Physical Health in Patients with Post-COVID-19 6 and 12 Months after an Inpatient Rehabilitation: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurements of Sociodemographic Variables, Post-COVID Symptoms, Functional Status and after Care
2.3. Physical Performance Measurements
2.4. Statistical Analyses
3. Results
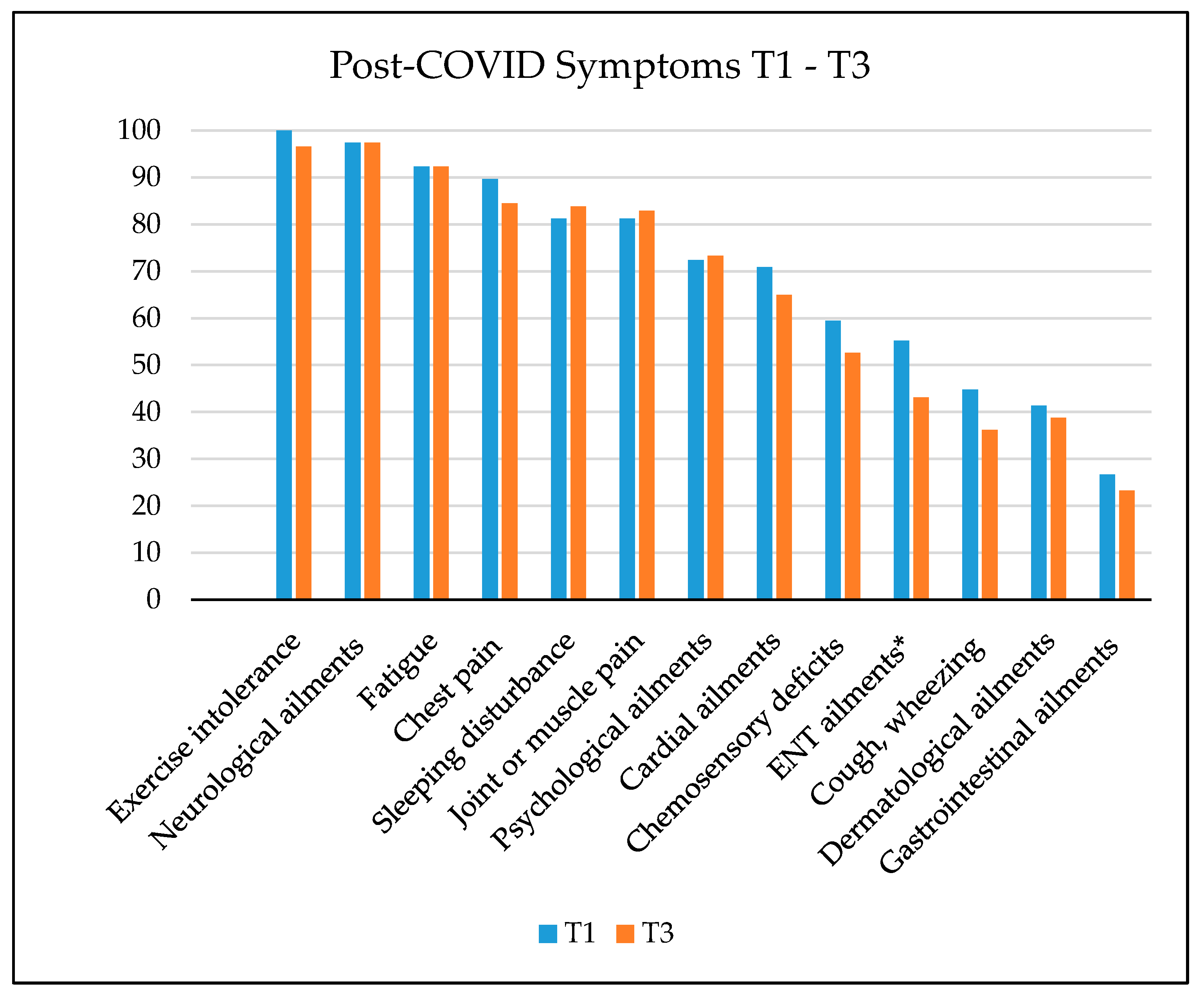
3.1. SARS-CoV-2 Infection and Post-COVID Symptoms
3.2. Analysis of Physical Performance between T1 and T3
Group Differences between T1 and T3
3.3. Analysis of Physical Performance between T1 and T4
Group Differences between T1 and T4
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willi, S.; Lüthold, R.; Hunt, A.; Hänggi, N.V.; Sejdiu, D.; Scaff, C.; Bender, N.; Staub, K.; Schlagenhauf, P. COVID-19 sequelae in adults aged less than 50 years: A systematic review. Travel Med. Infect. Dis. 2021, 40, 101995. [Google Scholar] [CrossRef]
- Wahrendorf, M.; Schaps, V.; Reuter, M.; Hoebel, J.; Wachtler, B.; Jacob, J.; Alibone, M.; Dragano, N. Occupational differences of COVID-19 morbidity and mortality in Germany. An analysis of health insurance data from 3.17 million insured persons. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2023, 66, 857–868. [Google Scholar] [CrossRef]
- Alshamrani, M.M.; El-Saed, A.; Al Zunitan, M.; Almulhem, R.; Almohrij, S. Risk of COVID-19 morbidity and mortality among healthcare workers working in a Large Tertiary Care Hospital. Int. J. Infect. Dis. 2021, 109, 238–243. [Google Scholar] [CrossRef]
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M.; Guevara, S.L.R.; Echeverría, L.E.; Glisic, M.; Muka, T. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2021, 190, 161–175. [Google Scholar] [CrossRef]
- Becker, H.; Franke, E.; Molkentin, T. (Eds.) Sozialgesetzbuch VII: Gesetzliche Unfallversicherung: Lehr- und Praxiskommentar, 5th ed.; Nomos: Baden-Baden, Germany, 2018. [Google Scholar]
- Deutsche Gesetzliche Unfallversicherung (DGUV). Berufskrankheiten und Arbeitsunfälle im Zusammenhang mit COVID-19. Available online: https://www.dguv.de/medien/inhalt/mediencenter/hintergrund/covid/dguv_zahlen_covid.pdf (accessed on 20 June 2023).
- Sivan, M.; Taylor, S. NICE guideline on long COVID. Br. Med. J. Publ. Group 2020, 371, m4938. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 408. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long COVID-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post-COVID-19 Condition: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef]
- Tziolos, N.R.; Ioannou, P.; Baliou, S.; Kofteridis, D.P. Long COVID-19 Pathophysiology: What Do We Know So Far? Microorganisms 2023, 11, 2458. [Google Scholar] [CrossRef]
- Perumal, R.; Shunmugam, L.; Naidoo, K.; Abdool Karim, S.S.; Wilkins, D.; Garzino-Demo, A.; Brechot, C.; Parthasarathy, S.; Vahlne, A.; Nikolich, J. Long COVID: A review and proposed visualization of the complexity of long COVID. Front. Immunol. 2023, 14, 1117464. [Google Scholar] [CrossRef]
