Abstract
Background and Objectives: Fibromyalgia is a multifaceted and frequently misunderstood chronic pain disease marked by widespread musculoskeletal pain and cognitive/somatic dysfunction. This trial aims to contribute to the existing knowledge on treating fibromyalgia (FM) with medical cannabis (Cannabis sativa L.) and explore a safer and more effective cannabis administration method. The goal is to provide evidence-based findings that can guide alternative treatment options for FM patients by assessing a pilot study. Materials and Methods: The trial was performed at the pain therapy unit of the San Carlo Hospital (Potenza, Italy) by administrating to 30 FM patients 100 mg/day of Bedrocan® (Bedrocan International, Veendam, The Netherlands) as a decoction. The Numerical Rating Scale (NRS) and SF-12 short-form health questionnaire were used to evaluate pain intensity and the quality of life at the beginning of the study and the 6th-month follow-up. A systematic review of all clinical studies investigating the use of cannabis to reduce FM was also undertaken to place this study in the context of the existing evidence base. Results: Pain intensity evaluated with the NRS lowered from a median of 8 [95% CI 7.66–8.54] at a baseline to a median of 4 (95% CI 3.28–4.79) after 6 months of follow-up (p-value < 0.001; t-test). Similarly, significant physical and mental state improvement, evaluated with the SF-12 questionnaire, was found in 96.67% and 82.33% of patients, respectively (95% CI 44.11–51.13 for the physical state, and 53.48–58.69 for mental state assessed after the 6th-month follow-up; p-value < 0.001; t-test). The systematic analysis of the literature identified 10 clinical trials concerning the treatment of fibromyalgia with cannabis. Conclusions: Considering results from the present pilot study and systematic review, it is possible to assume that medical cannabis may be considered an alternative therapy for FM patients who do not respond to conventional pharmacological therapy.
1. Introduction
Fibromyalgia (FM) is a term introduced in the early 1970s denoting a multifaceted and challenging clinical condition that transcends conventional medical classifications. FM definition has been revised significantly over the years, reflecting the evolving understanding of the condition. Initially considered mainly as a rheumatic disorder, it is now acknowledged as a pain processing disorder and sensitization of the central nervous system [1]. FM is, indeed, one of the most common causes of persistent chronic widespread pain (CWP). However, although pain is its main feature, it is represented by a complex polysymptomatology comprising fatigue, sleep disturbances, generalized hyperalgesia, stiffness, palpation-specific tender points, and cognitive and somatic dysfunction [2]. Pain might be initially generalized or localized in a specific body region, such as the neck or lower back. Fatigue is chronic in FM patients and is frequently referred to as moderate to severe, while cognitive problems are informally reported as part of the “fibro fog” and consist of compromised memory, attention, and concentration [3]. Due to FM’s multifaceted and complex nature, it poses a significant diagnostic challenge for doctors. For this reason, an FM diagnosis takes about two years, and patients usually have to see several medical specialists during this time [4]. Failure to diagnose FM in the past was exacerbated by the medical community’s skepticism, considering it as a psychosomatic disorder. This skepticism has hindered the recognition and understanding of FM due to the lack of objective diagnostic criteria and markers. However, as studies progressed, an expanding body of evidence illuminated the intricate interplay of psychological, biological, and social factors influencing the syndrome outbreak [1]. Nowadays, several diagnostic criteria have been developed, but those approved by the American College of Rheumatology (ACR) and first introduced in 1990 are normally used. Following the ACR, for the FM diagnosis, pain must be triggered by exerting pressure (tender points) up to 4 kg/cm2 in at least 11 of the 18 specified body points, and patients must have a multisite or widespread pain lasting more than 3 months [4]. In this context, widespread pain was labeled as right- and left-side pain, axial pain, and lower- and upper-segment pain [5]. However, the ACR classification criteria did not consider symptoms recently related to FM, such as cognitive problems and somatic symptoms. For this reason, in 2010, two variables best defined FM and its symptom aspect were identified: the widespread pain index (WPI) and the symptom severity (SS) scale. The WPI is strongly related to nineteen tender points (Figure 1), while the SS scale evaluates fatigue grade, unrefreshed sleep, and physician-rated cognitive symptoms (Table 1).
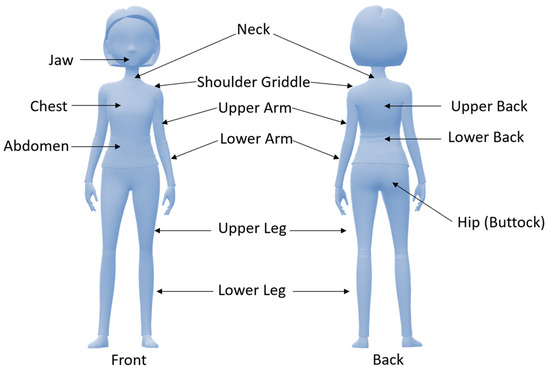
Figure 1.
Tender points used for the diagnosis of fibromyalgia with the widespread pain index (WPI).

Table 1.
Symptom severity (SS) scale.
These two criteria were combined to define a new FM case definition: WPI 7 and SS 7 or WPI 3–6 and SS 9 [5].
