Final Results from the First European Real-World Experience on Lusutrombopag Treatment in Cirrhotic Patients with Severe Thrombocytopenia: Insights from the REAl-World Lusutrombopag Treatment in ITalY Study
Abstract
1. Introduction
2. Patients and Methods
3. Results
3.1. Clinical Characteristics of the Patients
3.2. Effect of Lusutrombopag
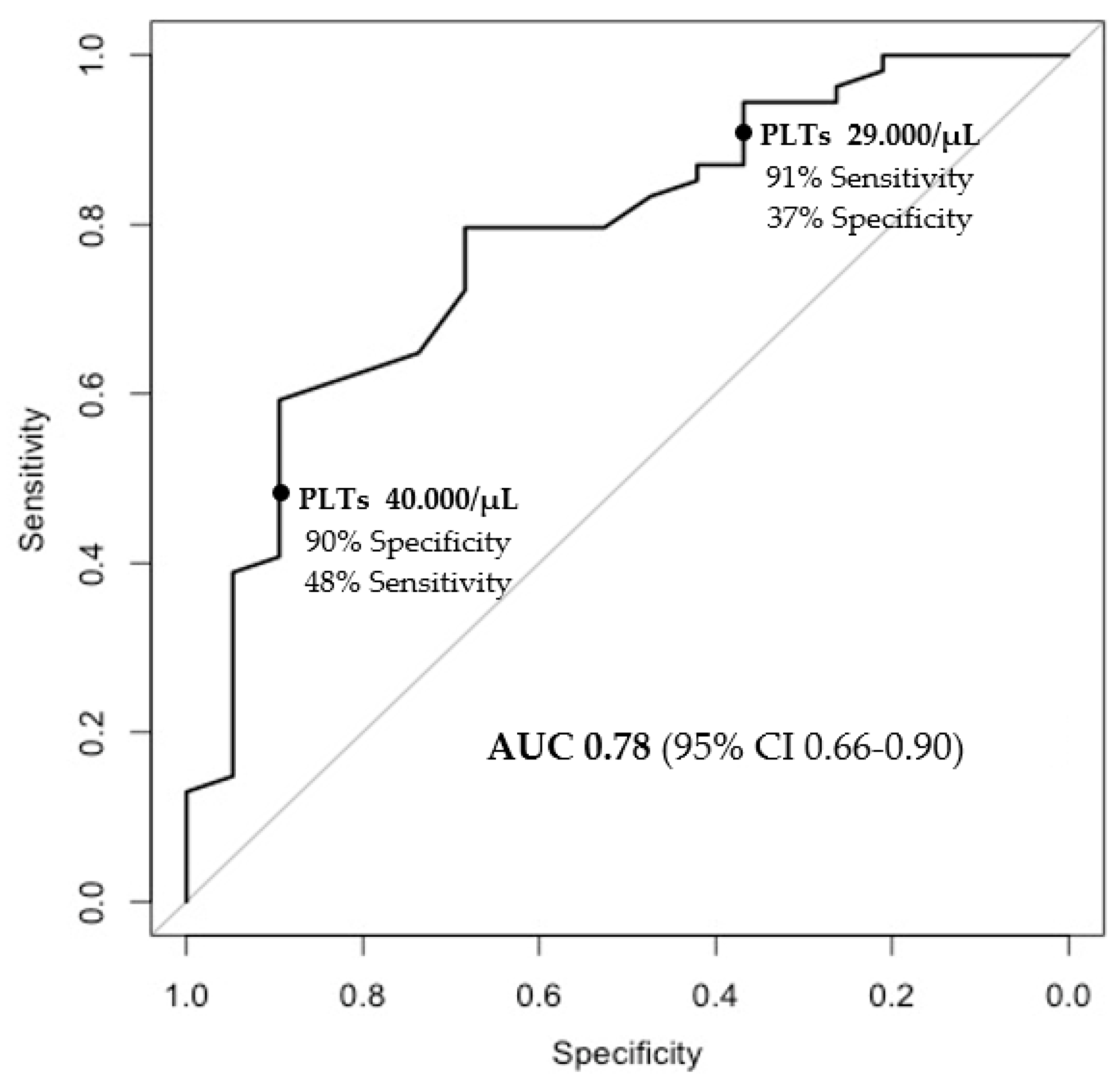
3.3. Factors Associated with Achieving a Platelet Count of ≥50,000 after Treatment with Lusutrombopag
3.4. Safety of Lusutrombopag
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallo, P.; Terracciani, F.; Di Pasquale, G.; Esposito, M.; Picardi, A.; Vespasiani-Gentilucci, U. Thrombocytopenia in chronic liver disease: Physiopathology and new therapeutic strategies before invasive procedures. World J. Gastroenterol. 2022, 28, 4061–4074. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.F.; Waters, A.H. Clinical aspects of platelet transfusions. Blood Coagul. Fibrinolysis 1991, 2, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Peck-Radosavljevic, M. Thrombocytopenia in chronic liver disease. Liver Int. 2017, 37, 778–793. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, H.; Kurosaki, M.; Tanaka, H.; Kudo, M.; Abiru, S.; Igura, T.; Ishikawa, T.; Seike, M.; Katsube, T.; Ochiai, T.; et al. Lusutrombopag Reduces Need for Platelet Transfusion in Patients with Thrombocytopenia Undergoing Invasive Procedures. Clin. Gastroenterol. Hepatol. 2019, 17, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Peck-Radosavljevic, M.; Simon, K.; Iacobellis, A.; Hassanein, T.; Kayali, Z.; Tran, A.; Makara, M.; Ben Ari, Z.; Braun, M.; Mitrut, P.; et al. Lusutrombopag for the Treatment of Thrombocytopenia in Patients with Chronic Liver Disease Undergoing Invasive Procedures (L-PLUS 2). Hepatology 2019, 70, 1336–1348. [Google Scholar] [CrossRef] [PubMed]
- Takada, H.; Kurosaki, M.; Nakanishi, H.; Takahashi, Y.; Itakura, J.; Tsuchiya, K.; Yasui, Y.; Tamaki, N.; Takaura, K.; Komiyama, Y.; et al. Real-life experience of lusutrombopag for cirrhotic patients with low platelet counts being prepared for invasive procedures. PLoS ONE 2019, 14, e0211122. [Google Scholar] [CrossRef]