- Peter, R.S.; Nieters, A.; Kräusslich, H.G.; Brockmann, S.O.; Göpel, S.; Kindle, G.; Merle, U.; Steinacker, J.M.; Rothenbacher, D.; Kern, W.V. Post-acute sequelae of COVID-19 six to 12 months after infection: Population based study. BMJ 2022, 379, e071050. [Google Scholar] [CrossRef]
- Wulf Hanson, S.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; Borzakova, S.; et al. Estimated Global Proportions of Individuals With Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar] [CrossRef]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of post-acute COVID-19 syndrome symptoms at different follow-up periods: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Tofiq, A.; Eriksson Crommert, M.; Zakrisson, A.B.; von Euler, M.; Nilsing Strid, E. Physical functioning post-COVID-19 and the recovery process: A mixed methods study. Disabil Rehabil 2023, 1–10. [Google Scholar] [CrossRef]
- de Oliveira Almeida, K.; Nogueira Alves, I.G.; de Queiroz, R.S.; de Castro, M.R.; Gomes, V.A.; Santos Fontoura, F.C.; Brites, C.; Neto, M.G. A systematic review on physical function, activities of daily living and health-related quality of life in COVID-19 survivors. Chronic Illn. 2023, 19, 279–303. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, D.C.; Normand, K.; Zhaoyun, Y.; Torres-Castro, R. Long-Term Impact of COVID-19: A Systematic Review of the Literature and Meta-Analysis. Biomedicines 2021, 9, 900. [Google Scholar] [CrossRef]
- Taboada, M.; Cariñena, A.; Moreno, E.; Rodríguez, N.; Domínguez, M.J.; Casal, A.; Riveiro, V.; Diaz-Vieito, M.; Valdés, L.; Álvarez, J.; et al. Post-COVID-19 functional status six-months after hospitalization. J. Infect. 2021, 82, e31–e33. [Google Scholar] [CrossRef]
- Wahlgren, C.; Forsberg, G.; Divanoglou, A.; Östholm Balkhed, Å.; Niward, K.; Berg, S.; Levi, R. Two-year follow-up of patients with post-COVID-19 condition in Sweden: A prospective cohort study. Lancet Reg. Health Eur. 2023, 28, 100595. [Google Scholar] [CrossRef]
- Gunnarsson, D.V.; Miskowiak, K.W.; Pedersen, J.K.; Hansen, H.; Podlekareva, D.; Johnsen, S.; Dall, C.H. Physical Function and Association with Cognitive Function in Patients in a Post-COVID-19 Clinic-A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 5866. [Google Scholar] [CrossRef]
- Vollrath, S.; Matits, L.; Schellenberg, J.; Kirsten, J.; Steinacker, J.M.; Bizjak, D.A. Decreased physical performance despite objective and subjective maximal exhaustion in post-COVID-19 individuals with fatigue. Eur. J. Med. Res. 2023, 28, 298. [Google Scholar] [CrossRef]
- Barbara, C.; Clavario, P.; De Marzo, V.; Lotti, R.; Guglielmi, G.; Porcile, A.; Russo, C.; Griffo, R.; Mäkikallio, T.; Hautala, A.J.; et al. Effects of exercise rehabilitation in patients with long coronavirus disease 2019. Eur. J. Prev. Cardiol. 2022, 29, e258–e260. [Google Scholar] [CrossRef]