For FM, a multidisciplinary intervention is advocated since pharmacological treatments are generally combined with approaches involving psychotherapy, patient education, physiotherapy, and pharmacological treatments [2,6]. The commonly prescribed drugs are antidepressants, anticonvulsants, muscle relaxants, analgesics, hypnotics, and antipsychotics [6,7]. Table 2 lists the main drugs used to treat FM [8,9].

Table 2.
FM pharmacological treatment.
However, many of these drugs offer few benefits and are frequently associated with side effects that may reduce patient compliance [10]. In the last year, particular attention was paid to the use of natural products in clinical practice [11,12,13]; specifically, cannabinoid-containing products were investigated in clinical settings due to their anti-inflammatory and immunomodulatory activity, making them useful for managing pain-associated symptoms [14]. The current literature has indeed demonstrated that medicinal cannabis (Cannabis sativa L.) can be associated with an improved quality of life for patients suffering from chronic pain [15]. Two different types of cannabinoid-containing products exist on the market: isolated compounds [delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD)] and plant-based (cannabis) products. In the present 6-month pilot study, the effect of a plant-based formulation (cannabis: Bedrocan®, 22% THC, <1% CBD) has been studied on a sample of 30 adult patients with FM resistant to opioid therapy. The choice of testing a plant-based formulation, and not an isolated compound, was made considering that cannabis, besides THC and CBD, contains other cannabinoid and non-cannabinoid molecules like terpenes that can synergically contribute to reducing pain [16,17]. In fact, for the so-called “entourage effect”, terpenes interact with cannabinoids, improving their pharmacodynamic effects by increasing cannabinoids’ charge binding to their receptors and pulmonary uptake [18]. Further, terpenes have their own effects, like analgesic, anti-inflammatory, and antidepressant effects [19]. Therefore, this study aims to evaluate the effectiveness of cannabis (Bedrocan®) therapy in fibromyalgia patients by analyzing their quality of life, as measured by the SF-12 short-form health questionnaire and by measuring pain intensity with the Numerical Pain Rating Scale (NRS). In addition, to place this study in the context of the existing evidence base, a systematic review of all clinical studies investigating the use of cannabis to reduce FM was also undertaken.
2. Materials and Methods
2.1. Study Design, Setting, and Outcomes
The current clinical trial is a pilot study (ClinicalTrials.gov Identifier: NCT05939466) investigating the analgesic effect of medical cannabis (MC) in adult Italian patients with a diagnosis of FM and who reported pain resistance to pharmacological treatments (e.g., Patrol, Busette, Arcoxia, Dulex, Depalgos, etc.).
Eligible subjects were those visiting the pain therapy unit of the San Carlo Hospital (Potenza, Italy) and fitted with the following inclusion criteria: informed written consent; age >18 years old; diagnosis of FM confirmed by a rheumatologist; persistent pain symptoms for at least three months without complaints that may otherwise explain the pain condition; persistent pain syndrome on conventional therapy with opioids or non-steroidal anti-inflammatory drugs; not having taken MC in the previous year since the start of the study; stopping drug therapy during the trial with cannabis (Bedrocan®).
Subjects were excluded from the trial if they agreed with the following criteria: specific contraindications to cannabinoid use; pain syndrome not associated with FM; major comorbidities like renal impairment, severe liver disease, chronic hepatitis C, history of alcohol or drug addiction; the presence of cognitive deficits that could impair understanding of the study, completion of questionnaires, or adherence to therapy; pregnant or planning pregnancy women and breastfeeding women.
This study was approved by the San Carlo Hospital ethics committee (n. of CEUR general register 37/2023, n. protocol of the Potenza’s AOR San Carlo referring to application for approval 20230021536, protocol date 11 May 2023) and was conducted in the pain therapy unit of the San Carlo Hospital (Potenza, Italy) from March 2021 to September 2021. During this period, 44 subjects who suffered from pain syndrome came to the pain therapy unit and were examined by a specialist. The presence of FM syndrome was validated by using the WPI and the SS scale (WPI ≥ 7 e SS ≥ 5 o WPI 3–6 e SS >9). Eligible subjects who provided informed written consent for starting MC treatment were given the prescription for Bedrocan® once a month for a total of 30 charts per month for 6 months. Specifically, 34 patients were recruited at the beginning of the study. However, 2 subjects did not start therapy in time to be included in the present study and were excluded, while 2 other patients dropped out of therapy due to side effects. Thus, the total number of analyzed FM subjects was 30 (Figure 2).
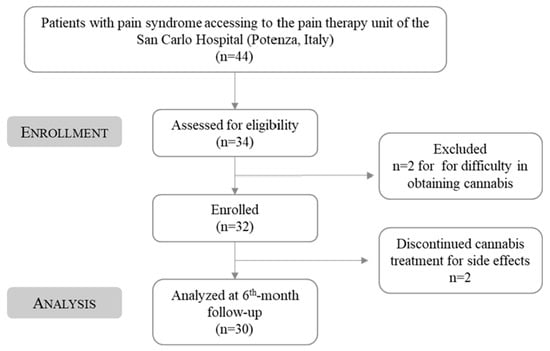
Figure 2.
Study flow-chart diagram.