- Nomoto, H.; Morimoto, N.; Miura, K.; Watanabe, S.; Takaoka, Y.; Maeda, H.; Sasaki, T.; Koyashiki, Y.; Kurata, H.; Numao, N.; et al. Lusutrombopag is effective and safe in patients with chronic liver disease and severe thrombocytopenia: A multicenter retrospective study. BMC Gastroenterol. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Ishikawa, T.; Okoshi, M.; Tomiyoshi, K.; Kojima, Y.; Horigome, R.; Imai, M.; Nozawa, Y.; Iwanaga, A.; Sano, T.; Honma, T.; et al. Efficacy and safety of repeated use of lusutrombopag prior to radiofrequency ablation in patients with recurrent hepatocellular carcinoma and thrombocytopenia. Hepatol. Res. 2019, 49, 590–593. [Google Scholar] [CrossRef]
- Kaneko, T.; Takano, Y.; Ishibashi, K. Lusutrombopag as a substitute for platelet transfusion for thrombocytopenia associated with chronic liver disease in a patient undergoing endoscopic spinal surgery: A case report. Medicine 2021, 100, e24094. [Google Scholar] [CrossRef]
- Kawata, Y.; Endou, M.; Isozaki, Y.; Kitamura, T.; Fukushima, Y.; Sato, T. Three cases of patients with chronic liver disease complicated by thrombocytopenia who were treated with lusutrombopag before tooth extraction. J. Oral Maxillofac. Surgery Med. Pathol. 2021, 33, 463–466. [Google Scholar] [CrossRef]
- Yoshida, M.; Tateishi, R.; Hiroi, S.; Hongo, Y.; Fujiwara, M.; Kitanishi, Y.; Iwasaki, K.; Takeshima, T.; Igarashi, A. Effects of Lusutrombopag on Post-invasive Procedural Bleeding in Thrombocytopenic Patients with Chronic Liver Disease. Adv. Ther. 2022, 39, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Barnish, M.S.; Turner, S. The value of pragmatic and observational studies in health care and public health. Pragmatic Obs. Res. 2017, 8, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Dionne, J.; Pinho, G.; Gignac, J.; Almirall, J.; Lapointe, L. Randomized Controlled Trials: Do They Have External Validity for Patients with Multiple Comorbidities? Ann. Fam. Med. 2006, 4, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Baba, T.; Yoshida, M.; Iguchi, M.; Sonoyama, T.; Fukuhara, T.; Kano, T. Relationship between baseline clinical characteristics and efficacy of lusutrombopag in thrombocytopenic patients with chronic liver disease: post hoc analysis of two placebo-controlled phase 3 trials. Curr. Med Res. Opin. 2022, 38, 303–310. [Google Scholar] [CrossRef]