- Koczulla, A.R.; Ankermann, T.; Behrends, U.; Berlit, P.; Brinkmann, F.; Frank, U.; Glöckl, R.; Gogoll, C.; Häuser, W.; Hohberger, B.; et al. S1-Leitlinie “Post-COVID/Long-COVID”. Available online: https://register.awmf.org/assets/guidelines/020-027l_S1_Long-Post-Covid_2024-06_1.pdf (accessed on 21 June 2024).
- WHO. Clinical Management of COVID-19: Living Guideline. 13 January 2023. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.1 (accessed on 22 March 2024).
- Ashra, F.; Jen, H.J.; Liu, D.; Lee, T.Y.; Pien, L.C.; Chen, R.; Lin, H.C.; Chou, K.R. Effectiveness of respiratory rehabilitation in patients with COVID-19: A meta-analysis. J. Clin. Nurs. 2023, 32, 4972–4987. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, M.; Rzepka-Cholasińska, A.; Pietrzykowski, Ł.; Michalski, P.; Kosobucka-Ozdoba, A.; Jasiewicz, M.; Kasprzak, M.; Kryś, J.; Kubica, A. Effects of Multidisciplinary Rehabilitation Program in Patients with Long COVID-19: Post-COVID-19 Rehabilitation (PCR SIRIO 8) Study. J. Clin. Med. 2023, 12, 420. [Google Scholar] [CrossRef]
- Martínez-Pozas, O.; Meléndez-Oliva, E.; Rolando, L.M.; Rico, J.A.Q.; Corbellini, C.; Sánchez Romero, E.A. The pulmonary rehabilitation effect on long COVID-19 syndrome: A systematic review and meta-analysis. Physiother. Res. Int. 2024, 29, e2077. [Google Scholar] [CrossRef]
- Ahmed, I.; Mustafaoglu, R.; Yeldan, I.; Yasaci, Z.; Erhan, B. Effect of Pulmonary Rehabilitation Approaches on Dyspnea, Exercise Capacity, Fatigue, Lung Functions, and Quality of Life in Patients With COVID-19: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 2051–2062. [Google Scholar] [CrossRef]
- Nopp, S.; Moik, F.; Klok, F.A.; Gattinger, D.; Petrovic, M.; Vonbank, K.; Koczulla, A.R.; Ay, C.; Zwick, R.H. Outpatient Pulmonary Rehabilitation in Patients with Long COVID Improves Exercise Capacity, Functional Status, Dyspnea, Fatigue, and Quality of Life. Respiration 2022, 101, 593–601. [Google Scholar] [CrossRef]
- Pouliopoulou, D.V.; Macdermid, J.C.; Saunders, E.; Peters, S.; Brunton, L.; Miller, E.; Quinn, K.L.; Pereira, T.V.; Bobos, P. Rehabilitation Interventions for Physical Capacity and Quality of Life in Adults With Post-COVID-19 Condition: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2333838. [Google Scholar] [CrossRef] [PubMed]
- Gloeckl, R.; Zwick, R.H.; Furlinger, U.; Schneeberger, T.; Leitl, D.; Jarosch, I.; Behrends, U.; Scheibenbogen, C.; Koczulla, A.R. Practical Recommendations for Exercise Training in Patients with Long COVID with or without Post-exertional Malaise: A Best Practice Proposal. Sports Med. Open 2024, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Zwingmann, K.; Auerswald, T.; Berger, I.; Thomas, A.; Schultz, A.L.; Wilhelm, E.; Weber, R.C.; Kolb, F.; Wastlhuber, A.; et al. Rehabilitation and Return-to-Work of Patients Acquiring COVID-19 in the Workplace: A Study Protocol for an Observational Cohort Study. Front. Rehabil. Sci. 2021, 2, 754468. [Google Scholar] [CrossRef]