Each subject was assigned a numerical identification code to ensure the patient’s anonymity.
The study’s primary outcomes include the evaluation of the Numerical Pain Rating Scale (NRS), a valuable tool in clinical practice for assessing and monitoring pain levels, guiding treatment decisions, and evaluating the effectiveness of pain management interventions (Figure 3).
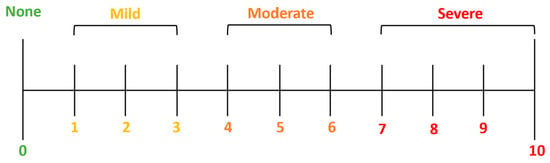
Figure 3.
Numerical Pain Rating Scale (NRS).
The study’s secondary outcomes include the evaluation of the short-form health survey (SF-12), a valuable tool for assessing health status and evaluating the impact of interventions on physical and mental well-being in clinical and research settings. The SF-12 consists of 12 questions that cover eight health domains: physical functioning, role limitations due to physical health, bodily pain, general health perceptions, vitality (energy/fatigue), social functioning, role limitations due to emotional problems, and mental health (psychological distress and well-being). Responses to the SF-12 are scored using a standardized method to derive two summary scores: physical component summary (PCS), reflecting the overall physical health status, and mental component summary (MCS), indicating the overall mental health status.
2.2. Treatment Regimen
Bedrocan®-type cannabis (22% THC, <1% CBD) was used because, compared to other formulations such as FM2 and Bediol (Bedrocan International), it ensured a more constant supply in Lucanian pharmacies, reducing the risk of therapy interruption due to the lack of availability of the drug. The MC was administered with herbal tea or decoction, as described by the Ministry of Health, through the Ministerial Decree of 9 November 2015. In the case of the present study, the Dutch protocol was used, which, in addition to the Italian protocol, provides for adding milk to form a lipophilic emulsion capable of increasing the extraction of the cannabinoids.
The nurses instructed the patients on how to prepare the decoction, as described below. Place the inflorescences and water in a container at the following ratio: 100 mL cold water for every 100 mg of cannabis. Heat to a boil and simmer for 15 min, stirring regularly. Allow the decoction to cool for about 15 min, then stir and strain by pressing the remaining residue on the filter with a spoon to recover more liquid and enrich the final solution. If the decoction is not consumed at the time of preparation, it can be stored in a closed container in the refrigerator for up to 24 h. When cannabis is taken orally by ingesting it, it takes 30–90 min before the effects are felt, maximum after about two to three hours and minimum after about six hours.
The therapy with Bedrocan® was started with 100 mg/day (1 chart) and was increased to 2 charts (200 mg/day) at the first-month follow-up in non-responsive patients.
2.3. Statistical Analysis
For the analysis of the data concerning the SF-12 questionnaire, the free source code made available by the Istituto di Ricerche Farmacologiche Mario Negri of Milan in Annex 4 of the manual Questionarioe Sullo Stato Di Salute SF-12 Italian version [20]. All clinical information collected during the clinical trial was stored and analyzed using Microsoft Excel (Microsoft Office Professional Plus 2021) and Minitab® (version 19). The final analysis was performed per protocol (PP) since only data from subjects who strictly adhered to the study protocol were analyzed. All data were expressed as median, interquartile range calculated at 25° percentile, mean ± standard deviation (SD), and 95% interval confidence (95% IC). Results with a p-value < 0.001 were considered significant.
2.4. Systematic Review
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a systematic literature search was performed in March 2023, including all clinical studies published until April 2023. Scopus (https://www.scopus.com/standard/marketing.uri) and PubMed (https://pubmed.ncbi.nlm.nih.gov/) were used as research databases. Only full texts were included, and those not available were not requested, as keywords were utilized: “cannabis” and “fibromyalgia”; “cannabis” and “fibromyalgia” and “Clinical trial”; “cannabis” and “fibromyalgia” and “Observational study”; “cannabis” and “fibromyalgia” and “Randomized study”.
The systematic research was performed by including only clinical investigation involving the use of cannabis for Fibromyalgia syndrome treatment. Only articles written in English and containing the keyword in the abstract or title were included, while review articles, meta-analyses, letters, editorials, abstracts, and manuscripts with a not available text were not used for writing the systematic review. Two independent investigators (Rocco Palmieri and Maria Ponticelli) selected documents based on the title and abstract adequacy and subsequently analyzed all full texts. In case of disagreement, other independent reviewers were involved (Luigi Milella and Antonio Giardina). All articles selected were closely reviewed for the inclusion or exclusion of manuscripts that did not comply with the described criteria.
Methodological Quality Assessment
Articles quality assessment was performed considering the Cochrane Methods Bias (https://www.riskofbias.info/welcome/home) for clinical studies. Specifically, the risk of bias evaluation was performed considering the absence or presence of the information reported in the Cochrane Handbook for Systematic Reviews of Interventions for randomized and non-randomized studies [21,22]. The robvis tool, including ROB2 for randomized clinical studies [23] and ROBINS-1 for non-randomized clinical study [24], was used to organize the data.
