Salivary Cortisol Levels after Hydrotherapy and Land-Based Therapy as a Marker of Stress in Children with Psychomotor Developmental Disorders: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
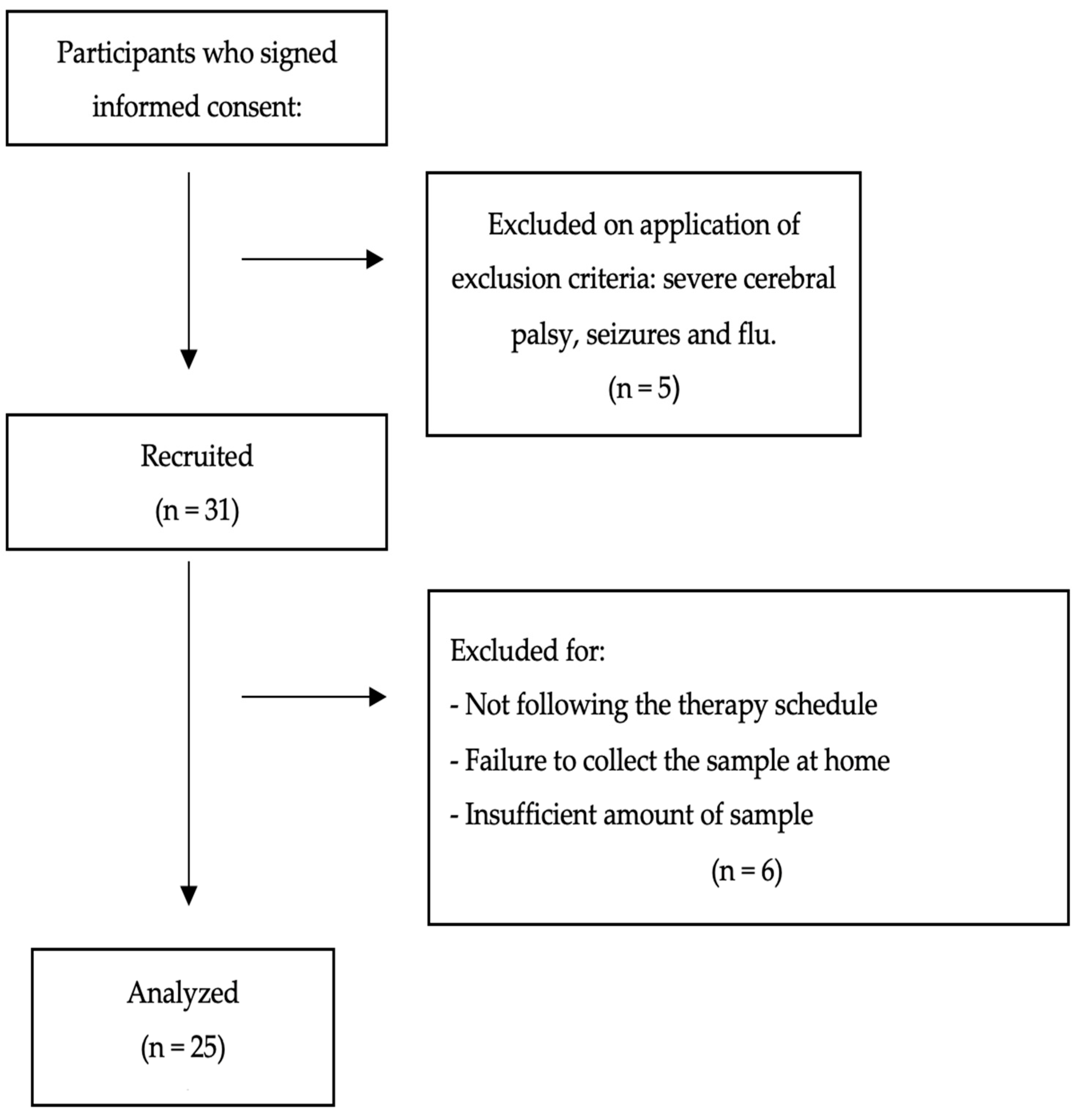
2.1. Design and Participants
2.2. Ethical Approval
2.3. Sample Collection and Transport to the Laboratory
2.4. Sample Analysis Procedure
2.5. Physiotherapy Interventions
2.5.1. Hydrotherapy
2.5.2. Land-Based Therapy
2.6. Statistical Analysis
3. Results
3.1. Demographic Information
3.2. Salivary Cortisol Levels after HYDRO and LBT
3.3. Statistical Group Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García Pérez, M.A.; Martínez Granero, M.A. Desarrollo psicomotor y signos de alarma. In Curso de Actualización Pediatría 2016; AEPap, Ed.; Lúa Ediciones 3.0: Madrid, Spain, 2016; pp. 81–93. [Google Scholar]
- Del Río, R.; Thió, M.; Bosio, M.; Figueras, J.; Iriondo, M. Prediction of mortality in premature neonates. An updated systematic review. An. De Pediatría (Engl. Ed.) 2020, 93, 24–33. [Google Scholar] [CrossRef]
- Kiebzak, W.; Zurawski, A.; Gluszek, S.; Koszto-lowicz, M.; Bialek, W.A. Cortisol levels in infants with central coordination disorders during VOJTA therapy. Children 2021, 8, 1113. [Google Scholar] [CrossRef]
- Campos-Berga, L.; Moreno-Giménez, A.; Sahuquillo-Leal, R.; Hervás, D.; Diago, V.; Navalón, P.; Vento, M.; García-Blanco, A. Emotional regulation and psychomotor development after threatening preterm labor: A prospective study. Eur. Child Adolesc. Psychiatry 2021, 31, 473–481. [Google Scholar] [CrossRef]
- Arslan, F.N.; Dogan, D.G.; Canaloglu, S.K.; Baysal, S.G.; Buyukavci, R.; Buyukavci, M.A. Effects of early physical therapy on motor development in children with Down syndrome. North. Clin. Istanb. 2022, 9, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Cazorla González, J.; Cornellà i Canals, J. Las posibilidades de la fisioterapia en el tratamiento multidisciplinar del autismo. Pediatría Atención Primaria 2014, 16, e37–e46. [Google Scholar] [CrossRef]
- Bourseul, J.S.; Brochard, S.; Houx, L.; Pons, C.; Bué, M.; Manesse, I.; Ropars, J.; Guyader, D.; Le Moine, P.; Dubois, A. Care-related pain and discomfort in children with motor disabilities in rehabilitation centres. Ann. Phys. Rehabil. Med. 2016, 59, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, L.E.; Guisado, C.G.; Fuentes, M.M. Efectividad de la hidroterapia en atención temprana. Fisioterapia 2012, 34, 79–86. [Google Scholar] [CrossRef]
