Safety of High-Dose Vitamin C in Non-Intensive Care Hospitalized Patients with COVID-19: An Open-Label Clinical Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
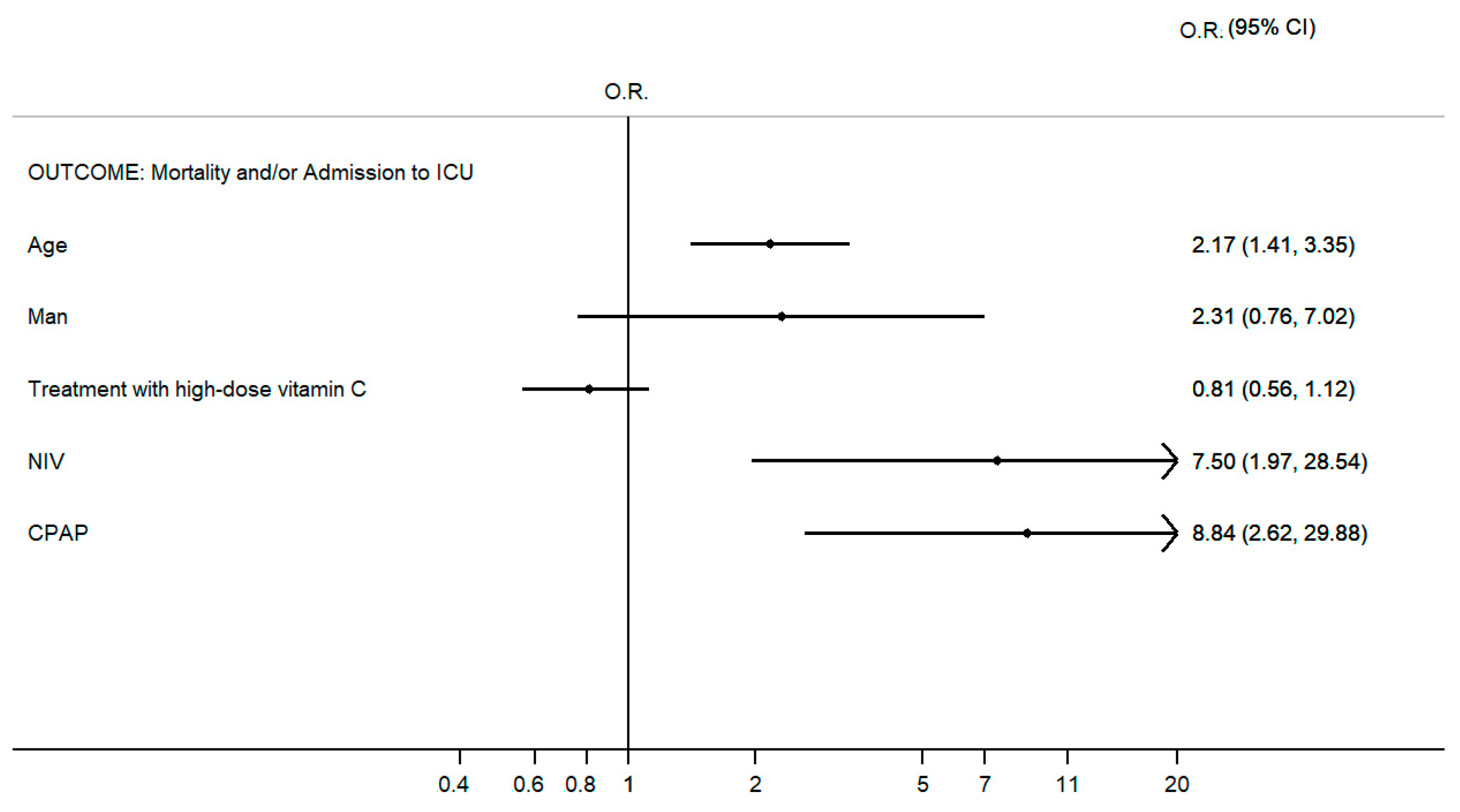
3. Results
4. Discussion
4.1. Vitamin C (Ascorbic Acid)
4.2. Role of Intravenous Vitamin C in Hospitalized Patients with COVID-19
4.3. The Immune System
4.4. Role of the Immune System in COVID-19
4.5. Cytokine Storm
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 14 October 2023).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 14 October 2023).
