0_9f35 (accessed on 8 April 2024).
- González-Pérez, R.; Poza-Guedes, P.; Pineda, F.; Galán, T.; Mederos-Luis, E.; Abel-Fernández, E.; Martínez, M.J.; Sánchez-Machín, I. Molecular Mapping of Allergen Exposome among Different Atopic Phenotypes. Int. J. Mol. Sci. 2023, 24, 10467. [Google Scholar] [CrossRef]
- Garcia-Marcos, L. Grand challenges in genetics and epidemiology of allergic diseases: From genome to exposome and back. Front. Allergy 2024, 5, 1368259. [Google Scholar] [CrossRef] [PubMed]
- Frix, A.N.; Heaney, L.G.; Dahlén, B.; Mihaltan, F.; Sergejeva, S.; Popović-Grle, S.; Sedlak, V.; Lehtimäki, L.; Bourdin, A.; Korn, S.; et al. Heterogeneity in the use of biologics for severe asthma in Europe: A SHARP ERS study. ERJ Open Res. 2022, 8, 00273–2022. [Google Scholar] [CrossRef] [PubMed]
- Casas-Maldonado, F.; Álvarez-Gutiérrez, F.J.; Blanco-Aparicio, M.; Domingo-Ribas, C.; Cisneros-Serrano, C.; Soto-Campos, G.; Román-Bernal, B.; González-Barcala, F.J. Monoclonal antibody treatment for severe uncontrolled asthma in Spain: Analytical map. J. Asthma 2022, 59, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.; Despiney, R.; Pfeffer, P. Case of paradoxical adverse response to mepolizumab with mepolizumab-induced alopecia in severe eosinophilic asthma. BMJ Case Rep. 2020, 13, e233161. [Google Scholar] [CrossRef]
- Park, S. Short communication: Comments on hair disorders associated with dupilumab based on VigiBase. PLoS ONE 2022, 17, e0270906. [Google Scholar] [CrossRef]
- Suzaki, I.; Tanaka, A.; Yanai, R.; Maruyama, Y.; Kamimura, S.; Hirano, K.; Kobayashi, H. Eosinophilic granulomatosis with polyangiitis developed after dupilumab administration in patients with eosinophilic chronic rhinosinusitis and asthma: A case report. BMC Pulm. Med. 2023, 23, 130. [Google Scholar] [CrossRef]
- Flanagan, K.; Sperling, L.; Lin, J. Drug-induced alopecia after dupilumab therapy. JAAD Case Rep. 2019, 5, 54–56. [Google Scholar] [CrossRef]
- Agache, I.; Beltran, J.; Akdis, C.; Akdis, M.; Canelo-Aybar, C.; Canonica, G.W.; Casale, T.; Chivato, T.; Corren, J.; Del Giacco, S.; et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines—Recommendations on the use of biologicals in severe asthma. Allergy 2020, 75, 1023–1042. [Google Scholar] [CrossRef]
- Casas-Maldonado, F.; Álvarez-Gutiérrez, F.J.; Aparicio, M.B.; Ribas, C.D.; Serrano, C.C.; Campos, G.S.; Bernal, B.R.; González-Barcala, F.J. Treatment Patterns of Monoclonal Antibodies in Patients with Severe Uncontrolled Asthma Treated by Pulmonologists in Spain. Open Respir. Arch. 2023, 5, 100252. [Google Scholar] [CrossRef]
- Caminati, M.; Fassio, A.; Alberici, F.; Baldini, C.; Bello, F.; Cameli, P.; Conticini, E.; Cottin, V.; Crimi, C.; Dagna, L.; et al. Eosinophilic granulomatosis with polyangiitis onset in severe asthma patients on monoclonal antibodies targeting type 2 inflammation: Report from the European EGPA study group. Allergy 2024, 79, 516–519. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.Y.; Gevaert, P.; Heffler, E.; Hopkins, C.; Tversky, J.R.; Barker, P.; Cohen, D.; Emson, C.; et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2022, 149, 1309–1317.e12. [Google Scholar] [CrossRef]
