Non-Invasive Biomarkers for Differentiating Alcohol Associated Hepatitis from Acute Decompensation in Patients with ALD
Abstract
1. Introduction
2. Natural History of ALD
2.1. ALD Early Stages
2.2. Alcohol-Related Liver Cirrhosis
Predicting Decompensation
3. Non-Invasive Assessment in Alcoholic-Related Hepatitis
3.1. Cross-Section Imaging in AH
3.2. Circulating Biomarkers in Alcohol-Associated Hepatitis
3.2.1. Serum Keratin 18
3.2.2. Sphingolipids
3.2.3. The Gut Microbiota
3.2.4. MicroRNAs
3.2.5. Breath Tests
3.2.6. Genetic Markers
3.2.7. Other Biomarkers
4. Non-Invasive Assessment of Corticotherapy Response
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Zhang, X.; Liu, M.; Zhu, L.; He, Z. Global, Regional, and National Burden of Cirrhosis and Other Chronic Liver Diseases Due to Alcohol Use, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. BMC Gastroenterol. 2022, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver Cirrhosis. Lancet Lond. Engl. 2021, 398, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Talbäck, M.; Andreasson, A.; Walldius, G.; Hammar, N. Repeated FIB-4 Measurements Can Help Identify Individuals at Risk of Severe Liver Disease. J. Hepatol. 2020, 73, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Lackner, C.; Tiniakos, D. Fibrosis and Alcohol-Related Liver Disease. J. Hepatol. 2019, 70, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Mathurin, P.; Beuzin, F.; Louvet, A.; Carrié-Ganne, N.; Balian, A.; Trinchet, J.C.; Dalsoglio, D.; Prevot, S.; Naveau, S. Fibrosis Progression Occurs in a Subgroup of Heavy Drinkers with Typical Histological Features. Aliment. Pharmacol. Ther. 2007, 25, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Bernardi, M.; Angeli, P. Towards a New Definition of Decompensated Cirrhosis. J. Hepatol. 2022, 76, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Fernandez, J.; Papp, M.; Caraceni, P.; Laleman, W.; Gambino, C.; Giovo, I.; Uschner, F.E.; Jimenez, C.; Mookerjee, R.; et al. The PREDICT Study Uncovers Three Clinical Courses of Acutely Decompensated Cirrhosis That Have Distinct Pathophysiology. J. Hepatol. 2020, 73, 842–854. [Google Scholar] [CrossRef]
- McPherson, S.; Lucey, M.R.; Moriarty, K.J. Decompensated Alcohol Related Liver Disease: Acute Management. Br. Med. J. 2016, 352, i124. [Google Scholar] [CrossRef] [PubMed]
- Mathurin, P.; Bataller, R. Trends in the Management and Burden of Alcoholic Liver Disease. J. Hepatol. 2015, 62, S38–S46. [Google Scholar] [CrossRef]
- Bataller, R.; Arab, J.P.; Shah, V.H. Alcohol-Associated Hepatitis. N. Engl. J. Med. 2022, 387, 2436–2448. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel; Chair:; EASL Governing Board representative:; Panel members: EASL Clinical Practice Guidelines on Non-Invasive Tests for Evaluation of Liver Disease Severity and Prognosis—2021 Update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Louvet, A.; Thursz, M.R.; Kim, D.J.; Labreuche, J.; Atkinson, S.R.; Sidhu, S.S.; O’Grady, J.G.; Akriviadis, E.; Sinakos, E.; Carithers, R.L.; et al. Corticosteroids Reduce Risk of Death Within 28 Days for Patients With Severe Alcoholic Hepatitis, Compared with Pentoxifylline or Placebo-a Meta-Analysis of Individual Data From Controlled Trials. Gastroenterology 2018, 155, 458–468.e8. [Google Scholar] [CrossRef]