- Afdhal, N.H.; Giannini, E.G.; Tayyab, G.; Mohsin, A.; Lee, J.-W.; Andriulli, A.; Jeffers, L.; McHutchison, J.; Chen, P.-J.; Han, K.-H.; et al. Eltrombopag before Procedures in Patients with Cirrhosis and Thrombocytopenia. New Engl. J. Med. 2012, 367, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Faccia, M.; Santopaolo, F.; Gasbarrini, A.; Pompili, M.; Zocco, M.A.; Ponziani, F.R. Risk factors for portal vein thrombosis or venous thromboembolism in a large cohort of hospitalized cirrhotic patients. Intern. Emerg. Med. 2022, 17, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Yerdel, M.A.; Gunson, B.; Mirza, D.; Karayalçin, K.; Olliff, S.; Buckels, J.; Mayer, D.; McMaster, P.; Pirenne, J. Portal vein thrombosis in adults undergoing liver transplantation: Risk factors, screening, management, and outcome. Transplantation 2000, 69, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, L.; Gao, F.; An, Y.; Yin, Y.; Guo, X.; Nery, F.G.; Yoshida, E.M.; Qi, X. Epidemiology of portal vein thrombosis in liver cirrhosis: A systematic review and meta-analysis. Eur. J. Intern. Med. 2022, 104, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Senzolo, M.; Shalaby, S.; Grasso, M.; Vitale, A.; Pizzirani, E.; Barbiero, G.; Zanetto, A.; Feltracco, P.; Simioni, P.; Burra, P.; et al. Role of nonneoplastic PVT in the natural history of patients with cirrhosis and first diagnosis of HCC. Hepatology 2024, 79, 355–367. [Google Scholar] [CrossRef]
- Bertot, L.C.; Sato, M.; Tateishi, R.; Yoshida, H.; Koike, K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: A systematic review. Eur. Radiol. 2011, 21, 2584–2596. [Google Scholar] [CrossRef]
- Kong, W.-T.; Zhang, W.-W.; Qiu, Y.-D.; Zhou, T.; Qiu, J.-L.; Ding, Y.-T. Major complications after radiofrequency ablation for liver tumors: Analysis of 255 patients. World J. Gastroenterol. 2009, 15, 2651–2656. [Google Scholar] [CrossRef] [PubMed]
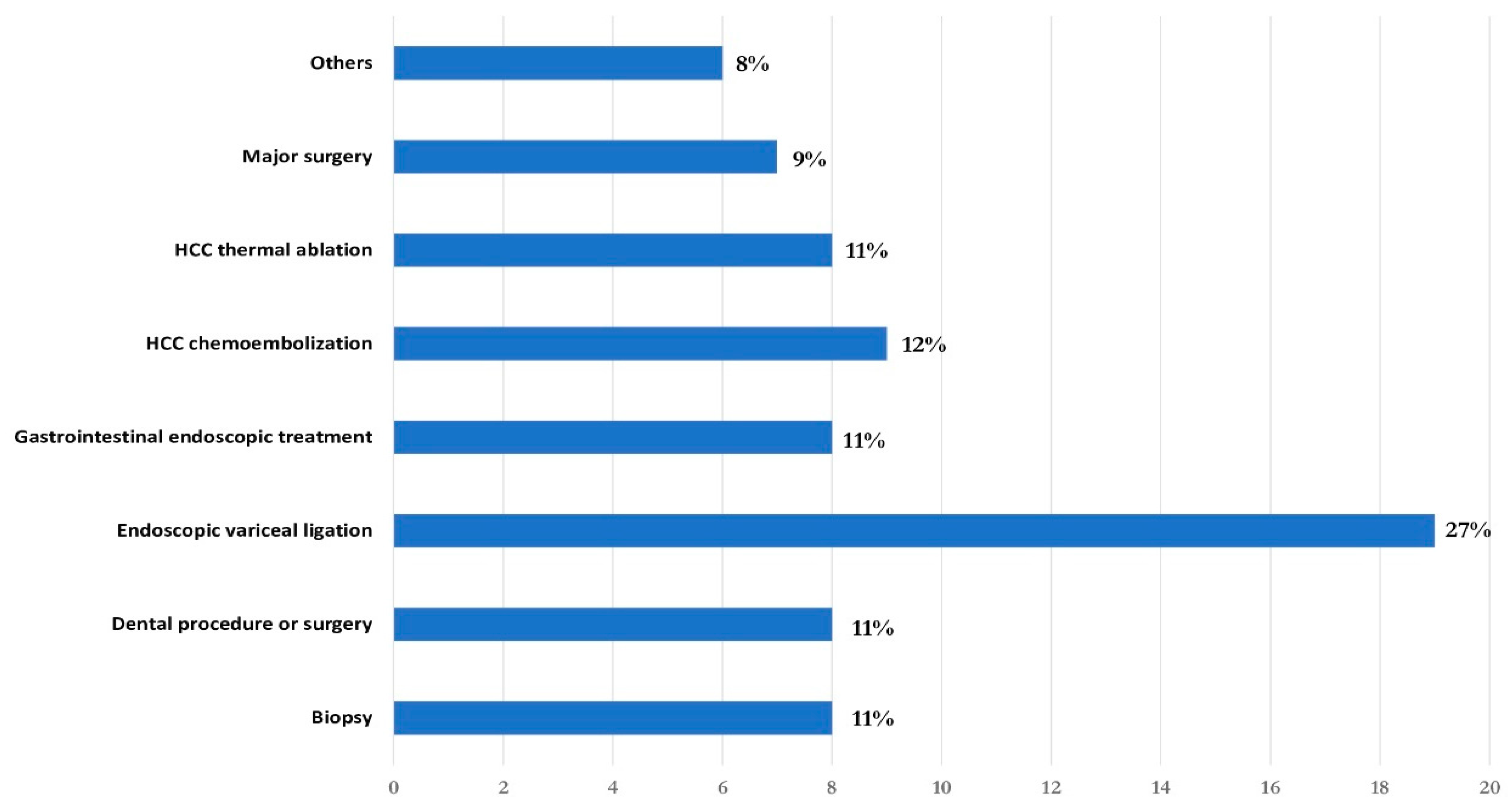

| Characteristics | n. 73 |
|---|---|
| Socio-demographics and comorbidities | |
| Age (years) | 66 (56–72) |
| Sex (female) | 23 (32%) |
| Body mass index (kg/m2) | 25 (21–28) |
| Diabetes mellitus | 27 (37%) |
| Liver disease etiology | |
| HCV | 29 (40%) |
| NASH | 16 (21%) |
| Alcohol | 10 (13%) |
| HBV | 11 (15%) |
| Others | 7 (11%) |