- Latorre-García, J.; Sánchez-López, A.M.; Baena García, L.; Noack Segovia, J.P.; Aguilar-Cordero, M.J. Influencia de la actividad física acuática sobre el neurodesarrollo de los bebés: Revisión sistemática. Nutr. Hosp. 2016, 33, 10–17. [Google Scholar] [CrossRef]
- McManus, B.M.; Kotelchuck, M. The effect of aquatic therapy on functional mobility of infants and toddlers in early intervention. Pediatr. Phys. Ther. 2007, 19, 275–282. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Tobinaga, W.C.; de Lima Marinho, C.; Abelenda, V.L.B.; de Sá, P.M.; Lopes, A.J. Short-term effects of hydrokinesiotherapy in hospitalized preterm newborns. Rehabil. Res. Pract. 2016, 2016, 9285056. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, M.; Du, S.; Li, H.; Li, X. Evaluation of stress and pain in young children with cerebral palsy during early developmental intervention programs: A descriptive study. Am. J. Phys. Med. Rehabil. 2015, 94, 169–179. [Google Scholar] [CrossRef]
- Macari, G.H.; Tapia, A.G.; Iniguez, G.; Weisstaub, G. Psychomotor development and cortisol salivary levels in infants that live with their inmate mothers. Rev. Chil. De Pediatría 2019, 90, 275–282. [Google Scholar]
- Oldehinkel, A.J.; Ormel, J.; Bosch, N.M.; Bouma, E.M.; Van Roon, A.M.; Rosmalen, J.G.; Riese, H. Stressed out? Associations between perceived and physiological stress responses in adolescents: The TRAILS study. Psychophysiology 2011, 48, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Antonini, S.R.; Jorge, S.M.; Moreira, A.C. The emergence of salivary cortisol circadian rhythm and its relationship to sleep activity in preterm infants. Clin. Endocrinol. 2000, 52, 423–426. [Google Scholar] [CrossRef]
- Vignochi, C.; Teixeira, P.P.; Nader, S.S. Effect of aquatic physical therapy on pain and state of sleep and wakefulness among stable preterm newborns in neonatal intensive care units. Braz. J. Phys. Ther. 2010, 14, 214–220. [Google Scholar] [CrossRef]
- Reguera Nieto, E.A. Apego, cortisol y estrés, en infantes: Una revisión narrativa. Rev. De La Asoc. Española De Neuropsiquiatría 2014, 34, 753–772. [Google Scholar] [CrossRef][Green Version]
- Wegner, M.; Koutsandréou, F.; Müller-Alcazar, A.; Lautenbach, F.; Budde, H. Effects of different types of exercise training on the cortisol awakening response in children. Front. Endocrinol. 2019, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.; Levitan, R.; Leung, E.; Masellis, M.; Basile, V.S.; Nemeroff, C.B.; Atkinson, L. Cortisol concentrations in 12-to 18-month-old infants: Stability over time, location, and stressor. Biol. Psychiatry 2003, 54, 719–726. [Google Scholar] [CrossRef]