- Mathurin, P. Early Liver Transplantation for Acute Alcoholic Hepatitis: We Can’t Say No. J. Hepatol. 2021, 75, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic Liver Disease. Nat. Rev. Dis. Primer 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Medina, C.; Melo, L.; Martí-Aguado, D.; Bataller, R. Subclinical versus Advanced Forms of Alcohol-Related Liver Disease: Need for Early Detection. Clin. Mol. Hepatol. 2023, 29, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Schwenzer, N.F.; Springer, F.; Schraml, C.; Stefan, N.; Machann, J.; Schick, F. Non-Invasive Assessment and Quantification of Liver Steatosis by Ultrasound, Computed Tomography and Magnetic Resonance. J. Hepatol. 2009, 51, 433–445. [Google Scholar] [CrossRef]
- Mueller, S.; Seitz, H.K.; Rausch, V. Non-Invasive Diagnosis of Alcoholic Liver Disease. World J. Gastroenterol. 2014, 20, 14626–14641. [Google Scholar] [CrossRef]
- Singal, A.K.; Mathurin, P. Diagnosis and Treatment of Alcohol-Associated Liver Disease: A Review. J. Am. Med. Assoc. 2021, 326, 165. [Google Scholar] [CrossRef]
- Crabb, D.W.; Bataller, R.; Chalasani, N.P.; Kamath, P.S.; Lucey, M.; Mathurin, P.; McClain, C.; McCullough, A.; Mitchell, M.C.; Morgan, T.R.; et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients with Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016, 150, 785–790. [Google Scholar] [CrossRef]
- Sersté, T.; Cornillie, A.; Njimi, H.; Pavesi, M.; Arroyo, V.; Putignano, A.; Weichselbaum, L.; Deltenre, P.; Degré, D.; Trépo, E.; et al. The Prognostic Value of Acute-on-Chronic Liver Failure during the Course of Severe Alcoholic Hepatitis. J. Hepatol. 2018, 69, 318–324. [Google Scholar] [CrossRef]
- Altamirano, J.; Miquel, R.; Katoonizadeh, A.; Abraldes, J.G.; Duarte-Rojo, A.; Louvet, A.; Augustin, S.; Mookerjee, R.P.; Michelena, J.; Smyrk, T.C.; et al. A Histologic Scoring System for Prognosis of Patients with Alcoholic Hepatitis. Gastroenterology 2014, 146, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Lackner, C.; Spindelboeck, W.; Haybaeck, J.; Douschan, P.; Rainer, F.; Terracciano, L.; Haas, J.; Berghold, A.; Bataller, R.; Stauber, R.E. Histological Parameters and Alcohol Abstinence Determine Long-Term Prognosis in Patients with Alcoholic Liver Disease. J. Hepatol. 2017, 66, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Lackner, C.; Stauber, R.E.; Davies, S.; Denk, H.; Dienes, H.P.; Gnemmi, V.; Guido, M.; Miquel, R.; Paradis, V.; Schirmacher, P.; et al. Development and Prognostic Relevance of a Histologic Grading and Staging System for Alcohol-Related Liver Disease. J. Hepatol. 2021, 75, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Pinzani, M. Hepatic Fibrosis 2022: Unmet Needs and a Blueprint for the Future. Hepatology 2022, 75, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Thiele, M.; Johansen, S.; Israelsen, M.; Trebicka, J.; Abraldes, J.G.; Gines, P.; Krag, A. Noninvasive assessment of hepatic decompensation. Hepatology 2023. [Google Scholar] [CrossRef] [PubMed]
- de Franchis, R.; Baveno, V.I. Faculty Expanding Consensus in Portal Hypertension: Report of the Baveno VI Consensus Workshop: Stratifying Risk and Individualizing Care for Portal Hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Semmler, G.; Yang, Z.; Fritz, L.; Köck, F.; Hofer, B.S.; Balcar, L.; Hartl, L.; Jachs, M.; Stopfer, K.; Schedlbauer, A.; et al. Dynamics in Liver Stiffness Measurements Predict Outcomes in Advanced Chronic Liver Disease. Gastroenterology 2023, 165, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver EASL Clinical Practice Guidelines for the Management of Patients with Decompensated Cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver EASL Clinical Practice Guidelines on the Management of Hepatic Encephalopathy. J. Hepatol. 