- Yonezawa, H.; Ohmura, S.I.; Ohkubo, Y.; Otsuki, Y.; Miyamoto, T. New-onset of eosinophilic granulomatosis with polyangiitis without eosinophilia and eosinophilic infiltration under benralizumab treatment: A case report. Mod. Rheumatol. Case Rep. 2023, 8, 145–149. [Google Scholar] [CrossRef]
- Caminati, M.; Menzella, F.; Guidolin, L.; Senna, G. Targeting eosinophils: Severe asthma and beyond. Drugs Context 2019, 8, 212587. [Google Scholar] [CrossRef]
- Kai, M.; Vion, P.A.; Boussouar, S.; Cacoub, P.; Saadoun, D.; Le Joncour, A. Eosinophilic granulomatosis polyangiitis (EGPA) complicated with periaortitis, precipitating role of dupilumab? A case report a review of the literature. RMD Open 2023, 9, e003300. [Google Scholar] [CrossRef]
- Mota, D.; Rama, T.A.; Severo, M.; Moreira, A. Potential cancer risk with omalizumab? A disproportionality analysis of the WHO’s VigiBase pharmacovigilance database. Allergy 2021, 76, 3209–3211. [Google Scholar] [CrossRef]
- Ferastraoaru, D.; Gross, R.; Rosenstreich, D. Increased malignancy incidence in IgE deficient patients not due to concomitant Common Variable Immunodeficiency. Ann. Allergy Asthma Immunol. 2017, 119, 267–273. [Google Scholar] [CrossRef]
- Ferastraoaru, D.; Rosenstreich, D. IgE deficiency and prior diagnosis of malignancy. Ann. Allergy Asthma Immunol. 2018, 121, 613–618. [Google Scholar] [CrossRef]
- Bagnasco, D.; Canevari, R.F.; Del Giacco, S.; Ferrucci, S.; Pigatto, P.; Castelnuovo, P.; Marseglia, G.L.; Yalcin, A.D.; Pelaia, G.; Canonica, G.W. Omalizumab and cancer risk: Current evidence in allergic asthma, chronic urticaria, and chronic rhinosinusitis with nasal polyps. World Allergy Organ. J. 2022, 15, 100721. [Google Scholar] [CrossRef]
- Jackson, K.; Bahna, S.L. Hypersensitivity and adverse reactions to biologics for asthma and allergic diseases. Expert Rev. Clin. Immunol. 2020, 16, 311–319. [Google Scholar] [CrossRef]
- FDA Drug Safety Communication: FDA Approves Label Changes for Asthma Drug Xolair (Omalizumab), Including Describing Slightly Higher Risk of Heart and Brain Adverse Events. 2014. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-approves-label-changes-asthma-drug-xolair-omalizumab-including (accessed on 8 April 2024).
- Iribarren, C.; Rahmaoui, A.; Long, A.A.; Szefler, S.J.; Bradley, M.S.; Carrigan, G.; Eisner, M.D.; Chen, H.; Omachi, T.A.; Farkouh, M.E.; et al. Cardiovascular and cerebrovascular events among patients receiving omalizumab: Results from EXCELS, a prospective cohort study in moderate to severe asthma. J. Allergy Clin. Immunol. 2017, 139, 1489–1495.e5. [Google Scholar] [CrossRef]
- Oblitas, C.M.; Galeano-Valle, F.; Vela-De La Cruz, L.; Del Toro-Cervera, J.; Demelo-Rodríguez, P. Omalizumab as a Provoking Factor for Venous Thromboembolism. Drug Target Insights 2019, 13, 1177392819861987. [Google Scholar] [CrossRef]
- Satake, Y.; Sakai, S.; Takao, T.; Saeki, T. A case of subarachnoid associated with MPO-ANCA-positive eosinophilic granulomatosis with polyangiitis, successfully treated with glucocorticoid, cyclophosphamide, and mepolizumab. Mod. Rheumatol. Case Rep. 2024, 8, 310–313. [Google Scholar] [CrossRef]