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- Parasher, A. COVID-19: Current Understanding of Its Pathophysiology, Clinical Presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef]
- Waterer, G. Understanding COVID-19 Pathophysiology: What Defines Progress? Am. J. Respir. Cell Mol. Biol. 2022, 66, 120–121. [Google Scholar] [CrossRef]
- Lui, G.; Guaraldi, G. Drug Treatment of COVID-19 Infection. Curr. Opin. Pulm. Med. 2023, 29, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.-G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging Treatment Strategies for COVID-19 Infection. Clin. Exp. Med. 2021, 21, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Szarpak, L.; Pruc, M.; Gasecka, A.; Jaguszewski, M.J.; Michalski, T.; Peacock, F.W.; Smereka, J.; Pytkowska, K.; Filipiak, K.J. Should We Supplement Zinc in COVID-19 Patients? Evidence from a Meta-Analysis. Pol. Arch. Intern. Med. 2021, 131, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Berti, A.D.; Kale-Pradhan, P.B.; Giuliano, C.A.; Aprilliano, B.N.; Miller, C.R.; Alyashae, B.T.; Bhargava, A.; Johnson, L.B. Clinical Outcomes of Zinc Supplementation Among COVID-19 Patients. Curr. Drug Saf. 2022, 17, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Corrao, S.; Mallaci Bocchio, R.; Lo Monaco, M.; Natoli, G.; Cavezzi, A.; Troiani, E.; Argano, C. Does Evidence Exist to Blunt Inflammatory Response by Nutraceutical Supplementation during COVID-19 Pandemic? An Overview of Systematic Reviews of Vitamin D, Vitamin C, Melatonin, and Zinc. Nutrients 2021, 13, 1261. [Google Scholar] [CrossRef]
- Argano, C.; Mallaci Bocchio, R.; Lo Monaco, M.; Scibetta, S.; Natoli, G.; Cavezzi, A.; Troiani, E.; Corrao, S. An Overview of Systematic Reviews of the Role of Vitamin D on Inflammation in Patients with Diabetes and the Potentiality of Its Application on Diabetic Patients with COVID-19. Int. J. Mol. Sci. 2022, 23, 2873. [Google Scholar] [CrossRef]
- Argano, C.; Mallaci Bocchio, R.; Natoli, G.; Scibetta, S.; Lo Monaco, M.; Corrao, S. Protective Effect of Vitamin D Supplementation on COVID-19-Related Intensive Care Hospitalization and Mortality: Definitive Evidence from Meta-Analysis and Trial Sequential Analysis. Pharmaceuticals 2023, 16, 130. [Google Scholar] [CrossRef] [PubMed]
- Milani, G.P.; Macchi, M.; Guz-Mark, A. Vitamin C in the Treatment of COVID-19. Nutrients 2021, 13, 1172. [Google Scholar] [CrossRef] [PubMed]
- Rawat, D.; Roy, A.; Maitra, S.; Shankar, V.; Khanna, P.; Baidya, D.K. Vitamin D Supplementation and COVID-19 Treatment: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. 2021, 15, 102189. [Google Scholar] [CrossRef]
- Olczak-Pruc, M.; Swieczkowski, D.; Ladny, J.R.; Pruc, M.; Juarez-Vela, R.; Rafique, Z.; Peacock, F.W.; Szarpak, L. Vitamin C Supplementation for the Treatment of COVID-19: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4217. [Google Scholar] [CrossRef] [PubMed]
- Linster, C.L.; Van Schaftingen, E. Vitamin C. Biosynthesis, Recycling and Degradation in Mammals. FEBS J. 2007, 274, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Shilotri, P.; Bhat, K. Effect of Mega Doses of Vitamin C on Bactericidal Ativity of Leukocytes. Am. J. Clin. Nutr. 1977, 30, 1077–1081. [Google Scholar] [CrossRef]
- Liugan, M.; Carr, A.C. Vitamin C and Neutrophil Function: Findings from Randomized Controlled Trials. Nutrients 2019, 11, 2102. [Google Scholar] [CrossRef]