- Müller, K.; Poppele, I.; Ottiger, M.; Zwingmann, K.; Berger, I.; Thomas, A.; Wastlhuber, A.; Ortwein, F.; Schultz, A.-L.; Weghofer, A.; et al. Impact of Rehabilitation on Physical and Neuropsychological Health of Patients Who Acquired COVID-19 in the Workplace. Int. J. Environ. Res. Public Health 2023, 20, 1468. [Google Scholar] [CrossRef]
- Lampert, T.; Kroll, L.E.; Müters, S.; Stolzenberg, H. Messung des sozioökonomischen Status in der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsbl 2013, 56, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Scheidt-Nave, C.; Kamtsiuris, P.; Gößwald, A.; Hölling, H.; Lange, M.; Busch, M.A.; Dahm, S.; Dölle, R.; Ellert, U.; Fuchs, J.; et al. German health interview and examination survey for adults (DEGS)—Design, objectives and implementation of the first data collection wave. BMC Public Health 2012, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Bahmer, T.; Borzikowsky, C.; Lieb, W.; Horn, A.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.F.; Maetzler, W.; Maetzler, C.; et al. Severity, predictors and clinical correlates of Post-COVID syndrome (PCS) in Germany: A prospective, multi-centre, population-based cohort study. EClinicalMedicine 2022, 51, 101549. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Boon, G.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Schneeberger, T.; Gloeckl, R.; Jarosch, I.; Drechsel, F.; Koczulla, A.R.; Kenn, K. The minimal important difference for the 1-min sit-to-stand test following pulmonary rehabilitation in patients with COPD—A prospective observational trial. Eur. Respir. J. 2018, 52, PA1431. [Google Scholar] [CrossRef]
- Theisen, D.; Wydra, G. Untersuchung der Gleichgewichtsfähigkeit. BG Bewegungstherapie Und Gesundheitssport 2011, 27, 231–239. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef]
- Poole-Wright, K.; Guennouni, I.; Sterry, O.; Evans, R.A.; Gaughran, F.; Chalder, T. Fatigue outcomes following COVID-19: A systematic review and meta-analysis. BMJ Open 2023, 13, e063969. [Google Scholar] [CrossRef]
- Kokolevich, Z.M.; Crowe, M.; Mendez, D.; Biros, E.; Reznik, J.E. Most Common Long COVID Physical Symptoms in Working Age Adults Who Experienced Mild COVID-19 Infection: A Scoping Review. Healthcare 2022, 10, 2577. [Google Scholar] [CrossRef]
- Delgado-Alonso, C.; Cuevas, C.; Oliver-Mas, S.; Díez-Cirarda, M.; Delgado-Álvarez, A.; Gil-Moreno, M.J.; Matías-Guiu, J.; Matias-Guiu, J.A. Fatigue and Cognitive Dysfunction Are Associated with Occupational Status in Post-COVID Syndrome. Int. J. Environ. Res. Public Health 2022, 19, 13368. [Google Scholar] [CrossRef]