2022, 77, 807–824. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; S Sulkowski, M.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a Simple Noninvasive Index to Predict Significant Fibrosis in Patients with HIV/HCV Coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Mauro, E.; Crespo, G.; Martinez-Garmendia, A.; Gutierrez-Acevedo, M.N.; Diaz, J.M.; Saidman, J.; Bermudez, C.; Ortiz-Patron, J.; Garcia-Olveira, L.; Zalazar, F.; et al. Cystatin C and Sarcopenia Predict Acute on Chronic Liver Failure Development and Mortality in Patients on the Liver Transplant Waiting List. Transplantation 2020, 104, e188–e198. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, D.N.; Thiele, M.; Johansen, S.; Kjærgaard, M.; Lindvig, K.P.; Israelsen, M.; Antonsen, S.; Detlefsen, S.; Krag, A.; GALAXY; et al. Prognostic Performance of 7 Biomarkers Compared to Liver Biopsy in Early Alcohol-Related Liver Disease. J. Hepatol. 2021, 75, 1017–1025. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, S.-D.; Wu, L.; Wang, S.-Q.; Chen, Y.; Liu, L.-L.; Li, J.; Yang, C.-Q.; Wang, J.-Y.; Jiang, W. The Prognostic Role of Liver Stiffness in Patients with Chronic Liver Disease: A Systematic Review and Dose-Response Meta-Analysis. Hepatol. Int. 2019, 13, 560–572. [Google Scholar] [CrossRef]
- Parkes, J.; Roderick, P.; Harris, S.; Day, C.; Mutimer, D.; Collier, J.; Lombard, M.; Alexander, G.; Ramage, J.; Dusheiko, G.; et al. Enhanced Liver Fibrosis Test Can Predict Clinical Outcomes in Patients with Chronic Liver Disease. Gut 2010, 59, 1245–1251. [Google Scholar] [CrossRef]
- Colli, A.; Massironi, S.; Faccioli, P.; Conte, D. “Pseudotumoral” Hepatic Areas in Acute Alcoholic Hepatitis: A Computed Tomography and Histological Study. Am. J. Gastroenterol. 2005, 100, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Gluskin, A.B.; Dueker, J.M.; El Hag, M.; Puthenpurayil, K.J.; Bataller, R. Alcoholic Hepatitis Masquerading as Tumor Infiltration: Reversibility after Abstinence. Clin. Case Rep. 2019, 7, 2174–2176. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.R.; Seibert, D.; Yang, M.; Salman, K.; Frick, M.P. Reversible Heterogeneous Arterial Phase Liver Perfusion Associated with Transient Acute Hepatitis: Findings on Gadolinium-Enhanced MRI. J. Magn. Reson. Imaging JMRI 2004, 20, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Woolbright, B.L.; Bridges, B.W.; Dunn, W.; Olson, J.C.; Weinman, S.A.; Jaeschke, H. Cell Death and Prognosis of Mortality in Alcoholic Hepatitis Patients Using Plasma Keratin-18. Gene Expr. 2017, 17, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Caulín, C.; Salvesen, G.S.; Oshima, R.G. Caspase Cleavage of Keratin 18 and Reorganization of Intermediate Filaments during Epithelial Cell Apoptosis. J. Cell Biol. 1997, 138, 1379–1394. [Google Scholar] [CrossRef]
- Atkinson, S.R.; Grove, J.I.; Liebig, S.; Astbury, S.; Vergis, N.; Goldin, R.; Quaglia, A.; Bantel, H.; Guha, I.N.; Thursz, M.R.; et al. In Severe Alcoholic Hepatitis, Serum Keratin-18 Fragments Are Diagnostic, Prognostic, and Theragnostic Biomarkers. Am. J. Gastroenterol. 2020, 115, 1857–1868. [Google Scholar] [CrossRef]