- Manning, J.; Mitchell, B.; Appadurai, D.A.; Shakya, A.; Pierce, L.J.; Wang, H.; Nganga, V.; Swanson, P.C.; May, J.M.; Tantin, D.; et al. Vitamin C Promotes Maturation of T-Cells. Antioxid. Redox Signal. 2013, 19, 2054–2067. [Google Scholar] [CrossRef]
- Hemilä, H.; Louhiala, P. Vitamin C May Affect Lung Infections. J. R. Soc. Med. 2007, 100, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Myint, P.K.; Wilson, A.M.; Clark, A.B.; Luben, R.N.; Wareham, N.J.; Khaw, K.-T. Plasma Vitamin C Concentrations and Risk of Incident Respiratory Diseases and Mortality in the European Prospective Investigation into Cancer-Norfolk Population-Based Cohort Study. Eur. J. Clin. Nutr. 2019, 73, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Louhiala, P. Vitamin C for Preventing and Treating Pneumonia. Cochrane Database Syst. Rev. 2013, 8, CD005532. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Sumanadasa, S.; Shahi, R.; Woodman, R.; Mangoni, A.A.; Bihari, S.; Thompson, C. Efficacy and Safety of Vitamin C Supplementation in the Treatment of Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Sci. Rep. 2024, 14, 11846. [Google Scholar] [CrossRef] [PubMed]
- Brant, E.B.; Angus, D.C. Is High-Dose Vitamin C Beneficial for Patients with Sepsis? JAMA 2019, 322, 1257–1258. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Litwak, J.J.; Cho, N.; Nguyen, H.B.; Moussavi, K.; Bushell, T. Vitamin C, Hydrocortisone, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Analysis of Real-World Application. J. Clin. Med. 2019, 8, 478. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.A.; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I Safety Trial of Intravenous Ascorbic Acid in Patients with Severe Sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Z.; Liao, X.; Guo, W.; Wen, J.-Y.; Qin, T.; Wang, S. Efficacy of Vitamin C in Patients with Sepsis: An Updated Meta-Analysis. Eur. J. Pharmacol. 2020, 868, 172889. [Google Scholar] [CrossRef]
- Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Zhang, M.; Jativa, D.F. Vitamin C Supplementation in the Critically Ill: A Systematic Review and Meta-Analysis. SAGE Open Med. 2018, 6, 2050312118807615. [Google Scholar] [CrossRef] [PubMed]
- Langlois, P.L.; Manzanares, W.; Adhikari, N.K.J.; Lamontagne, F.; Stoppe, C.; Hill, A.; Heyland, D.K. Vitamin C Administration to the Critically Ill: A Systematic Review and Meta-Analysis. J. Parenter. Enter. Nutr. 2019, 43, 335–346. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Vitamin C May Reduce the Duration of Mechanical Ventilation in Critically Ill Patients: A Meta-Regression Analysis. J. Intensive Care 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Bae, S.; Choi, J.; Lim, S.Y.; Lee, N.; Kong, J.M.; Hwang, Y.; Kang, J.S.; Lee, W.J. Vitamin C Is an Essential Factor on the Anti-Viral Immune Responses through the Production of Interferon-α/β at the Initial Stage of Influenza A Virus (H3N2) Infection. Immune Netw. 2013, 13, 70–74. [Google Scholar] [CrossRef]
- Teafatiller, T.; Agrawal, S.; De Robles, G.; Rahmatpanah, F.; Subramanian, V.S.; Agrawal, A. Vitamin C Enhances Anti-viral Functions of Lung Epithelial Cells. Biomolecules 2021, 11, 1148. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Vollbracht, C.; Kraft, K. Feasibility of Vitamin C in the Treatment of Post Viral Fatigue with Focus on Long COVID, Based on a Systematic Review of IV Vitamin C on Fatigue. Nutrients 2021, 13, 1154. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef]
- Corrao, S. Use of Ascorbic Acid in Patients with COVID 19. 2020. Available online: https://clinicaltrials.gov/ (accessed on 14 January 2024).