| Child-Pugh Class | |
| A | 45 (63%) |
| B | 20 (28%) |
| C | 6 (9%) |
| B-blocker treatment | 45 (62%) |
| Ultrasound parameters | |
| Spleen interpolar diameter (cm) | 18(16–20) |
| Portal vein diameter (mm) | 14 (13–17) |
| History of portal vein thrombosis | 14 (19%) |
| Biochemistry | |
| Baseline platelets (/μL) | 37,000 (33,000–44,000) |
| Baseline WBC (/μL) | 3470 (2495–4391) |
| Previous TPO-RA use | 11 (15%) |
| Platelets (/μL) | |
| Baseline (pre-treatment) | 37,000 (33,000–44,000) |
| Pre-procedure (post-treatment) | 58,000 (49,000–82,000) |
| 0–48 h after procedure | 56,000 (48,500–87,500) |
| 2–30 days after procedure | 51,499 (40,000–66,750) |
| Characteristics | n. 14 |
|---|---|
| Socio-demographics and comorbidities | |
| Age (years) | 72 (52–77) |
| Sex (female) | 5(36%) |
| Body mass index (kg/m2) | 25 (23–28) |
| Diabetes mellitus | 6 (43%) |
| Liver disease etiology | |
| HCV | 8 (57%) |
| NASH | 3 (21%) |
| Alcohol | 2 (14%) |
| HBV | 1 (8%) |
| Child-Pugh Class | |
| A | 9 (64%) |
| B | 4 (28%) |
| C | 1 (8%) |
| B-blocker treatment | 7 (50%) |
| History of portal vein thrombosis | |
| Acute portal vein thrombosis | 2 (14%) |
| Portal cavernous transformation | 12 (86%) |
| Ultrasound parameters | |
| Spleen interpolar diameter (cm) | 17 (14–22) |
| Portal vein diameter (mm) | 14 (13–19) |
| Biochemistry | |
| Baseline platelets (/μL) | 34,000 (26,000–46,500) |
| Baseline WBC (/μL) | 2720 (2300–2920) |
| Baseline INR | 1.4 (1.3–1.6) |
| Previous TPO-RA use | 1 (7%) |
| Platelets (/μL) | |
| Baseline (pre-treatment) | 34,000 (26,000–46,500) |
| Pre-procedure (post-treatment) | 53,000 (44,500–58,000) |
| 0–48 h after procedure | 54,499 (43,750–68,750) |
| 2–30 days after procedure | 50,000 (40,000–64,250) |
| Univariate | Multivariate * | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p Value | OR | 95%CI | p Value | |
| Age | 0.97 | 0.92–1.02 | 0.24 | |||
| Sex (female) | 1.4 | 0.45–4.88 | 0.57 | |||
| BMI | 0.99 | 0.94–1.03 | 0.51 | |||
| Diabetes mellitus | 1.01 | 0.35–3.10 | 0.99 | |||
| Child Pugh | 1.06 | 0.78–1.51 | 0.71 | |||
| Spleen diameter (cm) | 0.74 | 0.56–0.95 | 0.02 | 0.85 | 0.62–1.01 | 0.26 |
| Portal vein diameter (mm) | 0.95 | 0.80–1.13 | 0.52 | |||
| Beta blockers treatment | 0.70 | 0.22–2.08 | 0.54 | |||