- Cândia, M.F.; Osaku, E.F.; Leite, M.A.; Toccolini, B.; Costa, N.L.; Teixeira, S.N.; Osaku, N.O. Influência do posicionamento em prona sobre o estresse no recém-nascido prematuro avaliada pela dosagem de cortisol salivar: Um estudo piloto. Rev. Bras. De Ter. Intensiv. 2014, 26, 169–175. [Google Scholar]
- Aguilar-Cordero, M.; Sánchez López, A.; Mur Villar, N.; García García, I.; López, R.; Ortegón Piñero, A.; Cortés Castell, E. Cortisol salival como indicador de estrés fisiológico en niños y adultos: Revisión sistemática. Nutr. Hosp. 2014, 29, 960–968. [Google Scholar]
- Morera, L.P.; Tempesti, T.C.; Pérez, E.; Medrano, L.A. Biomarcadores en la medición del estrés: Una revisión sistemática. Ansiedad Y Estrés 2019, 25, 49–58. [Google Scholar] [CrossRef]
- De Freitas, P.; Bueno, M.; Holditch-Davis, D.; Santos, H.P.; Kimura, A.F. Biobehavioral responses of preterm infants to conventional and swaddled tub baths: A randomized crossover trial. J. Perinat. Neonatal Nurs. 2018, 32, 358–365. [Google Scholar] [CrossRef]
- Fogaça, M.d.C.; Carvalho, W.B.; Peres, C.d.A.; Lora, M.I.; Hayashi, L.F.; Verreschi, I.T.d.N. Salivary cortisol as an indicator of adrenocortical function in healthy infants, using massage therapy. Sao Paulo Med. J. 2005, 123, 215–218. [Google Scholar] [CrossRef]
- Papacosta, E.; Nassis, G.P. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J. Sci. Med. Sport 2011, 14, 424–434. [Google Scholar] [CrossRef]
- Ramsay, D.; Lewis, M. Reactivity and regulation in cortisol and behavioral responses to stress. Child Dev. 2003, 74, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Doan, B.K.; Newton, R.; Kraemer, W.; Kwon, Y.H.; Scheet, T. Salivary cortisol, testosterone, and T/C ratio responses during a 36-hole golf competition. Int. J. Sports Med. 2006, 28, 470–479. [Google Scholar] [CrossRef]
- Crewther, B.; Cronin, J.; Keogh, J.; Cook, C. The salivary testosterone and cortisol response to three loading schemes. J. Strength Cond. Res. 2008, 22, 250–255. [Google Scholar] [CrossRef]
- Nalla, A.A.; Thomsen, G.; Knudsen, G.M.; Frokjaer, V.G. The effect of storage conditions on salivary cortisol concentrations using an enzyme immunoassay. Scand. J. Clin. Lab. Investig. 2015, 75, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Garde, A.H.; Hansen, Å.M. Long-term stability of salivary cortisol. Scand. J. Clin. Lab. Investig. 2005, 65, 433–436. [Google Scholar] [CrossRef] [PubMed]