- Hosmer, D.W., Jr.; Lemeshow, S. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 978-0-471-65402-5. [Google Scholar]
- May, J.M.; Harrison, F.E. Role of Vitamin C in the Function of the Vascular Endothelium. Antioxid. Redox Signal 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Cerullo, G.; Negro, M.; Parimbelli, M.; Pecoraro, M.; Perna, S.; Liguori, G.; Rondanelli, M.; Cena, H.; D’Antona, G. The Long History of Vitamin C: From Prevention of the Common Cold to Potential Aid in the Treatment of COVID-19. Front. Immunol. 2020, 11, 574029. [Google Scholar] [CrossRef]
- Vissers, M.C.M.; Wilkie, R.P. Ascorbate Deficiency Results in Impaired Neutrophil Apoptosis and Clearance and Is Associated with Up-Regulation of Hypoxia-Inducible Factor 1α. J. Leukoc. Biol. 2007, 81, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- A Coordinated Global Research Roadmap. Available online: https://www.who.int/publications/m/item/a-coordinated-global-research-roadmap (accessed on 22 October 2023).
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot Trial of High-Dose Vitamin C in Critically Ill COVID-19 Patients. Ann. Intensive Care 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Dembra, S.; Dembra, P.; Bhawna, F.; Gul, A.; Ali, B.; Sohail, H.; Kumar, B.; Memon, M.K.; Rizwan, A. The Role of Vitamin C as Adjuvant Therapy in COVID-19. Cureus 2020, 12, e11779. [Google Scholar] [CrossRef]
- Li, M.; Ching, T.H.; Hipple, C.; Lopez, R.; Sahibzada, A.; Rahman, H. Use of Intravenous Vitamin C in Critically Ill Patients With COVID-19 Infection. J. Pharm. Pract. 2023, 36, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the Immune Response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host Cell Death and Inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Corrao, S.; Gervasi, F.; Di Bernardo, F.; Natoli, G.; Raspanti, M.; Catalano, N.; Argano, C. Immunological Characteristics of Non-Intensive Care Hospitalized COVID-19 Patients: A Preliminary Report. J. Clin. Med. 2021, 10, 849. [Google Scholar] [CrossRef]
- Corrao, S.; Gervasi, F.; Di Bernardo, F.; Argano, C. Immune Response Failure in Paucisymptomatic Long-Standing SARS-CoV-2 Spreaders. Clin. Pract. 2021, 11, 151–161. [Google Scholar] [CrossRef]
- Engelmann, B.; Massberg, S. Thrombosis as an Intravascular Effector of Innate Immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Di, B.; Xu, L. The NLRP3 Inflammasome and COVID-19: Activation, Pathogenesis and Therapeutic Strategies. Cytokine Growth Factor. Rev. 2021, 61, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Barker, B.R.; Taxman, D.J.; Ting, J.P.-Y. Cross-Regulation between the IL-1β/IL-18 Processing Inflammasome and Other Inflammatory Cytokines. Curr. Opin. Immunol. 2011, 23, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, L.; Lv, W.; Han, L.; Xiang, Y.; Fu, L.; Jin, M.; Zhou, R.; Chen, H.; Zhang, A. An NLRP3 Inflammasome-Triggered Cytokine Storm Contributes to Streptococcal Toxic Shock-like Syndrome (STSLS). PLoS Pathog. 2019, 15, e1007795. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes Are Activated in Response to SARS-CoV-2 Infection and Are Associated with COVID-19 Severity in Patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Xiang, P.; Pu, L.; Xiong, H.; Li, C.; Zhang, M.; Tan, J.; Xu, Y.; Song, R.; et al. Neutrophil-to-Lymphocyte Ratio Predicts Critical Illness Patients with 2019 Coronavirus Disease in the Early Stage. J. Transl. Med. 2020, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Cao, Z.; Xie, X.; Zhang, X.; Chen, J.Y.