- Haller, J.; Kocalevent, R.D.; Nienhaus, A.; Peters, C.; Bergelt, C.; Koch-Gromus, U. Persistent fatigue symptoms following COVID-19 infection in healthcare workers: Risk factors and impact on quality of life. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2022, 65, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Aschman, T.; Wyler, E.; Baum, O.; Hentschel, A.; Rust, R.; Legler, F.; Preusse, C.; Meyer-Arndt, L.; Buttnerova, I.; Forster, A.; et al. Post-COVID exercise intolerance is associated with capillary alterations and immune dysregulations in skeletal muscles. Acta Neuropathol. Commun. 2023, 11, 193. [Google Scholar] [CrossRef]
- Appelman, B.; Charlton, B.T.; Goulding, R.P.; Kerkhoff, T.J.; Breedveld, E.A.; Noort, W.; Offringa, C.; Bloemers, F.W.; van Weeghel, M.; Schomakers, B.V.; et al. Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat. Commun. 2024, 15, 17. [Google Scholar] [CrossRef]
- Islam, M.; Cotler, J.; Jason, L. Post-viral fatigue and COVID-19: Lessons from past epidemics. Fatigue Biomed. Health Behav. 2020, 8, 61–69. [Google Scholar] [CrossRef]
- Schaeffer, M.R.; Cowan, J.; Milne, K.M.; Puyat, J.H.; Voduc, N.; Corrales-Medina, V.; Lavoie, K.L.; Mulloy, A.; Chirinos, J.A.; Abdallah, S.J.; et al. Cardiorespiratory physiology, exertional symptoms, and psychological burden in post-COVID-19 fatigue. Respir. Physiol. Neurobiol. 2022, 302, 103898. [Google Scholar] [CrossRef]
- Kupferschmitt, A.; Langheim, E.; Tuter, H.; Etzrodt, F.; Loew, T.H.; Kollner, V. First results from post-COVID inpatient rehabilitation. Front. Rehabil. Sci. 2022, 3, 1093871. [Google Scholar] [CrossRef]
- Hasenoehrl, T.; Palma, S.; Huber, D.F.X.; Kastl, S.; Steiner, M.; Jordakieva, G.; Crevenna, R. Post-COVID: Effects of physical exercise on functional status and work ability in health care personnel. Disabil. Rehabil. 2023, 45, 2872–2878. [Google Scholar] [CrossRef]
- Ortiz-Ortigosa, L.; Galvez-Alvarez, P.; Vinolo-Gil, M.J.; Rodriguez-Huguet, M.; Gongora-Rodriguez, J.; Martin-Valero, R. Effectiveness of pulmonary rehabilitation programmes and/or respiratory muscle training in patients with post-COVID conditions: A systematic review. Respir. Res. 2024, 25, 248. [Google Scholar] [CrossRef]
- Kobelt-Pönicke, A.; Muschalla, B. Psychosomatische Nachsorge Nach Stationärer Psychosomatischer Rehabilitation (Psy-RENA). In Praxishandbuch Psychosomatische Medizin in der Rehabilitation; Köllner, V., Bassler, M., Eds.; Urban & Fischer: Amsterdam, The Netherlands, 2021; pp. 417–424. [Google Scholar]
- Rutsch, M.; Frommhold, J.; Buhr-Schinner, H.; Gross, T.; Schüller, P.O.; Deck, R. Pneumological Rehabilitation in Patients with Long COVID—Health Changes at the End of the Inpatient Rehabilitation Measure. Rehabilitation 2023, 62, 359–368. [Google Scholar] [CrossRef]