- Mutoh, T.; Shirai, T.; Sato, H.; Fujii, H.; Ishii, T.; Harigae, H. Multi-targeted therapy for refractory eosinophilic granulomatosis with polyangiitis characterized by intracerebral hemorrhage and cardiomyopathy: A case-based review. Rheumatol. Int. 2022, 42, 2069–2076. [Google Scholar] [CrossRef]
- Park, H.T.; Park, S.; Jung, Y.W.; Choi, S.A. Is Omalizumab Related to Ear and Labyrinth Disorders? A Disproportionality Analysis Based on a Global Pharmacovigilance Database. Diagnostics 2022, 12, 2434. [Google Scholar] [CrossRef] [PubMed]
- Namazy, J.A.; Blais, L.; Andrews, E.B.; Scheuerle, A.E.; Cabana, M.D.; Thorp, J.M.; Umetsu, D.T.; Veith, J.H.; Sun, D.; Kaufman, D.G.; et al. Pregnancy outcomes in the omalizumab pregnancy registry and a disease-matched comparator cohort. J. Allergy Clin. Immunol. 2020, 145, 528–536.e1. [Google Scholar] [CrossRef]
- Sitek, A.N.; Li, J.T.; Pongdee, T. Risks and safety of biologics: A practical guide for allergists. World Allergy Organ. J. 2023, 16, 100737. [Google Scholar] [CrossRef]
- Khurana, S.; Brusselle, G.G.; Bel, E.H.; FitzGerald, J.M.; Masoli, M.; Korn, S.; Kato, M.; Albers, F.C.; Bradford, E.S.; Gilson, M.J.; et al. Long-term Safety and Clinical Benefit of Mepolizumab in Patients with the Most Severe Eosinophilic Asthma: The COSMEX Study. Clin. Ther. 2019, 41, 2041–2056.e5. [Google Scholar] [CrossRef]
- Jackson, D.J.; Korn, S.; Mathur, S.K.; Barker, P.; Meka, V.G.; Martin, U.J.; Zangrilli, J.G. Safety of Eosinophil-Depleting Therapy for Severe, Eosinophilic Asthma: Focus on Benralizumab. Drug Saf. 2020, 43, 409–425. [Google Scholar] [CrossRef]
- Morikawa, K.; Toyoshima, M.; Koda, K.; Kamiya, Y.; Suda, T. Cryptogenic organizing pneumonia after withdrawal of systemic corticosteroids for chronic eosinophilic pneumonia and severe asthma under benralizumab treatment. Respir. Investig. 2024, 62, 231–233. [Google Scholar] [CrossRef]
- Tanaka, A.; Fujimura, Y.; Fuke, S.; Izumi, K.; Ujiie, H. A case of bullous pemphigoid developing under treatment with benralizumab for bronchial asthma. J. Dermatol. 2023, 50, 1199–1202. [Google Scholar] [CrossRef]
- Akenroye, A.; Lassiter, G.; Jackson, J.W.; Keet, C.; Segal, J.; Alexander, G.C.; Hong, H. Comparative efficacy of mepolizumab, benralizumab, and dupilumab in eosinophilic asthma: A Bayesian network meta-analysis. J. Allergy Clin. Immunol. 2022, 150, 1097–1105.e12. [Google Scholar] [CrossRef]
- Korn, S.; Bourdin, A.; Chupp, G.; Cosio, B.G.; Arbetter, D.; Shah, M.; Gil, E.G. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for up to 5 Years. J. Allergy Clin. Immunol. Pract. 2021, 9, 4381–4392.e4. [Google Scholar] [CrossRef]
- Kavanagh, J.E.; Hearn, A.P.; Dhariwal, J.; d’Ancona, G.; Douiri, A.; Roxas, C.; Fernandes, M.; Green, L.; Thomson, L.; Nanzer, A.M.; et al. Real-World Effectiveness of Benralizumab in Severe Eosinophilic Asthma. Chest 2021, 159, 496–506. [Google Scholar] [CrossRef]
- Mishra, A.K.; Sahu, K.K.; James, A. Disseminated herpes zoster following treatment with benralizumab. Clin. Respir. J. 2019, 13, 189–191. [Google Scholar] [CrossRef]
- Jackson, D.J.; Pavord, I.D. Living without eosinophils: Evidence from mouse and man. Eur. Respir. J. 2023, 61, 2201217. [Google Scholar] [CrossRef]