- Vatsalya, V.; Cave, M.C.; Kong, M.; Gobejishvili, L.; Falkner, K.C.; Craycroft, J.; Mitchell, M.; Szabo, G.; McCullough, A.; Dasarathy, S.; et al. Keratin 18 Is a Diagnostic and Prognostic Factor for Acute Alcoholic Hepatitis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, T.S.; Arab, J.P.; Liu, M.; Amrollahi, P.; Wan, M.; Fan, J.; Nakao, Y.; Pose, E.; Navarro-Corcuera, A.; Dasgupta, D.; et al. Circulating Extracellular Vesicles Carrying Sphingolipid Cargo for the Diagnosis and Dynamic Risk Profiling of Alcoholic Hepatitis. Hepatology 2021, 73, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, V.; Gabbert, C.; Raina, A.; Bell, L.N.; Cooper, S.; Malik, S.; Behari, J. Serum Metabolomic Profiling in Acute Alcoholic Hepatitis Identifies Multiple Dysregulated Pathways. PLoS ONE 2014, 9, e113860. [Google Scholar] [CrossRef] [PubMed]
- Horhat, A.; Fischer, P.; Nicoara-Farcau, O.; Rusu, I.; Morar, C.; Bumbu, A.; Ignat, M.; Procopet, B.; Socaciu, C.; Sparchez, Z.; et al. Enhanced Diagnosis and Prognosis of Severe Alcoholic Hepatitis Using Novel Metabolomic Biomarkers. Alcohol Alcohol. Oxf. Oxfs. 2023, 58, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Saha, B.; Kodys, K.; Catalano, D.; Satishchandran, A.; Szabo, G. Increased Number of Circulating Exosomes and Their microRNA Cargos Are Potential Novel Biomarkers in Alcoholic Hepatitis. J. Transl. Med. 2015, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Salameh, H.; Raff, E.; Erwin, A.; Seth, D.; Nischalke, H.D.; Falleti, E.; Burza, M.A.; Leathert, J.; Romeo, S.; Molinaro, A.; et al. PNPLA3 Gene Polymorphism Is Associated With Predisposition to and Severity of Alcoholic Liver Disease. Am. J. Gastroenterol. 2015, 110, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage Targeting of Gut Bacterium Attenuates Alcoholic Liver Disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lang, S.; Duan, Y.; Zhang, X.; Gao, B.; Chopyk, J.; Schwanemann, L.K.; Ventura-Cots, M.; Bataller, R.; Bosques-Padilla, F.; et al. Intestinal Virome in Patients With Alcoholic Hepatitis. Hepatology 2020, 72, 2182–2196. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Duan, Y.; Liu, J.; Torralba, M.G.; Kuelbs, C.; Ventura-Cots, M.; Abraldes, J.G.; Bosques-Padilla, F.; Verna, E.C.; Brown, R.S.; et al. Intestinal Fungal Dysbiosis and Systemic Immune Response to Fungi in Patients With Alcoholic Hepatitis. Hepatology 2020, 71, 522–538. [Google Scholar] [CrossRef]
- Castera, L.; Hartmann, D.J.; Chapel, F.; Guettier, C.; Mall, F.; Lons, T.; Richardet, J.P.; Grimbert, S.; Morassi, O.; Beaugrand, M.; et al. Serum Laminin and Type IV Collagen Are Accurate Markers of Histologically Severe Alcoholic Hepatitis in Patients with Cirrhosis. J. Hepatol. 2000, 32, 412–418. [Google Scholar] [CrossRef]
- Hanouneh, I.A.; Zein, N.N.; Cikach, F.; Dababneh, L.; Grove, D.; Alkhouri, N.; Lopez, R.; Dweik, R.A. The Breathprints in Patients with Liver Disease Identify Novel Breath Biomarkers in Alcoholic Hepatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, M.; Huang, E.; Pathak, V.; Bellar, A.; Welch, N.; Dasarathy, J.; Streem, D.; McClain, C.J.; Mitchell, M.C.; Barton, B.A.; et al. Proteomics Identifies Complement Protein Signatures in Patients with Alcohol-Associated Hepatitis. JCI Insight 2024, 9, e174127. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Mathurin, P. Alcoholic Liver Disease: Mechanisms of Injury and Targeted Treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef]