- García, J.L.; López, A.M.S.; García, L.B.; de Cabo, L.F.; Benítez, I.V.; Cordero, M.J.A. Actividad física en el agua para mejorar la psicomotricidad de los bebés sanos. Protocolo del estudio Babyswimming. J. Negat. No Posit. Results JONNPR 2017, 2, 186–193. [Google Scholar]
- Shaw, S.C.; Sankar, M.J.; Thukral, A.; Agarwal, R.; Deorari, A.K.; Paul, V.K. Assisted physical exercise and stress in preterm neonates. Indian Pediatr. 2018, 55, 679–682. [Google Scholar] [CrossRef]
- Durán-Carabali, L.E.; Henao-Pacheco, M.L.; González-Clavijo, A.M.; Dueñas, Z. Salivary alpha amylase and cortisol levels as stress biomarkers in children with cerebral palsy and their association with a physical therapy program. Res. Dev. Disabil. 2021, 108, 103807. [Google Scholar] [CrossRef] [PubMed]
- White-Traut, R.C.; Schwertz, D.; McFarlin, B.; Kogan, J. Salivary cortisol and behavioral state responses of healthy newborn infants to tactile-only and multisensory interventions. J. Obstet. Gynecol. Neonatal Nurs. 2009, 38, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.P.L.; Lau, W.K.W. Revisit the effectiveness of educational kinesiology on stress and anxiety amelioration in kindergarteners with special needs using biological measures. Front. Psychiatry 2021, 12, 773659. [Google Scholar] [CrossRef] [PubMed]
- Efe, Y.S.; Erdem, E.; Güneş, S.T. The effect of daily exercise program on bone mineral density and cortisol level in preterm infants with very low birth weight: A randomized controlled trial. J. Pediatr. Nurs. 2020, 51, e6–e12. [Google Scholar]
- Hayes, L.D.; Grace, F.M.; Baker, J.S.; Sculthorpe, N. Exercise-induced responses in salivary testosterone, cortisol, and their ratios in men: A meta-analysis. Sports Med. 2015, 45, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A. Exercise and circulating cortisol levels: The intensity threshold effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef]
- Ntovas, P.; Loumprinis, N.; Maniatakos, P.; Margaritidi, L.; Rahiotis, C. The effects of physical exercise on saliva composition: A comprehensive review. Dent. J. 2022, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, D.E.; Paul, E.E. Aquatic therapy for individuals with cerebral palsy across the lifespan. In Cerebral Palsy; Springer: Cham, Switzerland, 2020; pp. 2641–2660. [Google Scholar]
- Leo, I.; Leone, S.; Dicataldo, R.; Vivenzio, C.; Cavallin, N.; Taglioni, C.; Roch, M. A non-randomized pilot study on the benefits of baby swimming on motor development. Int. J. Environ. Res. Public Health 2022, 19, 9262. [Google Scholar] [CrossRef]