- Bylund, S.; Kobyletzki, L.; Svalstedt, M.; Svensson, A. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2020, 100, adv00160. [Google Scholar] [CrossRef]
- Akinlade, B.; Guttman-Yassky, E.; de Bruin-Weller, M.; Simpson, E.L.; Blauvelt, A.; Cork, M.J.; Prens, E.; Asbell, P.; Akpek, E.; Corren, J.; et al. Conjunctivitis in dupilumab clinical trials. Br. J. Dermatol. 2019, 181, 459–473. [Google Scholar] [CrossRef] [PubMed]
- De Bruin-Weller, M.; Thaçi, D.; Smith, C.A.; Reich, K.; Cork, M.J.; Radin, A.; Zhang, Q.; Akinlade, B.; Gadkari, A.; Eckert, L.; et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: A placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFÉ). Br. J. Dermatol. 2018, 178, 1083–1101. [Google Scholar] [PubMed]
- Blauvelt, A.; Teixeira, H.D.; Simpson, E.L.; Costanzo, A.; De Bruin-Weller, M.; Barbarot, S.; Prajapati, V.H.; Lio, P.; Hu, X.; Wu, T.; et al. Efficacy and Safety of Upadacitinib vs. Dupilumab in Adults with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2021, 157, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M. Alopecia areata related paradoxical reactions in patients on dupilumab therapy: A systematic review. J. Cutan. Med. Surg. 2021, 25, 451–452. [Google Scholar] [CrossRef]
- Beaziz, J.; Bouaziz, J.D. Dupilumab-induced psoriasis and alopecia areata: Case report and review of the literature. Ann. Dermatol. Venereol. 2021, 148, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.K.; Ivert, L.U.; Bradley, B.; Lundqvist, M.; Bradley, M. Weight gain in patients with severe atopic dermatitis treated with dupilumab: A cohort study. BMC Dermatol. 2020, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gonzalez, C.; Lopez-Gonzalez, E.; Herdeiro, M.T.; Figueiras, A. Strategies to improve adverse drug reaction reporting: A critical and systematic review. Drug Saf. 2013, 36, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Avedillo-Salas, A.; Pueyo-Val, J.; Fanlo-Villacampa, A.; Navarro-Pemán, C.; Lanuza-Giménez, F.J.; Ioakeim-Skoufa, I.; Vicente-Romero, J. Prescribed Drugs and Self-Directed Violence: A Descriptive Study in the Spanish Pharmacovigilance Database. Pharmaceuticals 2023, 16, 772. [Google Scholar] [CrossRef]
- Cutroneo, P.M.; Arzenton, E.; Furci, F.; Scapini, F.; Bulzomì, M.; Luxi, N.; Caminati, M.; Senna, G.; Moretti, U.; Trifirò, G. Safety of Biological Therapies for Severe Asthma: An Analysis of Suspected Adverse Reactions Reported in the WHO Pharmacovigilance Database. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2024, 38, 425–448. [Google Scholar] [CrossRef]
| Biological Drugs (Date of Approval) | ATC-GTER Classification | Approved Indications | Brand Name |
|---|---|---|---|
| Omalizumab (25 October 2005) | R03DX05—other systemic drugs for obstructive airway diseases | Allergic asthma convincingly mediated by IgE Chronic spontaneous urticaria Chronic rhinosinusitis with nasal polyps | Xolair® (Novartis International AG, Basel, Switzerland) |
| Mepolizumab (2 December 2015) | R03DX09 | Severe refractory eosinophilic asthma Chronic rhinosinusitis with nasal polyps Eosinophilic granulomatosis with polyangiitis Hyper-eosinophilic syndrome | Nucala® (GlaxoSmithKline plc, Brentford, UK) |