- Al Chikhanie, Y.; Veale, D.; Schoeffler, M.; Pepin, J.L.; Verges, S.; Herengt, F. Effectiveness of pulmonary rehabilitation in COVID-19 respiratory failure patients post-ICU. Respir. Physiol. Neurobiol. 2021, 287, 103639. [Google Scholar] [CrossRef] [PubMed]
- Cevei, M.; Onofrei, R.R.; Gherle, A.; Gug, C.; Stoicanescu, D. Rehabilitation of Post-COVID-19 Musculoskeletal Sequelae in Geriatric Patients: A Case Series Study. Int. J. Environ. Res. Public Health 2022, 19, 15350. [Google Scholar] [CrossRef] [PubMed]
- Nacul, L.C.; Mudie, K.; Kingdon, C.C.; Clark, T.G.; Lacerda, E.M. Hand Grip Strength as a Clinical Biomarker for ME/CFS and Disease Severity. Front. Neurol. 2018, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Jäkel, B.; Kedor, C.; Grabowski, P.; Wittke, K.; Thiel, S.; Scherbakov, N.; Doehner, W.; Scheibenbogen, C.; Freitag, H. Hand grip strength and fatigability: Correlation with clinical parameters and diagnostic suitability in ME/CFS. J. Transl. Med. 2021, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, C.; da Luz Goulart, C.; da Silva, B.M.; Valente, J.; Rezende, A.G.; Fernandes, E.; Cubas-Vega, N.; Borba, M.G.S.; Sampaio, V.; Monteiro, W.; et al. Low handgrip strength is associated with worse functional outcomes in long COVID. Sci. Rep. 2024, 14, 2049. [Google Scholar] [CrossRef] [PubMed]
- Ceravolo, M.G.; Anwar, F.; Andrenelli, E.; Udensi, C.; Qureshi, J.; Sivan, M.; Kiekens, C.; Zampolini, M. Evidence-based position paper on physical and rehabilitation medicine professional practice for persons with COVID-19, including post COVID-19 condition: The European PRM position (UEMS PRM Section). Eur. J. Phys. Rehabil. Med. 2023, 59, 789–799. [Google Scholar] [CrossRef]
- Gloeckl, R.; Leitl, D.; Schneeberger, T.; Jarosch, I.; Koczulla, A.R. Rehabilitative interventions in patients with persistent post COVID-19 symptoms-a review of recent advances and future perspectives. Eur. Arch. Psychiatry Clin. Neurosci. 2023. [Google Scholar] [CrossRef]
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- Pleguezuelos, E.; Del Carmen, A.; Moreno, E.; Miravitlles, M.; Serra, M.; Garnacho-Castaño, M.V. Effects of a telerehabilitation program and detraining on cardiorespiratory fitness in patients with post-COVID-19 sequelae: A randomized controlled trial. Scand. J. Med. Sci. Sports 2023, 34, e14543. [Google Scholar] [CrossRef]
- Rhead, R.; Wels, J.; Moltrecht, B.; Shaw, R.J.; Silverwood, R.; Zhu, J.; Hughes, A.; Chaturvedi, N.; Demou, E.; Katikireddi, S.V.; et al. Long COVID and financial outcomes: Evidence from four longitudinal population surveys. J. Epidemiol. Community Health 2024, 78, 458–465. [Google Scholar] [CrossRef]
- Haering, A.; Kottmann, R.; Ellert, C.; von Loga, I. Long/Post-COVID-Schweregrade und Ihre Gesellschaftlichen Folgen: Ergebnisse Einer Befragung. Available online: https://www.rwi-essen.de/fileadmin/user_upload/RWI/Publikationen/RWI_Materialien/rwi-materialien_156.pdf (accessed on 12 April 2024).