-C.; Wang, H.; Menachery, V.D.; Rajsbaum, R.; Shi, P.-Y. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020, 33, 108234. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, N.; Jin, R.; Feng, Y.; Wang, S.; Gao, S.; Gao, R.; Wu, G.; Tian, D.; Tan, W.; et al. Immune Suppression in the Early Stage of COVID-19 Disease. Nat. Commun. 2020, 11, 5859. [Google Scholar] [CrossRef]
- Noris, M.; Benigni, A.; Remuzzi, G. The Case of Complement Activation in COVID-19 Multiorgan Impact. Kidney Int. 2020, 98, 314–322. [Google Scholar] [CrossRef]
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 Puzzle: Deciphering Pathophysiology and Phenotypes of a New Disease Entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil Extracellular Traps in COVID-19. J.C.I. Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, Y.; Ling, Y.; Lu, G.; Liu, F.; Yi, Z.; Jia, X.; Wu, M.; Shi, B.; Xu, S.; et al. Viral and Host Factors Related to the Clinical Outcome of COVID-19. Nature 2020, 583, 437–440. [Google Scholar] [CrossRef]
- Van Eijk, L.E.; Binkhorst, M.; Bourgonje, A.R.; Offringa, A.K.; Mulder, D.J.; Bos, E.M.; Kolundzic, N.; Abdulle, A.E.; Van Der Voort, P.H.; Olde Rikkert, M.G.; et al. COVID-19: Immunopathology, Pathophysiological Mechanisms, and Treatment Options. J. Pathol. 2021, 254, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012. [Google Scholar] [CrossRef]
- Hewitt, J.; Carter, B.; Vilches-Moraga, A.; Quinn, T.J.; Braude, P.; Verduri, A.; Pearce, L.; Stechman, M.; Short, R.; Price, A.; et al. The Effect of Frailty on Survival in Patients with COVID-19 (COPE): A Multicentre, European, Observational Cohort Study. Lancet Public Health 2020, 5, e444–e451. [Google Scholar] [CrossRef]
- Argano, C.; Scichilone, N.; Natoli, G.; Nobili, A.; Corazza, G.R.; Mannucci, P.M.; Perticone, F.; Corrao, S.; REPOSI Investigators. Pattern of Comorbidities and 1-Year Mortality in Elderly Patients with COPD Hospitalized in Internal Medicine Wards: Data from the RePoSI Registry. Intern. Emerg. Med. 2021, 16, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Argano, C.; Natoli, G.; Mularo, S.; Nobili, A.; Monaco, M.L.; Mannucci, P.M.; Perticone, F.; Pietrangelo, A.; Corrao, S. Impact of Diabetes Mellitus and Its Comorbidities on Elderly Patients Hospitalized in Internal Medicine Wards: Data from the RePoSi Registry. Healthcare 2022, 10, 86. [Google Scholar] [CrossRef]
- Corrao, S.; Natoli, G.; Nobili, A.; Mannucci, P.M.; Pietrangelo, A.; Perticone, F.; Argano, C.; RePoSI Investigators. Comorbidity Does Not Mean Clinical Complexity: Evidence from the RePoSI Register. Intern. Emerg. Med. 2020, 15, 621–628. [Google Scholar] [CrossRef]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex Differences in Immune Responses That Underlie COVID-19 Disease Outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors Associated with COVID-19-Related Death Using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Van De Veerdonk, F.L.; Netea, M.G.; Van Deuren, M.; Van Der Meer, J.W.; De Mast, Q.; Brüggemann, R.J.; Van Der Hoeven, H. Kallikrein-Kinin Blockade in Patients with COVID-19 to Prevent Acute Respiratory Distress Syndrome. eLife 2020, 9, e57555. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Pregliasco, F.E.; Lombardi, G.; Perazzo, P.; Banfi, G. The Malnutritional Status of the Host as a Virulence Factor for New Coronavirus SARS-CoV-2. Front. Med. 2020, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine Release Syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current Concepts in the Diagnosis and Management of Cytokine Release Syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Kanneganti, T.-D. The “Cytokine Storm”: Molecular Mechanisms and Therapeutic Prospects. Trends Immunol. 2021, 42, 681–705. [Google Scholar] [CrossRef]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multiorgan Response. Curr. Probl. Cardiol. 2020, 45, 100618. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The Cytokine Storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef]
- Harker, J.A.; Lewis, G.M.; Mack, L.; Zuniga, E.I. Late Interleukin-6 Escalates T Follicular Helper Cell Responses and Controls a Chronic Viral Infection. Science 2011, 334, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune Response to SARS-CoV-2 and Mechanisms of Immunopathological Changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Recognition of Microorganisms and Activation of the Immune Response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Klinkhammer, J.; Schnepf, D.; Ye, L.; Schwaderlapp, M.; Gad, H.H.; Hartmann, R.; Garcin, D.; Mahlakõiv, T.; Staeheli, P. IFN-λ Prevents Influenza Virus Spread from the Upper Airways to the Lungs and Limits Virus Transmission. eLife 2018, 7, e33354. [Google Scholar] [CrossRef] [PubMed]
- Betakova, T.; Kostrabova, A.; Lachova, V.; Turianova, L. Cytokines Induced During Influenza Virus Infection. Curr. Pharm. Des. 2017, 23, 2616–2622. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Innate Immune Recognition of Viral Infection. Nat. Immunol. 2006, 7, 131–137. [Google Scholar] [CrossRef]
- Li, X.; Geng, M.; Peng, Y.; Meng, L.; Lu, S. Molecular Immune Pathogenesis and Diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Regulation of Adaptive Immunity by the Innate Immune System. Science 2010, 327, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-Cells and Inflammatory Monocytes Incite Inflammatory Storms in Severe COVID-19 Patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020, 221, jiaa150. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Braciale, T.J.; Sun, J.; Kim, T.S. Regulating the Adaptive Immune Response to Respiratory Virus Infection. Nat. Rev. Immunol. 2012, 12, 295–305. [Google Scholar] [CrossRef]
- D’Errico, S.; Zanon, M.; Montanaro, M.; Radaelli, D.; Sessa, F.; Di Mizio, G.; Montana, A.; Corrao, S.; Salerno, M.; Pomara, C. More than Pneumonia: Distinctive Features of SARS-CoV-2 Infection. From Autopsy Findings to Clinical Implications: A Systematic Review. Microorganisms 2020, 8, 1642. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.H.; Cardani, A.; Braciale, T.J. The Host Immune Response in Respiratory Virus Infection: Balancing Virus Clearance and Immunopathology. Semin. Immunopathol. 2016, 38, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Ascorbic Acid as Antioxidant. Vitam. Horm. 2023, 121, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Grudlewska-Buda, K.; Wiktorczyk-Kapischke, N.; Budzyńska, A.; Kwiecińska-Piróg, J.; Przekwas, J.; Kijewska, A.; Sabiniarz, D.; Gospodarek-Komkowska, E.; Skowron, K. The Variable Nature of Vitamin C—Does It Help When Dealing with Coronavirus? Antioxidants 2022, 11, 1247. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-Y.; Jo, E.-J.; Eom, J.S.; Mok, J.; Kim, M.-H.; Kim, K.U.; Park, H.-K.; Lee, M.K.; Lee, K. Combined Vitamin C, Hydrocortisone, and Thiamine Therapy for Patients with Severe Pneumonia Who Were Admitted to the Intensive Care Unit: Propensity Score-Based Analysis of a before-after Cohort Study. J. Crit. Care 2018, 47, 211–218. [Google Scholar] [CrossRef]
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00299-20. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Xu, M.; Wang, G.; Lv, J.; Ma, X.; Guo, Y.; Zhang, D.; Yang, H.; Jiang, W.; Deng, F.; et al. The Efficiency and Safety of High-Dose Vitamin C in Patients with COVID-19: A Retrospective Cohort Study. Aging 2021, 13, 7020–7034. [Google Scholar] [CrossRef]
- JamaliMoghadamSiahkali, S.; Zarezade, B.; Koolaji, S.; SeyedAlinaghi, S.; Zendehdel, A.; Tabarestani, M.; Sekhavati Moghadam, E.; Abbasian, L.; Dehghan Manshadi, S.A.; Salehi, M.; et al. Safety and Effectiveness of High-Dose Vitamin C in Patients with COVID-19: A Randomized Open-Label Clinical Trial. Eur. J. Med. Res. 2021, 26, 20. [Google Scholar] [CrossRef]
- Lee, Z.-Y.; Ortiz-Reyes, L.; Lew, C.C.H.; Hasan, M.S.; Ke, L.; Patel, J.J.; Stoppe, C.; Heyland, D.K. Intravenous Vitamin C Monotherapy in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with Trial Sequential Analysis. Ann. Intensive Care 2023, 13, 14. [Google Scholar] [CrossRef] [PubMed]
| N | 146 |
|---|---|
| Patients undergoing Intravenous High-Dose Vitamin C (IHDVC) | 104 |
| Patients not undergoing Intravenous High-Dose Vitamin C (IHDVC) | 42 |
| Age § | 64.3 (54.9–76.0) |
| Men (%) * | 59.6 (51.3–67.2) |
| Pre-existing comorbidities * | 80.4 (73.0–86.0) |
| Hypertension * | 70.0 (61.8–76.9) |
| Obesity (BMI ≥ 30 Kg/m2) * | 40.8 (33.0–49.2) |
| Diabetes mellitus * | 35.6 (28.2–43.9) |
| Chronic ischemic heart disease * | 29.3 (22.4–37.4) |
| Chronic cerebrovascular disease * | 29.1 (22.1–36.9) |
| Chronic renal failure * | 14.7 (9.7–21.5) |
| Neoplasm (active or previous) * | 11.1 (6.9–17.5) |
| Atrial fibrillation * | 9.0 (5.3–15.1) |
| Body temperature at admission * | 37.7 (37.1–38.4) |
| Oxygen support at admission: None * | 37 (29.5–45.2) |
| Oxygen support at admission: Nose cannulas * | 10.2 (6.3–16.4) |
| Oxygen support at admission: Venturi mask * | 25.3 (18.9–33.1) |
| Oxygen support at admission: CPAP * | 15.8 (10.7–22.7) |
| Oxygen support at admission: NIV S/T * | 11.0 (6.8–17.2) |
| Hospitalization days § | 19.0 (13.0–29.5) |
| Admission to intensive care/ICU (%) | 8.3 |
| Death (%) | 10.2 |
| Variable | Patients Undergoing IHDVC (n = 104) | Patients Not Undergoing IHDVC (n = 42) | p |
|---|---|---|---|
| Age § | 64 (53–76) | 64 (56–76) | 0.8086 |
| Men (%) | 61.5 | 54.8 | 0.4500 |
| Pre-existing comorbidities (%) | 79.6 | 82.5 | 0.6960 |
| Hypertension (%) | 71.8 | 65.0 | 0.4230 |
| Obesity (BMI ≥ 30 Kg/m2) (%) | 39.2 | 45.0 | 0.5282 |
| Diabetes mellitus (%) | 35.9 | 35.0 | 0.9177 |
| Chronic ischemic heart disease or Chronic cerebrovascular disease (%) | 29.1 | 30.0 | 0.9180 |
| Chronic renal failure (%) | 13.6 | 17.5 | 0.5535 |
| Neoplasm (active or previous) (%) | 10.7 | 12.5 | 0.7566 |