| Reslizumab (15 May 2016) | R03DX08 | Insufficiently controlled severe eosinophilic asthma | Cinqaero® ▼ (Teva Pharmaceutical Industries, Petah Tikva, Israel) |
| Benralizumab (12 February 2018) | R03DX10 | Severe eosinophilic asthma | Fasenra® (AstraZeneca PLC, Cambridge, UK) |
| Tezepelumab (19 September 2022) | R03DX11 | Severe asthma | Tezspire® ▼ (Amgen Inc., Thousand Oaks, CA, USA and AstraZeneca PLC, Cambridge, UK) |
| Dupilumab (26 September 2017) | D11AH—agents for dermatitis, excluding corticosteroids | Moderate to severe atopic dermatitis Severe uncontrolled eosinophilic asthma Chronic rhinosinusitis with nasal polyps Prurigo nodularis Eosinophilic esophagitis | Dupixent® (Sanofi, Paris, France, and Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA) |
| Origin of Cases (n) | Non-Serious n (%) | Serious * n (%) |
|---|---|---|
| SEFV-H (361) | 154 (43%) | 207 (57%) |
| MAH (1985) | 1506 (76%) | 479 (24%) |
| Origin of Cases (n) | Recovered | In Recovery | Recovered with after-Effects | Not Recovered | Mortal | Unknown |
|---|---|---|---|---|---|---|
| SEFV-H (361) | 40% | 25% | 2% | 16% | 1% | 17% |
| MAH (1985) | 25% | 8% | 0.40% | 22% | 1% | 43% |
| Biological Drug | FEDRA (n) | Serious * Cases | Males | Females | Warning ** Cases |
|---|---|---|---|---|---|
| Omalizumab | 672 | 61% | 27% | 65% | 10.1% |
| Mepolizumab | 497 | 32% | 24% | 66% | 3.6% |
| Reslizumab | 35 | 54% | 17% | 74% | 17% |
| Benralizumab | 588 | 18% | 23% | 75% | 2.2% |
| Tezepelumab | 3 | 33% | 33% | 66% | - |
| Dupilumab | 536 | 29% | 47% | 45% | 5,6% |
| Omalizumab | Mepolizumab | Reslizumab | Benralizumab | Dupilumab | |
|---|---|---|---|---|---|
| Symptom Grouping | Diagnosis (No. of Cases) | Diagnosis (No. of Cases) | Diagnosis (No. of Cases) | Diagnosis (No. of cases) | Diagnosis (No. of Cases) |
| Blood and lymphatic system disorders | Anemic disorders (7) Lymphadenopathy (4) | Lymphadenopathy (11) | |||
| Cardiac disorders | Ischemic coronary artery disorders (six cases, including acute myocardial infarction) Pericarditis (4) | Ischemic coronary artery disorders (17) | |||
| Ear and labyrinth disorders | Deafness (four, two of them specified as “Deafness neurosensory”) | ||||
| Eye disorders | Eyelid edema (4) | ||||
| Gastrointestinal disorders | Abdominal pain or discomfort (11) | Abdominal pain or discomfort (14) | Vomiting and nausea (13) Diarrhea (5) Crohn’s disease (3) | ||
| General disorders and administration site conditions | Fatigue–asthenic conditions (43) | Fatigue–asthenic conditions (55) | Fatigue–asthenic conditions (18) Pyrexia (14) Malaise (12) | ||
| Immune system disorders | Multiple sclerosis (3) Sjögren’s syndrome (3) | Eosinophilic granulomatosis with polyangiitis (EGPA) (3) | |||
| Infections and infestations | Herpes viral infect. (8) Lung and lower resp. tract infection (10) | Herpes viral infect * (7) | Infective pneumonia (9) | Infective pneumonia (7) Cellulitis (3) | |
| Metabolism and nutrition disorders | Decreased appetite (3) Decreased weight (4) | Decreased appetite (2) Decreased weight (2) | |||