3. Results
3.1. Patients Characteristics
Forty-four patients (34 women and 10 men) with a median age of 51.5 years were visited for pain conditions in the pain therapy unit of the San Carlo Hospital (Potenza, Italy). Among these 44 subjects, 34 (28 women and 6 men) were considered eligible for study participation since a correlation between pain and FM was diagnosed. Two subjects, due to supply problems, did not start therapy in time to be included in the present study and were excluded, while two other patients dropped out of therapy due to side effects (both due to psychomotor agitation) at the first administration and were excluded from the study. Psychomotor agitation appeared with repetitive, involuntary movements, a condition that may be related to the active ingredient tested. It is indeed seen that while cannabis is commonly associated with relaxation and sedation, it can also induce psychomotor agitation in some individuals, especially in susceptible ones [25].
The total number of recruited and analyzed FM subjects was 30 (24 women and 6 men) with a median age of 50.5 years (range 32–82 years): 12.5% of subjects were <40 years old, 25% were 41–50 years old, 25% were 51–69 years old, and 12.5% were >70 years old (Table 3).

Table 3.
Patients characteristics.
Comorbidities and pain syndromes associated with FM were evaluated for all considered subjects. The main comorbidities were hypertension (20%) and hypothyroidism (10%), while the most frequent pain syndromes associated with FM were polydistractual pain (40%) and migraine (6.67%) (Table 4).

Table 4.
Comorbidities and pain syndrome associated with FM.
The most assumed therapies included for reducing FM symptoms were opiates (60%), non-steroidal anti-inflammatory drugs (23.33%), and antidepressants (23.33%). Before starting the current trial, all patients stopped to assume the drug therapy they were following.
3.2. Numerical Pain Rating Scale (NRS)
The NRS is commonly used in a clinical setting to evaluate patients’ pain intensity through a pain scale from 0 to 10, where 0 indicates “no pain” while 10 is “the worst imaginable pain”. The present study evaluated the pain intensity at baseline and the sixth month of follow-up (Table 5).

Table 5.
Effect of medical cannabis (MC), Bedrocan®, on pain assessment and quality of life before treatment (Prior MC) and at 6th month of follow-up (After MC).
Prior to the treatment with MC, 24 patients (80%) reported an NRS score comprised between 8 and 10. As reported in Figure 4, after 6 months, only 3 patients (10%) reported a mild pain improvement (NRS score from 10 to 8 at 6th-month follow-up), while 1 subject (3.33%) did not show pain amelioration (NRS score of 6 at baseline and after 6th-month follow-up).
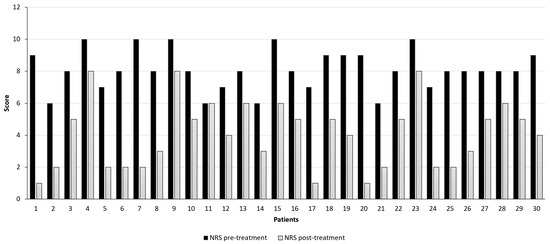
Figure 4.
Pain intensity assessment was evaluated with the Numerical Pain Rating Scale (NRS) before (black bars) and after 6 months (grey bars) of therapy with Bedrocan® decoction. (p-value < 0.001; t-test).
Overall, pain intensity lowered from a median of 8 [95% confidence interval (CI) 7.66–8.54] at a baseline to a median of 4 (95% CI 3.28–4.79) after 6 months of follow-up (p-value < 0.001; t-test). Hence, a total NRS score reduction of 50% was evidenced after 6 months of treatment with Bedrocan®.
3.3. Short-Form Health Survey (SF-12)
The SF-12 is a questionnaire designed to investigate the perception of individuals’ psychophysical conditions. The synthesis of the scores makes it possible to construct two health status indices, one concerning the physical state (physical component summary, PCS-12) and the other the psychological state (mental component summary, MCS-12). Synthetic indices values may vary from 10.5 to 69.7 for the PCS and 7.4 to 72.1 for the MCS index, where psychophysical health conditions improve as they increase. PCS levels, approximately below 20 points, correspond to a condition of “substantial limitations in self-care and physical, social and personal activity; significant physical pain; frequent fatigue; poor health”. Similarly, a low psychological health index shows “frequent psychological distress; significant social and personal impairment due to emotional problems; poor health” [26].
Regarding the physical state evaluation before the treatment with Bedrocan®, 20.00% of patients reported a PCS score lower than 20, indicative of a scarce quality of life (Table 3; Figure 5).
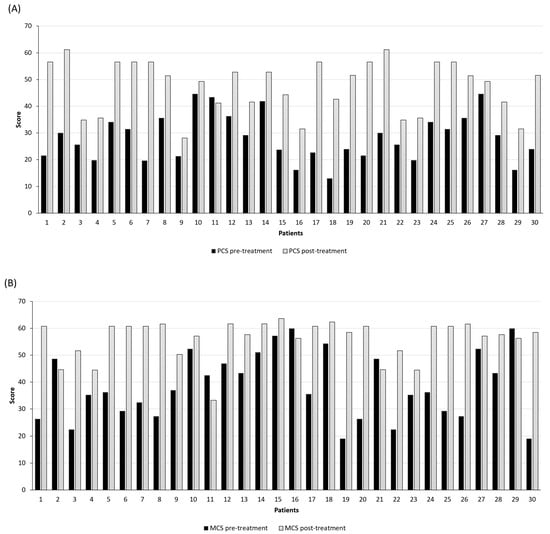
Figure 5.