| Oxygen support at admission: None (%) | 39.4 | 30.9 | 0.3372 |
| Oxygen support at admission: Nose cannulas (%) | 11.5 | 7.1 | 0.4284 |
| Oxygen support at admission: Venturi mask (%) | 23.1 | 30.9 | 0.3220 |
| Oxygen support at admission: CPAP (%) | 12.5 | 23.8 | 0.0895 |
| Oxygen support at admission: NIV S/T (%) | 12.5 | 7.1 | 0.3482 |
| Hospitalization days § | 18 (13–27) | 24 (13–31) | 0.1596 |
| Admission to intensive care/ICU (%) | 9.7 | 5.0 | 0.3620 |
| Death (%) | 8.6 | 14.3 | 0.3103 |
| Variable | Patients Undergoing High-Dose Vitamin C Treatment (n = 104) | Patients Not Undergoing High-Dose Vitamin C Treatment (n = 42) | p |
|---|---|---|---|
| Hemoglobin (gr/dL) | −5.2 (−10.9–1.8) | −3.6 (−14.9–4.4) | 0.5064 |
| White cells (cell/uL) | 24.4 (−6.8–75.9) | 1.1 (−23.7–52.5) | 0.0656 |
| Neutrophils (cell/uL) | 16.2 (−22.9–81.5) | −18.6 (−40.6–31.8) | 0.0126 |
| Linfocytes (cell/uL) | 43.2 (2.8–99.6) | 38.6 (−7.4–125) | 0.9161 |
| Platelets (cell/µL) | 12.1 (−19.4–48.6) | 0 (−15.8–27.7) | 0.1735 |
| PCR (mg/dL) | −83.9 (−95.0–−10.1) | −82.5 (−95.0–−19.2) | 0.7957 |
| Procalcitonin (PCT) (of/L) | 0 (−51.4–0) | 0 (−63.1–0) | 0.5827 |
| eGFR (mL/min/m2) | 7.7 (0.4–24.2) | 4.5 (−7.7–31.0) | 0.3599 |
| D-Dimer (ng/mL) | −2.6 (−50.3–40.7) | −14.1 (−58.6–2.1) | 0.2604 |
| Hematocrit (%) | −4.1 (−9.6–2.4) | −1.3 (−10.1–4.9) | 0.3407 |
| Monocytes (cell/µL) | 21.6 (−16.0–71.9) | 18.7 (−15.0–103.7) | 0.9335 |
| Sodium (mmol/L) | 0 (−2.1–2.2) | 1.4 (−0.7–2.9) | 0.1853 |
| Glycemia (mg/dL) | −11.2 (−29.9–24.5) | −10.2 (−28.8–16.5) | 0.9702 |
| Outcome: Length of Hospital Stays | ||
|---|---|---|
| Variables | Coefficient (I.C. 95%) | p Value |
| Age | 0.13 (−0.02–0.28) | 0.089 |
| Male sex | 1.26 (−3.06–5.59) | 0.565 |
| High-dose Vitamin C treatment | −4.95 (−0.21–−9.69) | 0.041 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrao, S.; Raspanti, M.; Agugliaro, F.; Gervasi, F.; Di Bernardo, F.; Natoli, G.; Argano, C., on behalf of Internal Medicine IGR COVID-19 Investigators. Safety of High-Dose Vitamin C in Non-Intensive Care Hospitalized Patients with COVID-19: An Open-Label Clinical Study. J. Clin. Med. 2024, 13, 3987. https://doi.org/10.3390/jcm13133987
Corrao S, Raspanti M, Agugliaro F, Gervasi F, Di Bernardo F, Natoli G, Argano C on behalf of Internal Medicine IGR COVID-19 Investigators. Safety of High-Dose Vitamin C in Non-Intensive Care Hospitalized Patients with COVID-19: An Open-Label Clinical Study. Journal of Clinical Medicine. 2024; 13(13):3987. https://doi.org/10.3390/jcm13133987
Chicago/Turabian StyleCorrao, Salvatore, Massimo Raspanti, Federica Agugliaro, Francesco Gervasi, Francesca Di Bernardo, Giuseppe Natoli, and Christiano Argano on behalf of Internal Medicine IGR COVID-19 Investigators. 2024. "Safety of High-Dose Vitamin C in Non-Intensive Care Hospitalized Patients with COVID-19: An Open-Label Clinical Study" Journal of Clinical Medicine 13, no. 13: 3987. https://doi.org/10.3390/jcm13133987
APA StyleCorrao, S., Raspanti, M., Agugliaro, F., Gervasi, F., Di Bernardo, F., Natoli, G., & Argano, C., on behalf of Internal Medicine IGR COVID-19 Investigators. (2024). Safety of High-Dose Vitamin C in Non-Intensive Care Hospitalized Patients with COVID-19: An Open-Label Clinical Study. Journal of Clinical Medicine, 13(13), 3987. https://doi.org/10.3390/jcm13133987