- Gandjour, A. Long COVID: Costs for the German economy and health care and pension system. BMC Health Serv. Res. 2023, 23, 641. [Google Scholar] [CrossRef] [PubMed]
- Karasu, A.U.; Karatas, L.; Yildiz, Y.; Gunendi, Z. Natural Course of Muscular Strength, Physical Performance, and Musculoskeletal Symptoms in Hospitalized Patients With COVID-19. Arch. Phys. Med. Rehabil. 2023, 104, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, C.; Paneroni, M.; Vitacca, M.; Ambrosino, N. Measures of physical performance in COVID-19 patients: A mapping review. Pulmonology 2021, 27, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Pujol, J.C.; Spector, T.D.; Ourselin, S.; Steves, C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022, 399, 2263–2264. [Google Scholar] [CrossRef] [PubMed]

| N = 121 1 | Mean | SD 2 | NA 3 | |
|---|---|---|---|---|
| Sex | - | |||
| 29 (24%) | |||
| 92 (76%) | |||
| Age [years] | 50.62 | 10.87 | ||
| BMI [kg/m2] | - | |||
| 17 (14%) | |||
| 39 (32%) | |||
| 35 (29%) | |||
| 20 (17%) | |||
| 10 (8%) | |||
| Smoking status | - | |||
| 5 (4%) | |||
| 4 (3%) | |||
| 46 (38%) | |||
| 66 (55%) | |||
| COVID-19 severity | - | |||
| 85 (70%) | |||
| 30 (25%) | |||
| 6 (5%) | |||
| Hospitalisation due to COVID-19 | 32 (26%) | - | ||
| 13.86 | 19.32 | ||
| 9 (7%) | |||
| Pneumonia due to COVID-19 | 36 (30%) | 1 | ||
| Interval COVID-19—Rehabilitation [days] | 409.52 | 143.97 | - | |
| Rehabilitation duration [days] | 28.99 | 5.22 | - | |
| PCFS score before Rehabilitation | - | |||
| 11 (9%) | |||
| 2 (2%) | |||
| 43(36%) | |||
| 63 (52%) | |||
| 2 (2%) | |||
| Occupation | - | |||
| 85 (70%) | |||
| 36 (30%) | |||
| Socio-economic status | 1 | |||
| 41 (34%) | |||
| 79 (66%) | |||
| Pre-existing conditions | - | |||
| 79 (65%) | |||
| 58 (48%) | |||
| 52 (43%) | |||
| 23 (19%) | |||
| 40 (33%) | |||
| 80 (66%) | |||
| Aftercare interventions | ||||
| 96 (79%) | 9 | ||
| 28 (23%) | 10 | ||
| 18 (15%) | 16 | ||
| 95 (79%) | 11 |
| T1 | T3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Min | Max | Median (IQR) | Min | Max | Median (IQR) | Z | p | r | |
| 6MWD [m] | 108 | 202.00 | 701.00 | 519.50 (448.50–575.25) | 310.00 | 766.00 | 588.00 (519.00–644.00) | −7.841 | <0.001 ** | −0.755 |
| Gait velocity [m/s] | 108 | 0.84 | 2.10 | 1.48 (1.33–1.66) | 1.11 | 2.53 | 1.69 (1.51–1.88) | −7.113 | <0.001 ** | −0.684 |
| Wattmax [W] | 50 | 32.00 | 288.00 | 96.50 (76.25–113.00) | 26.00 | 324.00 | 108.00 (88.75–136.25) | −3.350 | <0.001 ** | −0.474 |
| VO2max [L/Min] | 50 | 0.66 | 3.29 | 1.44 (1.21–1.56) | 0.69 | 3.83 | 1.52 (1.34–1.81) | −3.316 | <0.001 ** | −0.469 |
| 1MSTS [n/Min] | 107 | 9.00 | 43.00 | 20.00 (16.00–24.00) | 9.00 | 45.00 | 21.00 (17.00–26.00) | −2.981 | 0.003 * | −0.288 |
| Quadricep strength [kg] | 108 | 33.02 | 252.09 | 97.78 (74.08–133.51) | 28.83 | 268.34 | 109.09 (89.41–141.00) | −5.766 | <0.001 ** | −0.555 |
| Handgrip strength [kg] | 108 | 11.60 | 70.13 | 28.20 (21.32–35.06) | 7.67 | 70.60 | 29.17 (22.87–35.17) | −0.884 | 0.377 | −0.085 |
| Balance function [0–36 points] | 108 | 3.00 | 35.00 | 25.00 (21.25–27.00) | 11.00 | 35.00 | 26.50 (22.00–30.00) | −3.072 | 0.002 * | −0.297 |