Evaluation of the quality of life with the short-form health survey (SF-12): (A) physical component summary, PCS-12; (B) mental component, MCS-12. Black bars represent the score before starting the treatment, while grey bars indicate the score at the 6th month of follow-up (p-value < 0.001; t-test).
However, after treatment, a significant quality of life improvement was found in 96.67% of patients with a median of 27.40 at baseline and 51.46 after 6th-month follow-up (95% CI 25.09–31.33 and 44.11–51.13, respectively; p-value < 0.001; t-test). Contrarily, 1 subject (3.33%) assessed only a slight reduction in the PCS score (from 43.44 at baseline to 41.21 after 6 months).
Regarding the physical state, a significant mental state amelioration was seen for 83.33% of patients, with a median of 36.22 at baseline and 58.46 after the 6th month of follow-up (95% CI 34.12–43.00 and 53.48–58.69, respectively; p-value < 0.001; t-test).
3.4. Collateral Effects
During the treatment with cannabis, 2 patients out of 32 first included subjects (6.25%) dropped out of the study due to side effects related to psychomotor agitation. The remaining 30 patients (93.75%) who completed the study up to 6 months experienced no side effects.
3.5. Systematic Review
To insert this study in the context of the existing clinical trials, a systematic review of the literature was performed. Specifically, the systematic review was performed by following the PRISMA statement recommendation [27], including studies published between 2000 and 2023 using Scopus and Pubmed as databases. The initial manuscripts selection provided 392 studies, of which 305 were found on Scopus and 87 on PubMed. Among the 392 studies, 161 are duplicates, resulting in 231 studies out of those 213 not fitting with the considered inclusion criteria and 8 not containing information congruent with the study topic. Thus, the final reference list includes 10 clinical studies (Figure 6), of which 8 observational studies and 2 randomized clinical trials have been published since 2000 regarding the use of cannabis in the treatment of FM.
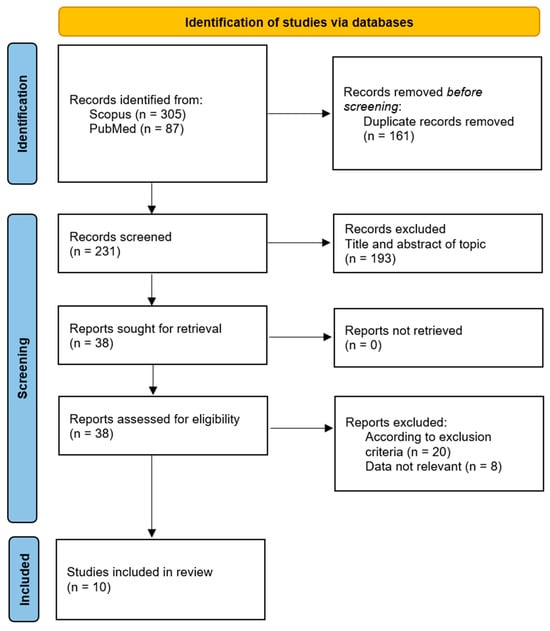
Figure 6.
Systematic review literature research based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements [27,28].
Overall, as reported in the present study, a significant reduction in pain and an amelioration of quality of life were recorded. Table 6 summarizes the data from the included clinical trials.

Table 6.
Clinical trials included in the systematic review.
Risk of Bias Assessment
Detailed results of the risk of bias analysis are reported in Figure 7 and Figure 8. Of the eight non-randomized clinical trials (Figure 7), six have an overall medium risk of bias predominantly due to bias in the classification of intervention (D3) and deviation from the intended intervention (D4). On the contrary, the two randomized clinical trials have a low risk of bias (Figure 8).
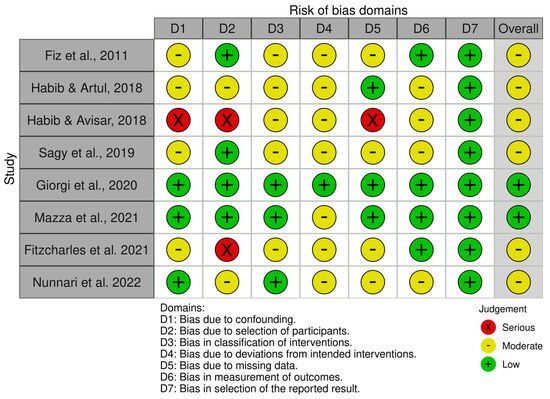
Figure 7.
Risk of Bias analysis for non-randomized studies made using the ROBINS-1 tool (https://www.riskofbias.info/welcome/home) [29,30,31,32,34,36,37,38].
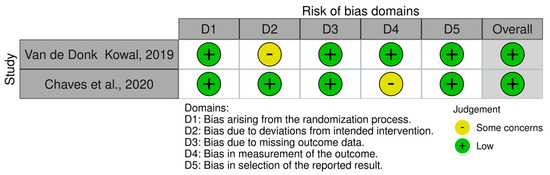
Figure 8.