| Baseline platelets (1000/μL) | 1.13 | 1.06–1.23 | <0.001 | 1.13 | 1.04–1.26 | 0.01 |
| Baseline white blood cells (1000/μL) | 1.51 | 1.02–2.43 | 0.06 | 1.09 | 0.74–1.74 | 0.67 |
| Previous TPO-RA use | 0.57 | 0.15–2.42 | 0.42 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, P.; De Vincentis, A.; Terracciani, F.; Falcomatà, A.; Pace Palitti, V.; Russello, M.; Vignone, A.; Alvaro, D.; Tortora, R.; Biolato, M.; et al. Final Results from the First European Real-World Experience on Lusutrombopag Treatment in Cirrhotic Patients with Severe Thrombocytopenia: Insights from the REAl-World Lusutrombopag Treatment in ITalY Study. J. Clin. Med. 2024, 13, 3965. https://doi.org/10.3390/jcm13133965
Gallo P, De Vincentis A, Terracciani F, Falcomatà A, Pace Palitti V, Russello M, Vignone A, Alvaro D, Tortora R, Biolato M, et al. Final Results from the First European Real-World Experience on Lusutrombopag Treatment in Cirrhotic Patients with Severe Thrombocytopenia: Insights from the REAl-World Lusutrombopag Treatment in ITalY Study. Journal of Clinical Medicine. 2024; 13(13):3965. https://doi.org/10.3390/jcm13133965
Chicago/Turabian StyleGallo, Paolo, Antonio De Vincentis, Francesca Terracciani, Andrea Falcomatà, Valeria Pace Palitti, Maurizio Russello, Anthony Vignone, Domenico Alvaro, Raffaella Tortora, Marco Biolato, and et al. 2024. "Final Results from the First European Real-World Experience on Lusutrombopag Treatment in Cirrhotic Patients with Severe Thrombocytopenia: Insights from the REAl-World Lusutrombopag Treatment in ITalY Study" Journal of Clinical Medicine 13, no. 13: 3965. https://doi.org/10.3390/jcm13133965
APA StyleGallo, P., De Vincentis, A., Terracciani, F., Falcomatà, A., Pace Palitti, V., Russello, M., Vignone, A., Alvaro, D., Tortora, R., Biolato, M., Pompili, M., Calvaruso, V., Marzia, V., Tizzani, M., Caneglias, A., Frigo, F., Gesualdo, M., Marzano, A., Rosato, V., ... Vespasiani-Gentilucci, U. (2024). Final Results from the First European Real-World Experience on Lusutrombopag Treatment in Cirrhotic Patients with Severe Thrombocytopenia: Insights from the REAl-World Lusutrombopag Treatment in ITalY Study. Journal of Clinical Medicine, 13(13), 3965. https://doi.org/10.3390/jcm13133965