- Thorpe, D.E.; Reilly, M.; Case, L. The effects of an aquatic resistive exercise program on ambulatory children with cerebral palsy. J. Aquat. Phys. Ther. 2005, 13, 21–34. [Google Scholar]
- Sadeh, A. A brief screening questionnaire for infant sleep problems: Validation and findings for an Internet sample. Pediatrics 2004, 113, e570–e577. [Google Scholar] [CrossRef]
- Backhaus, J.; Junghanns, K.; Hohagen, F. Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology 2004, 29, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Scher, A.; Hall, W.A.; Zaidman-Zait, A.; Weinberg, J. Sleep quality, cortisol levels, and behavioral regulation in toddlers. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2010, 52, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Ivars, K.; Nelson, N.; Theodorsson, A.; Theodorsson, E.; Ström, J.O.; Mörelius, E. Development of salivary cortisol circadian rhythm and reference intervals in full-term infants. PLoS ONE 2015, 10, e0129502. [Google Scholar] [CrossRef]
- Urfer, A.; Turpin, H.; Dimitrova, N.; Borghini, A.; Plessen, K.J.; Morisod Harari, M.; Urben, S. Consequences of prematurity on cortisol regulation and adjustment difficulties: A 9-year longitudinal study. Children 2021, 9, 9. [Google Scholar] [CrossRef]
- Van Bodegom, M.; Homberg, J.R.; Henckens, M.J. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front. Cell. Neurosci. 2017, 11, 87. [Google Scholar] [CrossRef]
- Gao, H.; Xu, G.; Li, F.; Lv, H.; Rong, H.; Mi, Y.; Li, M. Effect of combined pharmacological, behavioral, and physical interventions for procedural pain on salivary cortisol and neurobehavioral development in preterm infants: A randomized controlled trial. Pain 2021, 162, 253–262. [Google Scholar] [CrossRef]
- Singer, H.S.; Kossoff, E.H.; Hartman, A.L.; Thomas, O. Crawford. Treatment of Pediatric Neurologic Disorders, 2nd ed.; Taylor & Francis Group: Abingdon, UK, 2019; pp. 117–128. [Google Scholar]
- Kuo, P.X.; Saini, E.K.; Tengelitsch, E.; Volling, B.L. Is one secure attachment enough? Infant cortisol reactivity and the security of infant-mother and infant-father attachments at the end of the first year. Attach. Hum. Dev. 2019, 21, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Granger, D.A.; Blair, C.; Willoughby, M.; Kivlighan, K.T.; Hibel, L.C.; Fortunato, C.K.; Wiegand, L.E. Individual differences in salivary cortisol and alpha-amylase in mothers and their infants: Relation to tobacco smoke exposure. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2007, 49, 692–697. [Google Scholar] [CrossRef]