| Musculoskeletal and connective tissue disorders | Back pain (9) Muscular weakness (4) Muscle spasms (6) Musculoskeletal stiffness (5) | Arthralgia * (27) | Arthralgia (11) | Arthralgia (29) Myalgia (18) Muscle spasms (3) | Myalgia (5) Bone pain (4) Limb pain (5) |
| Neoplasms benign, malignant, and unspecified (incl cysts and polyps) | Breast and nipple neoplasm malignant (16) Lymphomas unspecified (4) Colorectal neoplasm malignant (7) Respiratory tract neoplasm malignant cell type unspecified. (7) | Breast and nipple neoplasm malignant (3, male breast cancer 2) Malignant neo. GI NEC (4) Respiratory tract neoplasm malignant cell type unspecified (3) | Glial tumors malignant (2) | Malignant neo.GI NEC (3) Respiratory tract neoplasm malignant cell type unspecified (2) | |
| Nervous system disorders | Cerebrovascular accident (8) Gait disturbance (3) Gait inability (1) Movement disturbances (eleven cases, including three cases of hypokinesia and five of tremor) Optic neuritis (3) | Cerebrovascular accident (3) Paraesthesias (8) Dysaesthesias (4) Hypoaesthesias (4) Tremor (5) | Headaches (25) Dizziness (10) Syncope (5) | ||
| Pregnancy, puerperium, and perinatal conditions | Abortion spontaneous and gestational fetal death (8) | ||||
| Psychiatric disorders | Anxiety, stress, and nervousness (4) | Sleep disorders (21) | Anxiety, stress, and nervousness (11) | ||
| Reproductive system and breast disorders | Menstrual cycle disorders (5) | ||||
| Respiratory, thoracic, and mediastinal disorders | Pulmonary embolism and thrombosis (5) | Pulmonary embolism and thrombosis (1) | Pulmonary embolism and thrombosis (2) | Eosinophilic pneumonia (1) | |
| Skin and subcutaneous tissue disorders | Atopic dermatitis (4) Purpura (3) Hyperhidrosis (3) | Alopecia (8) | Alopecia (1) | Alopecia (4) | Alopecia (15) |
| Vascular disorders | Deep vein thrombosis (5) | Deep vein thrombosis (3) | Hypertension (5) Hypotension (5) | Vasculitis (5) |
| Association | N | Lower Limit ROR * | Lower Limit CI ^ | X2 † |
|---|---|---|---|---|
| Omalizumab–Acute myocardial infarction (PT: acute myocardial infarction, acute coronary syndrome, and Kounis’ syndrome) | 6 | 1.48 | 0.23 | 9.25 |
| Omalizumab–Angina pectoris (PT) | 4 | 2.97 | 0.45 | 23.05 |
| Omalizumab–Pericarditis (PT: pericarditis; pleuropericarditis) | 4 | 2.86 | 0.43 | 21.95 |
| Omalizumab–Deafness (PT: deafness; neurosensorial deafness) | 4 | 3.69 | 0.54 | 30.19 |
| Omalizumab–Multiple sclerosis (PT) | 3 | 2.75 | 0.13 | 18.45 |
| Omalizumab–Sjögren’s syndrome (PT) | 3 | 15.15 | 0.42 | 122.99 |
| Omalizumab–Optic neuritis (PT) | 3 | 3.28 | 0.19 | 22.95 |
| Omalizumab–Herpes viral infection (PT: oral herpes, herpes zoster, eczema herpeticum, and herpes virus infections.) | 8 | 1.10 | 0.00 | 5.7 |
| Omalizumab–Muscular weakness; back pain; muscle spasms; joint stiffness (PTs) | 18 | 1.11 | 0.09 | 5.85 |
| Omalizumab–Breast and nipple neoplasm malignant (PTs: hormone receptor-positive breast cancer, breast cancer, invasive lobular breast carcinoma, invasive breast carcinoma, and invasive ductal breast cancer) | 16 | 23.33 | 2.84 | 540.78 |
| Omalizumab–Colon cancer (PT) | 7 | 12.49 | 1.62 | 162.52 |
| Omalizumab–Lymphoma (PT) | 4 | 1.64 | 0.10 | 9.78 |
| Omalizumab–Lung adenocarcinoma and lung malignant neoplasm (PTs) | 7 | 8.27 | 1.48 | 104.16 |