| Subjective physical ability [0–10 points] | 117 | 1.33 | 8.00 | 4.67 (3.44–6.00) | 1.33 | 9.33 | 5.11 (3.78–6.44) | −3.514 | <0.001 ** | −0.325 |
| PCFS [grade 0–4] | 117 | 0.00 | 4.00 | 3.00 (2.00–3.00) | 0.00 | 4.00 | 3.00 (2.00–3.00) | −0.266 | 0.790 | −0.219 |
| Subjective post-COVID symptoms [0–10 points] | 117 | 1.00 | 10.00 | 7.00 (4.00–8.00) | 0.00 | 10.00 | 7.00 (4.00–8.00) | −0.873 | 0.383 | −0.081 |
| T1 | T4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Min | Max | Median (IQR) | Min | Max | Median (IQR) | Z | p | r | |
| 6MWD [m] | 95 | 202.00 | 701.00 | 521.00 (455.00–581.00) | 330.00 | 852.00 | 571.00 (502.00–628.00) | −6.635 | <0.001 ** | −0.681 |
| Gait velocity [m/s] | 95 | 0.99 | 2.10 | 1.47 (1.35–1.68) | 0.92 | 2.51 | 1.65 (1.47–1.83) | −5.668 | <0.001 ** | −0.582 |
| Wattmax [W] | 51 | 37.00 | 177.00 | 97.00 (77.00–113.00) | 30.00 | 190.00 | 108.00 (84.00–139.00) | −3.005 | 0.003 * | −0.421 |
| VO2max [L/Min] | 51 | 0.96 | 2.27 | 1.41 (1.21–1.56) | 0.71 | 2.43 | 1.50 (1.28–1.81) | −3.117 | 0.002 * | −0.437 |
| 1MSTS [n/Min] | 96 | 8.00 | 43.00 | 20.00 (16.00–23.75) | 5.00 | 42.00 | 21.00 (17.00–26.00) | −3.840 | <0.001 ** | −0.392 |
| Quadricep strength [kg] | 96 | 33.02 | 252.09 | 98.53 (74.08–134.20) | 26.46 | 273.45 | 115.78 (87.47–158.79) | −5.525 | <0.001 ** | −0.564 |
| Handgrip strength [kg] | 98 | 9.67 | 70.13 | 27.47 (20.51–35.50) | 7.97 | 67.57 | 28.92 (21.16–35.68) | −0.085 | 0.932 | −0.009 |
| Balance function [0–36 points] | 96 | 3.00 | 35.00 | 25.00 (22.00–27.00) | 6.00 | 36.00 | 26.00 (23.00–29.00) | −2.552 | 0.011 * | −0.261 |
| Subjective physical ability [0–10 points] | 112 | 1.33 | 8.00 | 4.78 (3.47–6.11) | 0.89 | 9.44 | 5.33 (4.03–6.56) | −3.496 | <0.001 ** | −0.330 |
| PCFS [grade 0–4] | 112 | 0.00 | 4.00 | 3.00 (2.00–3.00) | 0.00 | 4.00 | 3.00 (2.00–3.00) | −1.997 | 0.046 * | −0.189 |
| Subjective post-COVID symptoms [0–10 points] | 112 | 1.00 | 10.00 | 7.00 (4.00–8.00) | 0.00 | 10.00 | 6.00 (3.00–8.00) | −1.675 | 0.094 | −0.158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, K.; Ottiger, M.; Poppele, I.; Wastlhuber, A.; Stegbauer, M.; Schlesinger, T. Physical Health in Patients with Post-COVID-19 6 and 12 Months after an Inpatient Rehabilitation: An Observational Study. J. Clin. Med. 2024, 13, 3988. https://doi.org/10.3390/jcm13133988
Müller K, Ottiger M, Poppele I, Wastlhuber A, Stegbauer M, Schlesinger T. Physical Health in Patients with Post-COVID-19 6 and 12 Months after an Inpatient Rehabilitation: An Observational Study. Journal of Clinical Medicine. 2024; 13(13):3988. https://doi.org/10.3390/jcm13133988
Chicago/Turabian StyleMüller, Katrin, Marcel Ottiger, Iris Poppele, Alois Wastlhuber, Michael Stegbauer, and Torsten Schlesinger. 2024. "Physical Health in Patients with Post-COVID-19 6 and 12 Months after an Inpatient Rehabilitation: An Observational Study" Journal of Clinical Medicine 13, no. 13: 3988. https://doi.org/10.3390/jcm13133988
APA StyleMüller, K., Ottiger, M., Poppele, I., Wastlhuber, A., Stegbauer, M., & Schlesinger, T. (2024). Physical Health in Patients with Post-COVID-19 6 and 12 Months after an Inpatient Rehabilitation: An Observational Study. Journal of Clinical Medicine, 13(13), 3988. https://doi.org/10.3390/jcm13133988