- Phillips, R.; Ursell, T.; Wiggins, P.; Sens, P. Emerging Roles for Lipids in Shaping Membrane-Protein Function. Nature 2009, 459, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Thiele, M.; Suvitaival, T.; Trošt, K.; Kim, M.; de Zawadzki, A.; Kjaergaard, M.; Rasmussen, D.N.; Lindvig, K.P.; Israelsen, M.; Detlefsen, S.; et al. Sphingolipids Are Depleted in Alcohol-Related Liver Fibrosis. Gastroenterology 2023, 164, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Mundra, P.A.; Wong, G.; Rahman, K.; Huynh, K.; Barlow, C.K.; Duly, A.M.P.; Haber, P.S.; Whitfield, J.B.; Seth, D. Circulating Lipids Are Associated with Alcoholic Liver Cirrhosis and Represent Potential Biomarkers for Risk Assessment. PLoS ONE 2015, 10, e0130346. [Google Scholar] [CrossRef] [PubMed]
- Clària, J.; Curto, A.; Moreau, R.; Colsch, B.; López-Vicario, C.; Lozano, J.J.; Aguilar, F.; Castelli, F.A.; Fenaille, F.; Junot, C.; et al. Untargeted Lipidomics Uncovers Lipid Signatures That Distinguish Severe from Moderate Forms of Acutely Decompensated Cirrhosis. J. Hepatol. 2021, 75, 1116–1127. [Google Scholar] [CrossRef]
- Rachakonda, V.; Argemi, J.; Borhani, A.A.; Bataller, R.; Tevar, A.; Behari, J. Reduced Serum Sphingolipids Constitute a Molecular Signature of Malnutrition in Hospitalized Patients With Decompensated Cirrhosis. Clin. Transl. Gastroenterol. 2019, 10, e00013. [Google Scholar] [CrossRef]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The Gut-Liver Axis and the Intersection with the Microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef]
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut Microbiome and Liver Diseases. Gut 2016, 65, 2035–2044. [Google Scholar] [CrossRef]
- Trebicka, J.; Bork, P.; Krag, A.; Arumugam, M. Utilizing the Gut Microbiome in Decompensated Cirrhosis and Acute-on-Chronic Liver Failure. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; White, M.B.; Monteith, P.; Noble, N.A.; Unser, A.B.; Daita, K.; Fisher, A.R.; et al. Altered Profile of Human Gut Microbiome Is Associated with Cirrhosis and Its Complications. J. Hepatol. 2014, 60, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Thacker, L.R.; Fagan, A.; White, M.B.; Gavis, E.A.; Hylemon, P.B.; Brown, R.; Acharya, C.; Heuman, D.M.; Fuchs, M.; et al. Gut Microbial RNA and DNA Analysis Predicts Hospitalizations in Cirrhosis. JCI Insight 2018, 3, e98019. [Google Scholar] [CrossRef] [PubMed]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of Ethanol-Induced Akkermansia Muciniphila Depletion Ameliorates Alcoholic Liver Disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Llopis, M.; Cassard, A.M.; Wrzosek, L.; Boschat, L.; Bruneau, A.; Ferrere, G.; Puchois, V.; Martin, J.C.; Lepage, P.; Le Roy, T.; et al. Intestinal Microbiota Contributes to Individual Susceptibility to Alcoholic Liver Disease. Gut 2016, 65, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Ciocan, D.; Voican, C.S.; Wrzosek, L.; Hugot, C.; Rainteau, D.; Humbert, L.; Cassard, A.-M.; Perlemuter, G. Bile Acid Homeostasis and Intestinal Dysbiosis in Alcoholic Hepatitis. Aliment. Pharmacol. Ther. 2018, 48, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S. Alcohol, Liver Disease and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Liangpunsakul, S.; Christensen, J.E.; Shah, V.H.; Kamath, P.S.; Gores, G.J.; Walker, S.; Comerford, M.; Katz, B.; Borst, A.; et al. The Circulating Microbiome Signature and Inferred Functional Metagenomics in Alcoholic Hepatitis. Hepatology 2018, 67, 1284–1302. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Croce, C.M. MicroRNAs in Cancer: Small Molecules with a Huge Impact. J. Clin. Oncol. 2009, 27, 5848–5856. [Google Scholar] [CrossRef]