Risk of Bias analysis for randomized studies made using the ROB2 tool (https://www.riskofbias.info/welcome/rob-2-0-tool) [33,35].
4. Discussion
In this study, the efficacy of MC in FM patients was evaluated, obtaining results that suggest a possible implication of MC as an alternative treatment for patients with FM not responsive to conventional pharmacological therapy. As in other studies [29,30,31,32,36], in the current pilot study, most of the patients were women since they represent 89.3% of the patients. Considering that, as previously stated, the male-to-female ratio with FM syndrome is 1:3 [39], the current investigation confirmed that FM patients with clinical pain are women. Noteworthy is the NRS at baseline since patients with a pain score comprised between 8 and 10, representing the maximum level of pain, were 24 (80% of subjects). This basal pain score is higher than that registered in other clinical trials investigating the effect of a synthetic derivative of Δ-9-THC, nabilone [40,41], while it is in line with that registered by Mazza et al. and Sagy et al. [32,36]. As in the last clinical study [32], the current one revealed a significant reduction in NRS from a median of 8 (95% CI 7.66–8.54) at a baseline to a median of 4 (95% CI 3.28–4.79) after 6 months (p-value < 0.001; t-test). Hence, there was a reduction in pain score in the 6th-month follow-up of 4 points, comparable to the pain decrease that was assessed by Mazza et al. in the 12th month (4.3 points) [36]. An improvement in pain symptoms was also assessed in two observational studies [30,34] and two randomized, double-blind, placebo-controlled clinical trials [33,35]. Even if the data obtained in these investigations are not comparable with those of the current one since another evaluation parameter was used, the Fibromyalgia Impact Questionnaire (FIQ) and the pressure pain test. Finally, a reduction in pain evaluated with the VAS score was detected in an observational cross-sectional survey investigating the short-term effect of cannabis inhalation, while only a low analgesic effect was detected [29]. In the complex, these results may indicate a potential application of cannabis in reducing pain. In the current pilot study, the improvement of pain symptoms was corroborated by the data from the SF-12 consisting of 12 items (taken from the 36 items of the original SF-36 questionnaire) that produce two measures relating to two different aspects of health: physical health (PCS-12) and mental health (MCS-12). In the current trial, the PCS-12 was significantly improved from the baseline to the 6th month for 96.67% of FM subjects [mean (M) = 28.21 ± 8.72 to 47.62 ± 9.81 for baseline and 6th-month evaluation, respectively, p-value < 0.001; t-test]. In the same way, the MCS-12 increase significantly for 83.33% of FM patients (M = 38.56 ± 12.41 to 56.09 ± 7.28 for baseline and 6th-month evaluation, respectively, p-value < 0.001; t-test). These results are indicative of an improvement in the quality of life and are in line with those of previous clinical trials since an increase in mental score was also seen when it was evaluated with the SF-36 [29]. SF-36 is the SF-12 extended version and has been demonstrated to be complementary to the SF-12, confirming the comparable nature of results obtained from the SF-36 and SF-12 questionnaire [42]. Enhanced mental health was further evidenced in another survey, where 85% and 62% of FM patients ameliorated depressive and anxious states after cannabis assumption [31]. Contrarily, no differences in mental mood were observed when two synthetic Δ-9-THC derivatives, nabilone and amitriptyline, were administered [43]. Improved quality of life was also seen in two more observational studies when it was evaluated with the Likert scale regarding appetite, sleep quality, and sexual activity [15,32]. Thus, based on these results, it is possible to assert the potential therapeutic effect of cannabis for the treatment of FM. This assumption was further supported by the demonstrated safety of MC taken as decoction since only 6.25% of subject reported psychomotor agitation, while no other side effects were reported.
One strength of the current Pilot Study was the evaluation of the cannabis effect in a controlled hospital setting by using only a type of administration mood and not a combination of administration mood (e.g., decoction and oil or smoke) to reduce the risk of bias. Furthermore, it was demonstrated that decoction is patients’ preferred cannabis mode of assumption since it is easy to prepare and has a good safety profile, thus avoiding accidental overdoses of THC and the consequent psychotropic effect [44]. Another strength was the low dosage of 100 mg (1 sachet) per day, which was increased to 200 mg (2 sachets) per day for only two patients (7.14%) who were not responsive to the low dosage. This administered cannabis amount, corresponding approximately to 3.0 g per month, was definitively lower than that used in other investigations (about 31.4 ± 16.3 g per month) [30,31,32,36]. Finally, in the present clinical study, before starting MC therapy, all patients stopped the assumption of conventional drugs, while in all other studies, the pharmacological therapy was continued during the evaluation of MC efficacy [30,32,34,35]. This may suggest that MC can represent a suitable and efficacious alternative to conventional drugs. However, due to the small number of FM patients investigated, the risk of bias is high, and the results obtained should be interpreted carefully. It is also important to take into consideration the limitations of this study since the use of self-administered questionnaires may represent a source of bias. Furthermore, the absence of a control group in the present investigation can represent an additional limitation. Hence, further randomized placebo control clinical trials with a higher number of participants should be needed to better assess the role of MC, administered as a decoction, in FM symptom reduction.