| Omalizumab–Cerebrovascular accident, ischemic stroke, and transient ischemic attack (PTs) | 8 | 2.48 | 0.82 | 24.74 |
| Omalizumab–Gait disturbance, gait inability, mobility decreased, and hypokinesia (PTs) | 8 | 1.44 | 0.29 | 9.54 |
| Omalizumab–Spontaneous abortion and gestational fetal death (PTs) | 8 | 4.05 | 1.21 | 48.52 |
| Omalizumab–Atopic dermatitis (PT) | 4 | 8.06 | 0.77 | 73.97 |
| Omalizumab–Deep vein thrombosis (PT) | 5 | 1.47 | 0.14 | 8.71 |
| Omalizumab–Embolic and thrombotic events (SMQ) | 25 | 1.51 | 0.50 | 16.69 |
| Mepolizumab–Arthralgia (PT) ** | 27 | 2.22 | 0.95 | 39.15 |
| Mepolizumab–Herpes zoster (PT) ** | 6 | 2.42 | 0.63 | 20.81 |
| Mepolizumab–Breast and nipple neoplasm malignant (HLT) and Breast malignant tumors (SMQ) | 3 | 5.36 | 0.32 | 40.85 |
| Mepolizumab–Gastrointestinal neoplasms malignant and unspecified (HLGT) | 4 | 5.41 | 0.67 | 47.55 |
| Mepolizumab–Respiratory and mediastinal neoplasms malignant and unspecified (HLGT) | 3 | 5.98 | 0.34 | 46.21 |
| Mepolizumab–Malignancies (SMQ) | 16 | 2.86 | 1.19 | 44.32 |
| Mepolizumab–Alopecia (PT) | 8 | 3.88 | 1.17 | 45.66 |
| Reslizumab–Arthralgia (PT) | 10 | 6.28 | 1.42 | 77.96 |
| Benralizumab–Alopecia (PT) | 4 | 2.68 | 0.38 | 20.05 |
| Dupilumab–Crohn’s disease (PT) | 3 | 3.67 | 0.22 | 26.23 |
| Dupilumab–Infective pneumonia (SMQ) [excluded COVID-19] | 7 | 1.92 | 0.52 | 15.47 |
| Dupilumab–Bone pain (PT) | 4 | 2.02 | 0.23 | 13.52 |
| Dupilumab–Alopecia (PT) | 15 | 5.00 | 1.76 | 92.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boada-Fernández-del-Campo, C.; García-Sánchez-Colomer, M.; Fernández-Quintana, E.; Poza-Guedes, P.; Rolingson-Landaeta, J.L.; Sánchez-Machín, I.; González-Pérez, R. Real-World Safety Profile of Biologic Drugs for Severe Uncontrolled Asthma: A Descriptive Analysis from the Spanish Pharmacovigilance Database. J. Clin. Med. 2024, 13, 4192. https://doi.org/10.3390/jcm13144192
Boada-Fernández-del-Campo C, García-Sánchez-Colomer M, Fernández-Quintana E, Poza-Guedes P, Rolingson-Landaeta JL, Sánchez-Machín I, González-Pérez R. Real-World Safety Profile of Biologic Drugs for Severe Uncontrolled Asthma: A Descriptive Analysis from the Spanish Pharmacovigilance Database. Journal of Clinical Medicine. 2024; 13(14):4192. https://doi.org/10.3390/jcm13144192
Chicago/Turabian StyleBoada-Fernández-del-Campo, Carlos, Marcelino García-Sánchez-Colomer, Eduardo Fernández-Quintana, Paloma Poza-Guedes, Jaime Leonardo Rolingson-Landaeta, Inmaculada Sánchez-Machín, and Ruperto González-Pérez. 2024. "Real-World Safety Profile of Biologic Drugs for Severe Uncontrolled Asthma: A Descriptive Analysis from the Spanish Pharmacovigilance Database" Journal of Clinical Medicine 13, no. 14: 4192. https://doi.org/10.3390/jcm13144192
APA StyleBoada-Fernández-del-Campo, C., García-Sánchez-Colomer, M., Fernández-Quintana, E., Poza-Guedes, P., Rolingson-Landaeta, J. L., Sánchez-Machín, I., & González-Pérez, R. (2024). Real-World Safety Profile of Biologic Drugs for Severe Uncontrolled Asthma: A Descriptive Analysis from the Spanish Pharmacovigilance Database. Journal of Clinical Medicine, 13(14), 4192. https://doi.org/10.3390/jcm13144192