- Blaya, D.; Coll, M.; Rodrigo-Torres, D.; Vila-Casadesús, M.; Altamirano, J.; Llopis, M.; Graupera, I.; Perea, L.; Aguilar-Bravo, B.; Díaz, A.; et al. Integrative microRNA Profiling in Alcoholic Hepatitis Reveals a Role for microRNA-182 in Liver Injury and Inflammation. Gut 2016, 65, 1535–1545. [Google Scholar] [CrossRef]
- Tang, Y.; Banan, A.; Forsyth, C.B.; Fields, J.Z.; Lau, C.K.; Zhang, L.J.; Keshavarzian, A. Effect of Alcohol on miR-212 Expression in Intestinal Epithelial Cells and Its Potential Role in Alcoholic Liver Disease. Alcohol. Clin. Exp. Res. 2008, 32, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Schnabl, B. Acute Alcohol-Associated Hepatitis: Latest Findings in Non-Invasive Biomarkers and Treatment. Liver Int. 2023; ahead of print. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, T.; Kusumanchi, P.; Tang, Q.; Sun, Z.; Radaeva, S.; Peiffer, B.; Shah, V.H.; Kamath, P.; Gores, G.J.; et al. Transcriptomic Analysis Reveals the MicroRNAs Responsible for Liver Regeneration Associated With Mortality in Alcohol-Associated Hepatitis. Hepatology 2021, 74, 2436–2451. [Google Scholar] [CrossRef] [PubMed]
- Yeligar, S.; Tsukamoto, H.; Kalra, V.K. Ethanol-Induced Expression of ET-1 and ET-BR in Liver Sinusoidal Endothelial Cells and Human Endothelial Cells Involves Hypoxia-Inducible Factor-1alpha and microrNA-199. J. Immunol. 2009, 183, 5232–5243. [Google Scholar] [CrossRef] [PubMed]
- Im, G.Y. Emerging Biomarkers in Alcohol-Associated Hepatitis. J. Clin. Exp. Hepatol. 2023, 13, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Q.; Mitchell, S.C.; Smith, R.L. Dietary Precursors of Trimethylamine in Man: A Pilot Study. Food Chem. Toxicol. 1999, 37, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Trépo, E.; Goossens, N.; Fujiwara, N.; Song, W.-M.; Colaprico, A.; Marot, A.; Spahr, L.; Demetter, P.; Sempoux, C.; Im, G.Y.; et al. Combination of Gene Expression Signature and Model for End-Stage Liver Disease Score Predicts Survival of Patients With Severe Alcoholic Hepatitis. Gastroenterology 2018, 154, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Johansson, L.E.; Johansson, L.M.; Roos, C.; Westerbacka, J.; Hamsten, A.; Bergholm, R.; Arkkila, P.; Arola, J.; Kiviluoto, T.; et al. A Common Variant in PNPLA3, Which Encodes Adiponutrin, Is Associated with Liver Fat Content in Humans. Diabetologia 2009, 52, 1056–1060. [Google Scholar] [CrossRef]
- Kollerits, B.; Coassin, S.; Kiechl, S.; Hunt, S.C.; Paulweber, B.; Willeit, J.; Brandstätter, A.; Lamina, C.; Adams, T.D.; Kronenberg, F. A Common Variant in the Adiponutrin Gene Influences Liver Enzyme Values. J. Med. Genet. 2010, 47, 116–119. [Google Scholar] [CrossRef]
- Atkinson, S.R.; Way, M.J.; McQuillin, A.; Morgan, M.Y.; Thursz, M.R. Homozygosity for Rs738409:G in PNPLA3 Is Associated with Increased Mortality Following an Episode of Severe Alcoholic Hepatitis. J. Hepatol. 2017, 67, 120–127. [Google Scholar] [CrossRef]