5. Conclusions
FM is one of the most common causes of persistent chronic and widespread pain. However, although pain is its main feature, it is represented by a complex polysymptomatology comprising fatigue, sleep disturbances, generalized hyperalgesia, stiffness, palpation-specific tender points, and cognitive and somatic dysfunction. The current pilot study evidenced a positive effect of a low dosage of MC (Bedrocan®; 100 mg/day) in treating FM symptomatology. Likewise, data from the literature demonstrated that cannabis administration could be associated with an improved quality of life for patients suffering from chronic pain. Hence, it is possible to conclude that cannabinoids may represent an effective alternative to conventional pharmacological therapy for reducing pain and mind disorders in FM subjects. Further investigations like randomized, placebo-controlled clinical trials are needed to corroborate these findings.
Author Contributions
Conceptualization, methodology, software, validation, formal analysis, investigation, data curation, A.G., R.P., C.A., M.C., A.D.F., L.M. and M.P.; visualization, supervision, writing—original draft preparation, L.M. and M.P.; writing—review and editing, M.P., V.C. and N.B.; project administration, A.G. and L.M.; funding acquisition, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the project “VALBIOECONOMIA: valorizzazione di biomasse vegetali per un’economia circolare a scarto zero” PO FESR 2014–2020—Azione 1B.1.2.2 prov. 15/2019/0214 CUP: G49J19001420004. Project “SPIA—Valorization of by-products from the agro-food chain” CUP: G49J19001350004; and DGR n. 527/2019 “PO FESR BASILICATA 2014–2020—Axis I—Research, Innovation Action and Technological Development—Action 1B.1.2.1”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional After the San Carlo Hospital ethics committee’s (n. of CEUR general register 37/2023, n. protocol of the Potenza’s AOR San Carlo referring to application for approval 20230021536, protocol date 11 May 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Al Sharie, S.; Varga, S.J.; Al-Husinat, L.i.; Sarzi-Puttini, P.; Araydah, M.; Bal’awi, B.R.; Varrassi, G. Unraveling the Complex Web of Fibromyalgia: A Narrative Review. Medicina 2024, 60, 272. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann. Intern. Med. 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.; Perrot, S.; Leon, T.; Kaplan, J.; Petersel, D.; Ginovker, A.; Kramer, E. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv. Res. 2010, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Wiffen, P.J.; Derry, S.; Rice, A.S. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst. Rev. 2014, 6, CD007938. [Google Scholar] [CrossRef]
- Aster, H.; Evdokimov, D.; Braun, A.; Üçeyler, N.; Sommer, C. Analgesic Medication in Fibromyalgia Patients: A Cross-Sectional Study. Pain Res. Manag. J. Can. Pain Soc. 2022, 2022, 1217717. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.E.; Guimarães, I. Fibromyalgia—Are there any new approaches? Best Pract. Res. Clin. Rheumatol. 2024, 101933. [Google Scholar] [CrossRef] [PubMed]
- Argenbright, C.M.; Bertlesman, A.M.; Russell, I.M.; Greer, T.L.; Peng, Y.B.; Fuchs, P.N. The Fibromyalgia Pain Experience: A Scoping Review of the Preclinical Evidence for Replication and Treatment of the Affective and Cognitive Pain Dimensions. Biomedicines 2024, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Walitt, B.; Fitzcharles, M.-A.; Sommer, C. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Res. Ther. 2014, 16, 201. [Google Scholar] [CrossRef] [PubMed]
- Melnikovova, I.; Russo, D.; Fait, T.; Kolarova, M.; Tauchen, J.; Kushniruk, N.; Falabella, R.; Milella, L.; Fernández Cusimamani, E. Evaluation of the effect of Lepidium meyenii Walpers in infertile patients: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2021, 35, 6359–6368. [Google Scholar] [CrossRef] [PubMed]
- Campesi, I.; Milella, L.; Palermo, M.; Sotgiu, G.; Reggiardo, G.; Franconi, F. Cigarette smoking affects the differences between male and female phenotypes. Am. J. Transl. Res. 2020, 12, 2998. [Google Scholar] [PubMed]
- Melnikovova, I.; Fait, T.; Kolarova, M.; Fernandez, E.C.; Milella, L. Effect of Lepidium meyenii Walp. on semen parameters and serum hormone levels in healthy adult men: A double-blind, randomized, placebo-controlled pilot study. Evid.-Based Complement. Altern. Med. 2015, 2015, 324369. [Google Scholar] [CrossRef] [PubMed]