| Outcome | Description | N | % | |
|---|---|---|---|---|
| Sex | Female | 12 | 48 | |
| Male | 13 | 52 | ||
| Gestational age, mean *(SD) | 35.52 | (5.67) weeks. | ||
| Premature | 11 | 44 | ||
| Age, mean (SD) | 7.46 | (7.54) months. | ||
| Birth weight, mean (SD) | 2496 | (1022.59) grams. | ||
| Functional diagnosis | Neurological risk | 21 | 84 | |
| GDD | 4 | 16 | ||
| COVID-19 infection | 12 | 48 | ||
| Nutrition | Breastfeeding | 11 | 44 | |
| Mixed | 9 | 36 | ||
| Formula | 5 | 20 | ||
| Maternal age, mean (SD) | 33.48 | (5.14) years. | ||
| Parents’ educational level | Basic/high school | 6 | 24 | |
| University | 19 | 76 | ||
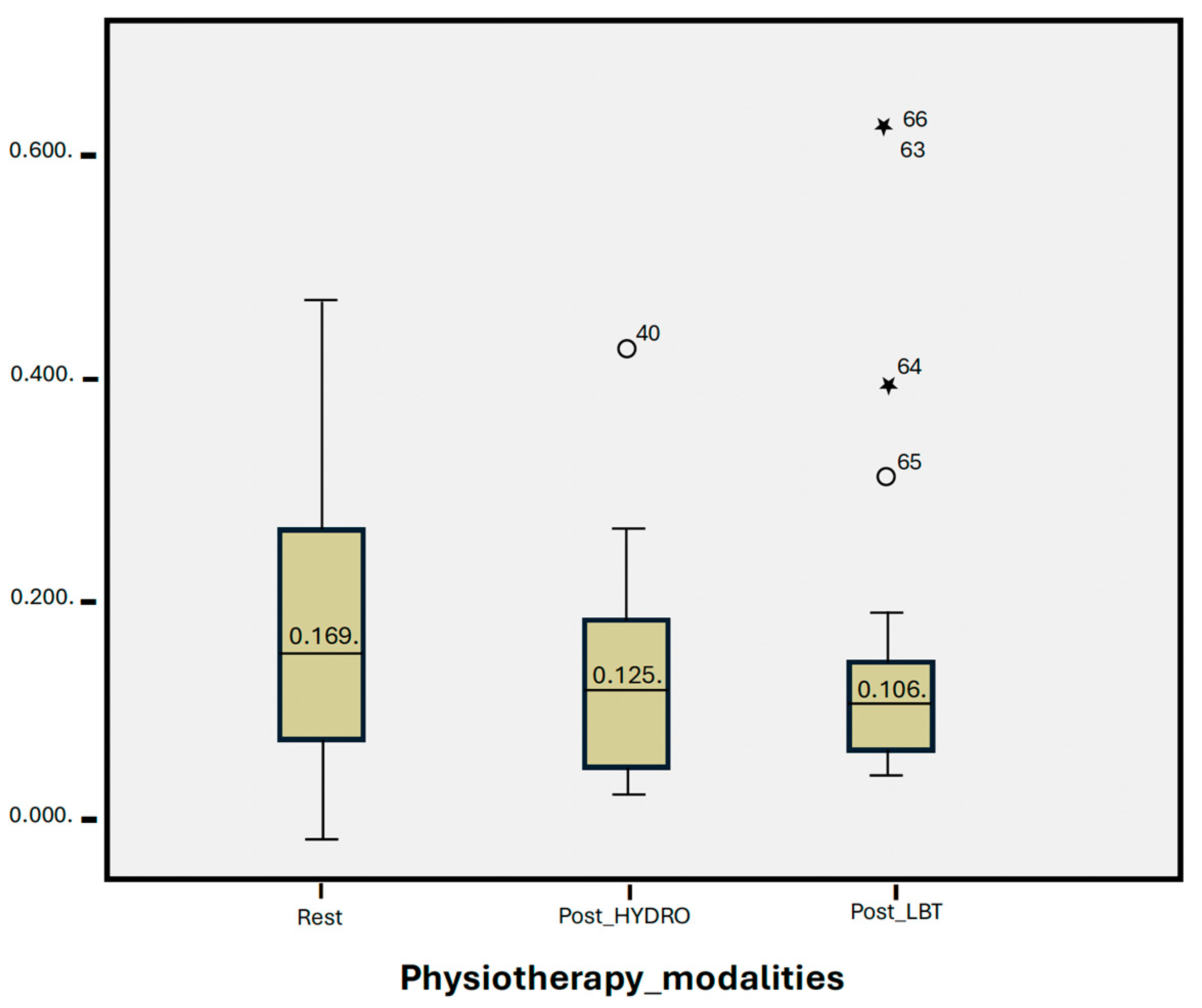
| Median | * SD | p Value | |
|---|---|---|---|
| Resting | 0.169 | 0.13 | |
| Post-HYDRO | 0.125 | 0.09 | 0.107 |
| Post-LBT | 0.106 | 0.32 | 0.271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Cordero, M.J.; Michel-Araya, S.; Noack Segovia, J.P.; Latorre-García, J.; Rojas-Carvajal, A.M.; Castillos, R.F. Salivary Cortisol Levels after Hydrotherapy and Land-Based Therapy as a Marker of Stress in Children with Psychomotor Developmental Disorders: A Pilot Study. J. Clin. Med. 2024, 13, 4147. https://doi.org/10.3390/jcm13144147
Aguilar-Cordero MJ, Michel-Araya S, Noack Segovia JP, Latorre-García J, Rojas-Carvajal AM, Castillos RF. Salivary Cortisol Levels after Hydrotherapy and Land-Based Therapy as a Marker of Stress in Children with Psychomotor Developmental Disorders: A Pilot Study. Journal of Clinical Medicine. 2024; 13(14):4147. https://doi.org/10.3390/jcm13144147
Chicago/Turabian StyleAguilar-Cordero, María José, Sabina Michel-Araya, Jessica Pamela Noack Segovia, Julio Latorre-García, Ana María Rojas-Carvajal, and Rafael Fernández Castillos. 2024. "Salivary Cortisol Levels after Hydrotherapy and Land-Based Therapy as a Marker of Stress in Children with Psychomotor Developmental Disorders: A Pilot Study" Journal of Clinical Medicine 13, no. 14: 4147. https://doi.org/10.3390/jcm13144147
APA StyleAguilar-Cordero, M. J., Michel-Araya, S., Noack Segovia, J. P., Latorre-García, J., Rojas-Carvajal, A. M., & Castillos, R. F. (2024). Salivary Cortisol Levels after Hydrotherapy and Land-Based Therapy as a Marker of Stress in Children with Psychomotor Developmental Disorders: A Pilot Study. Journal of Clinical Medicine, 13(14), 4147. https://doi.org/10.3390/jcm13144147