- Stickel, F.; Lutz, P.; Buch, S.; Nischalke, H.D.; Silva, I.; Rausch, V.; Fischer, J.; Weiss, K.H.; Gotthardt, D.; Rosendahl, J.; et al. Genetic Variation in HSD17B13 Reduces the Risk of Developing Cirrhosis and Hepatocellular Carcinoma in Alcohol Misusers. Hepatology 2020, 72, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Ghebrehiwet, B. The Complement System: An Evolution in Progress. F1000Research 2016, 5, 2840. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, V.; Gabbert, C.; Raina, A.; Li, H.; Malik, S.; DeLany, J.P.; Behari, J. Stratification of Risk of Death in Severe Acute Alcoholic Hepatitis Using a Panel of Adipokines and Cytokines. Alcohol. Clin. Exp. Res. 2014, 38, 2712–2721. [Google Scholar] [CrossRef] [PubMed]
- Affò, S.; Morales-Ibanez, O.; Rodrigo-Torres, D.; Altamirano, J.; Blaya, D.; Dapito, D.H.; Millán, C.; Coll, M.; Caviglia, J.M.; Arroyo, V.; et al. CCL20 Mediates Lipopolysaccharide Induced Liver Injury and Is a Potential Driver of Inflammation and Fibrosis in Alcoholic Hepatitis. Gut 2014, 63, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Vergis, N.; Atkinson, S.R.; Knapp, S.; Maurice, J.; Allison, M.; Austin, A.; Forrest, E.H.; Masson, S.; McCune, A.; Patch, D.; et al. In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated with High Circulating Levels of Bacterial DNA. Gastroenterology 2017, 152, 1068–1077.e4. [Google Scholar] [CrossRef] [PubMed]
- Sukriti, S.; Maras, J.S.; Bihari, C.; Das, S.; Vyas, A.K.; Sharma, S.; Hussain, S.; Shasthry, S.; Choudhary, A.; Premkumar, M.; et al. Microvesicles in Hepatic and Peripheral Vein Can Predict Nonresponse to Corticosteroid Therapy in Severe Alcoholic Hepatitis. Aliment. Pharmacol. Ther. 2018, 47, 1151–1161. [Google Scholar] [CrossRef]
- Bihari, C.; Anand, L.; Rooge, S.; Kumar, D.; Saxena, P.; Shubham, S.; Sukriti; Trehanpati, N.; Kumar, G.; Pamecha, V.; et al. Bone Marrow Stem Cells and Their Niche Components Are Adversely Affected in Advanced Cirrhosis of the Liver. Hepatology 2016, 64, 1273–1288. [Google Scholar] [CrossRef] [PubMed]
- Michelena, J.; Altamirano, J.; Abraldes, J.G.; Affò, S.; Morales-Ibanez, O.; Sancho-Bru, P.; Dominguez, M.; García-Pagán, J.C.; Fernández, J.; Arroyo, V.; et al. Systemic Inflammatory Response and Serum Lipopolysaccharide Levels Predict Multiple Organ Failure and Death in Alcoholic Hepatitis. Hepatology 2015, 62, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Frenette, C.T.; Morelli, G.; Shiffman, M.L.; Frederick, R.T.; Rubin, R.A.; Fallon, M.B.; Cheng, J.T.; Cave, M.; Khaderi, S.A.; Massoud, O.; et al. Emricasan Improves Liver Function in Patients with Cirrhosis and High Model for End-Stage Liver Disease Scores Compared with Placebo. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 774–783.e4. [Google Scholar] [CrossRef]
- Philips, C.A.; Pande, A.; Shasthry, S.M.; Jamwal, K.D.; Khillan, V.; Chandel, S.S.; Kumar, G.; Sharma, M.K.; Maiwall, R.; Jindal, A.; et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin. Gastroenterol. Hepatol. 2017, 15, 600–602. [Google Scholar] [CrossRef]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Brusnic, O.; Onișor, D.M. Fecal Microbiota Transplantation in Liver Cirrhosis. Biomedicines 2023, 11, 2930. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.A.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, D.; Wang, J.; Gurry, T.; et al. Fecal Microbiota Transplant from a Rational Stool Donor Improves Hepatic Encephalopathy: A Randomized Clinical Trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.; Enki, D.G.; Thursz, M.; Cramp, M.E.; Dhanda, A.D. Systematic Review with Meta-Analysis: High Mortality in Patients with Non-Severe Alcoholic Hepatitis. Aliment. Pharmacol. Ther. 2019, 50, 249–257. [Google Scholar] [CrossRef] [PubMed]