- Alciati, A.; Nucera, V.; Masala, I.F.; Giallanza, M.; La Corte, L.; Giorgi, V.; Sarzi-Puttini, P.; Atzeni, F. One year in review 2021: Fibromyalgia. Clin. Exp. Rheumatol. 2021, 39, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Boehnke, K.F.; Litinas, E.; Clauw, D.J. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J. Pain 2016, 17, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Savage, S.R.; Romero-Sandoval, A.; Schatman, M.; Wallace, M.; Fanciullo, G.; McCarberg, B.; Ware, M. Cannabis in pain treatment: Clinical and research considerations. J. Pain 2016, 17, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.G.; Serafini, M.R.; Quintans-Júnior, L.J. Terpenes and derivatives as a new perspective for pain treatment: A patent review. Expert Opin. Ther. Pat. 2014, 24, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Apolone, G.; Mosconi, P.; Quattrociocchi, L.; Gianicolo, E.; Groth, N.; Ware, J. Questionario Sullo Stato di Salute SF-12. Versione Italiana; SF-12 questionnaire; Italian Version; IRFMN: Milano, Italy, 2005. [Google Scholar]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Chapter 8: Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Sterne, J.A.C.; Higgins, J.; Reeves, B.C. A Cochrane Risk Of Bias Assessment Tool: For Non Randomized Studies of Interventions (ACROBAT-NRSI). Version 2014, 1, 24. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Atakan, Z.; Martin-Santos, R.; Crippa, J.A.; Kambeitz, J.; Prata, D.; Williams, S.; Brammer, M.; Collier, D.A.; McGuire, P.K. Preliminary report of biological basis of sensitivity to the effects of cannabis on psychosis: AKT1 and DAT1 genotype modulates the effects of δ-9-tetrahydrocannabinol on midbrain and striatal function. Mol. Psychiatry 2012, 17, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Istituto Nazionale di Statistica (ISTAT). Stato di Salute. 2011. Available online: https://www.istat.it/it/files/2011/02/metadati.pdf (accessed on 15 April 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Lerose, V.; Ponticelli, M.; Benedetto, N.; Carlucci, V.; Lela, L.; Tzvetkov, N.T.; Milella, L. Withania somnifera (L.) Dunal, a Potential Source of Phytochemicals for Treating Neurodegenerative Diseases: A Systematic Review. Plants 2024, 13, 771. [Google Scholar] [CrossRef]
- Fiz, J.; Durán, M.; Capellà, D.; Carbonell, J.; Farré, M. Cannabis use in patients with fibromyalgia: Effect on symptoms relief and health-related quality of life. PLoS ONE 2011, 6, e18440. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Artul, S. Medical cannabis for the treatment of fibromyalgia. JCR J. Clin. Rheumatol. 2018, 24, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Avisar, I. The consumption of cannabis by fibromyalgia patients in Israel. Pain Res. Treat. 2018, 2018, 7829427. [Google Scholar] [CrossRef] [PubMed]
- Sagy, I.; Bar-Lev Schleider, L.; Abu-Shakra, M.; Novack, V. Safety and efficacy of medical cannabis in fibromyalgia. J. Clin. Med. 2019, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Van de Donk, T.; Niesters, M.; Kowal, M.A.; Olofsen, E.; Dahan, A.; van Velzen, M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain 2019, 160, 860. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, V.; Bongiovanni, S.; Atzeni, F.; Marotto, D.; Di Carlo, M.; Galli, M.; Giovanioni, A.; Salaffi, F.; Sarzi-Puttini, P. Adding medical cannabis to standard analgesic treatment for fibromyalgia: A prospective observational study. Clin. Exp. Rheumatol. 2020, 38, 53–59. [Google Scholar] [PubMed]
- Chaves, C.; Bittencourt, P.C.T.; Pelegrini, A. Ingestion of a THC-rich cannabis oil in people with fibromyalgia: A randomized, double-blind, placebo-controlled clinical trial. Pain Med. 2020, 21, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M. Medical cannabis for the treatment of fibromyalgia syndrome: A retrospective, open-label case series. J. Cannabis Res. 2021, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.-A.; Rampakakis, E.; Sampalis, J.; Shir, Y.; Cohen, M.; Starr, M.; Häuser, W. Use of medical cannabis by patients with fibromyalgia in Canada after cannabis legalisation: A cross-sectional study. Clin. Exp. Rheumatol. 2021, 39, S115–S119. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, P.; Ladiana, N.; Ceccarelli, G.; Notaro, P. Long-term Cannabis-based oil therapy and pain medications prescribing patterns: An Italian observational study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, L.P. Worldwide epidemiology of fibromyalgia. Curr. Pain Headache Rep. 2013, 17, 356. [Google Scholar] [CrossRef] [PubMed]
- Walitt, B.; Klose, P.; Fitzcharles, M.-A.; Phillips, T.; Haeuser, W. Cannabinoids for fibromyalgia. Cochrane Database Syst. Rev. 2016, 7, CD011694. [Google Scholar] [CrossRef] [PubMed]
- Skrabek, R.Q.; Galimova, L.; Ethans, K.; Perry, D. Nabilone for the treatment of pain in fibromyalgia. J. Pain 2008, 9, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Pezzilli, R.; Morselli-Labate, A.; Frulloni, L.; Cavestro, G.; Ferri, B.; Comparato, G.; Gullo, L.; Corinaldesi, R. The quality of life in patients with chronic pancreatitis evaluated using the SF-12 questionnaire: A comparative study with the SF-36 questionnaire. Dig. Liver Dis. 2006, 38, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Fitzcharles, M.-A.; Joseph, L.; Shir, Y. The effects of nabilone on sleep in fibromyalgia: Results of a randomized controlled trial. Anesth. Analg. 2010, 110, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Del Rio, A. Herbal preparations of medical cannabis: A vademecum for prescribing doctors. Medicina 2020, 56, 237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).