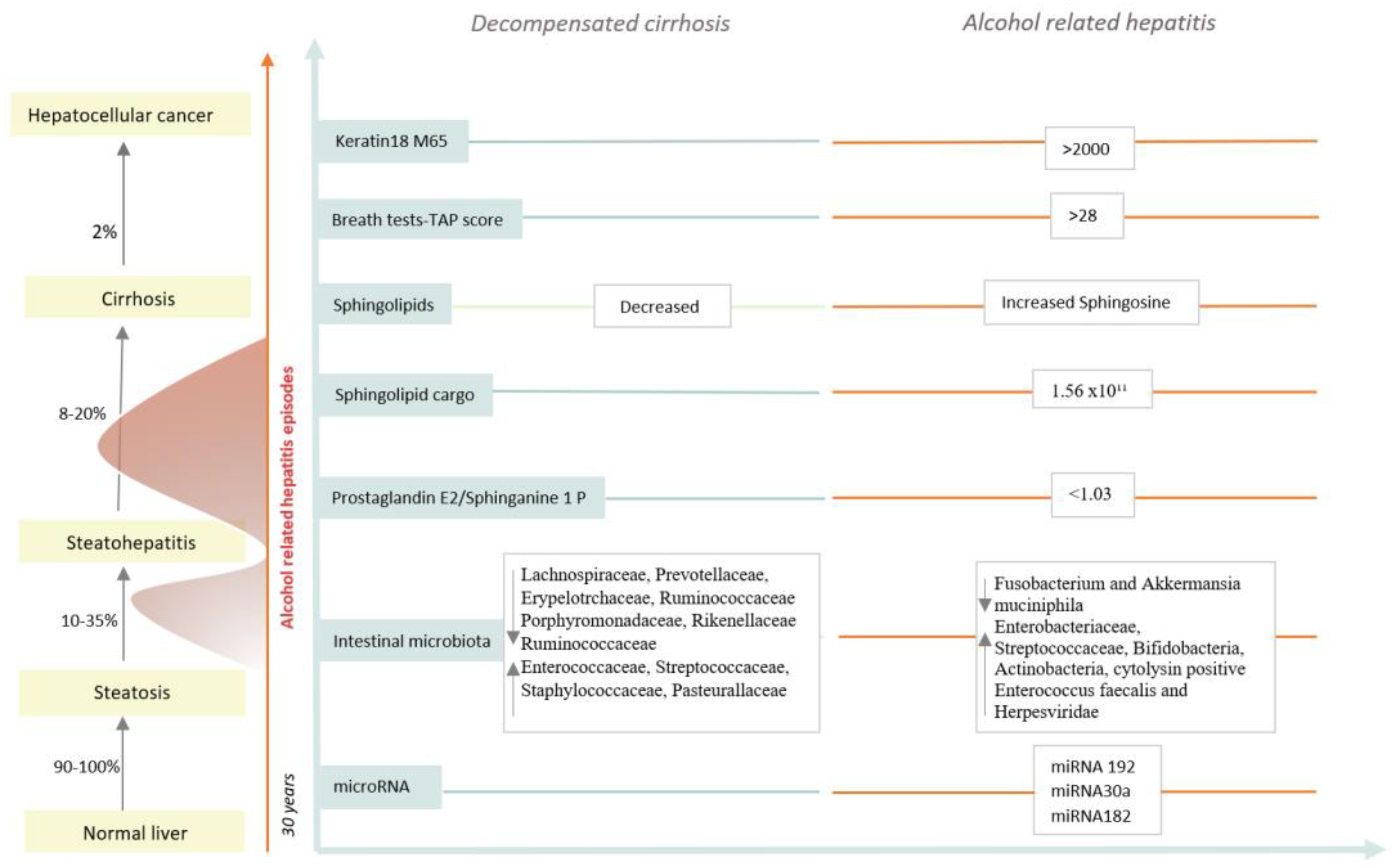
| Classification | Biomarkers | Diagnostic Ability | References |
|---|---|---|---|
| Cytokeratin 18 components | Cytokeratin 18 M65 | M65 > 2000 IU/L sensibility = 67%, specificity = 92% M65 < 641 IU/L sensibility = 93%, specificity = 62% | Atkinson et al. [40] |
| Sphingolipids | Ev sphingolipid cargo Sphingolipids Prostaglandin E2/Sphinganine 1 P | >1.56 × 1011 particles/mL, discriminate AH from DC sensibility = 0.92%, specificity = 0.94% high levels of sphingosine PGE2/S1P < 1.03 discriminate AH from DC, AUC = 0.96 | Sehrawat et al. [42] Rachkonda et al. [43] Horhat et al. [44] |
| microRNAs | miRNA 192 | AUC = 0.96 for distinguishing AH from controls | Momen Heravi et al. [45] |
| miRNA 30a | AUC = 0.85 for distinguishing AH from controls | ||
| Genetics | PNPLA3 | Homozygosity for Rs 738409:G—risk factor for AH occurrences | Salameh et al. [46] |
| Microbiota | Cytolysin positivity Enterococcus faecalis Mammalian viruses | Cytolysin-positive Enterococcus faecalis, Herpesviridae and Anti serum Saccharomyces cerevisiae antibodies are associated with mortality | Duan et al. [47] Jiang et al. [48] Lang et al. [49] |
| Extracellular matrix | Laminin | 90% sensibility and 77% specificity for the diagnosis of AH, using a cut-off of 4.1 UI/mL | Forrest et al. [50] |
| Collagen type IV | 89% sensibility and 77% specificity for the diagnosis of AH, using a cut-off of >150 ng/mL | ||
| Others | TAP score Serum collectin 11 | sensibility = 90%, specificity = 80% discriminate AH from AC discriminate between sAH and AC; AUC = 0.77 | Hanouneh et al. [51] Taiwo et al. [52] |
| Comparation | Alterd Phyla | Taxa Enriched | Taxa Depleted | References |
|---|---|---|---|---|
| Alcohol consumption | ↑ Proteobacteria ↓ Bacteroidetes | Tilg et al. [60] | ||
| Compensated and decompensated cirrhosis | Enterococcaceae | Lachnospiraceae | Bajaj et al. [63] | |
| Peptostreptococcaceae | Ruminococcaceae | |||
| Streptococcaceae | Erysipelotrchaceae | |||
| Staphylococcaceae | Prevotellaceae | |||
| Porphyromonadaceae | ||||
| Rikenellaceae | ||||
| Alcohol-associated hepatitis | Enteriobacteriaceae Streptococcaceae Bifidobacteria Actinobacteria | Akkermansia muciniphilia | Grander et al. [64] Llopis et al. [65] Ciocan et al. [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignat, M.; Stefanescu, H. Non-Invasive Biomarkers for Differentiating Alcohol Associated Hepatitis from Acute Decompensation in Patients with ALD. J. Clin. Med. 2024, 13, 3747. https://doi.org/10.3390/jcm13133747
Ignat M, Stefanescu H. Non-Invasive Biomarkers for Differentiating Alcohol Associated Hepatitis from Acute Decompensation in Patients with ALD. Journal of Clinical Medicine. 2024; 13(13):3747. https://doi.org/10.3390/jcm13133747
Chicago/Turabian StyleIgnat, Mina, and Horia Stefanescu. 2024. "Non-Invasive Biomarkers for Differentiating Alcohol Associated Hepatitis from Acute Decompensation in Patients with ALD" Journal of Clinical Medicine 13, no. 13: 3747. https://doi.org/10.3390/jcm13133747
APA StyleIgnat, M., & Stefanescu, H. (2024). Non-Invasive Biomarkers for Differentiating Alcohol Associated Hepatitis from Acute Decompensation in Patients with ALD. Journal of Clinical Medicine, 13(13), 3747. https://doi.org/10.3390/jcm13133747








