Comparative Uptake Patterns of Radioactive Iodine and [18F]-Fluorodeoxyglucose (FDG) in Metastatic Differentiated Thyroid Cancers
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort Selection
2.2. Histology and Molecular Data Classification
3. Image Review
3.1. Statistical Analyses
3.2. Agreement Analyses
4. Results
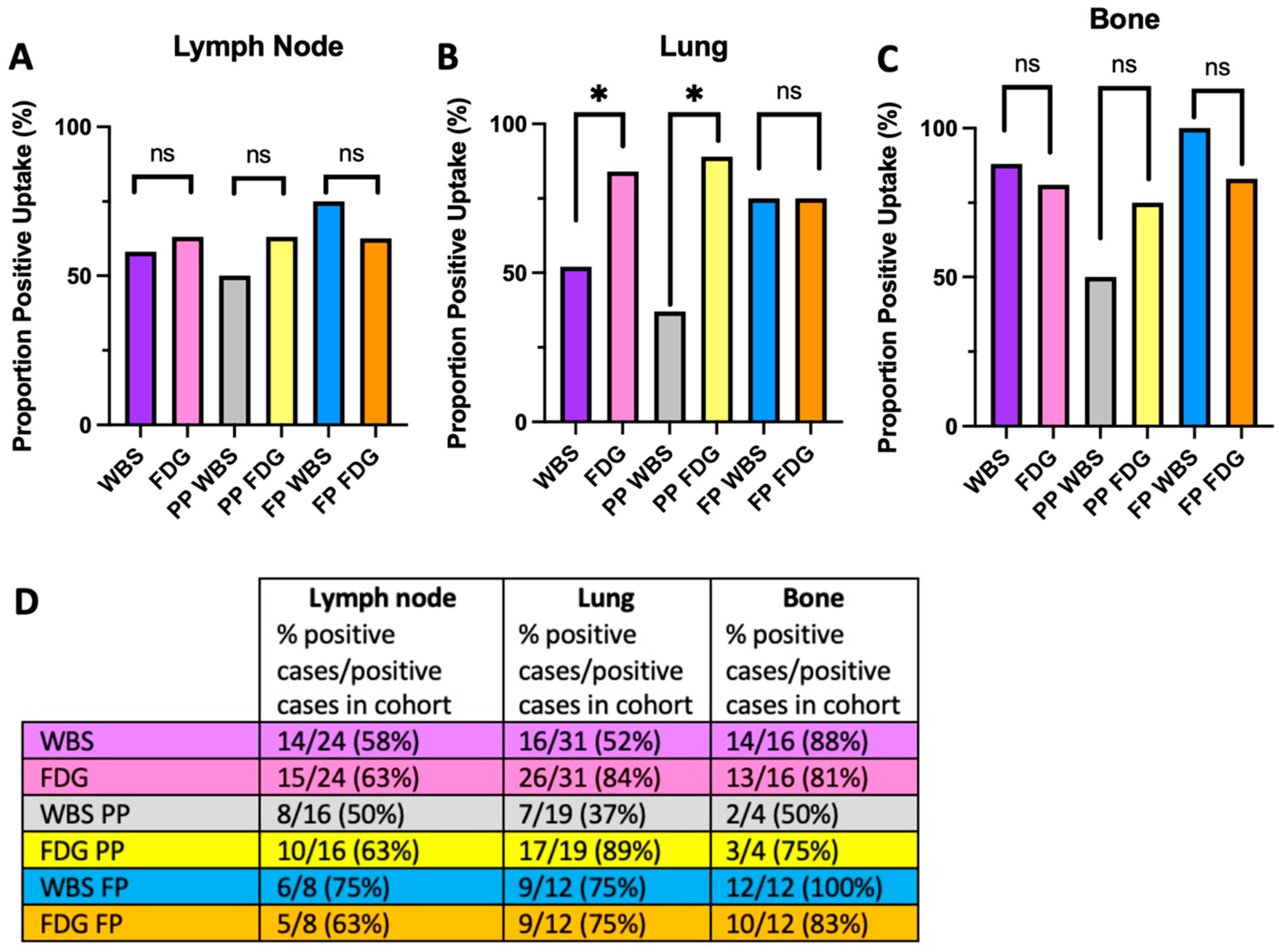
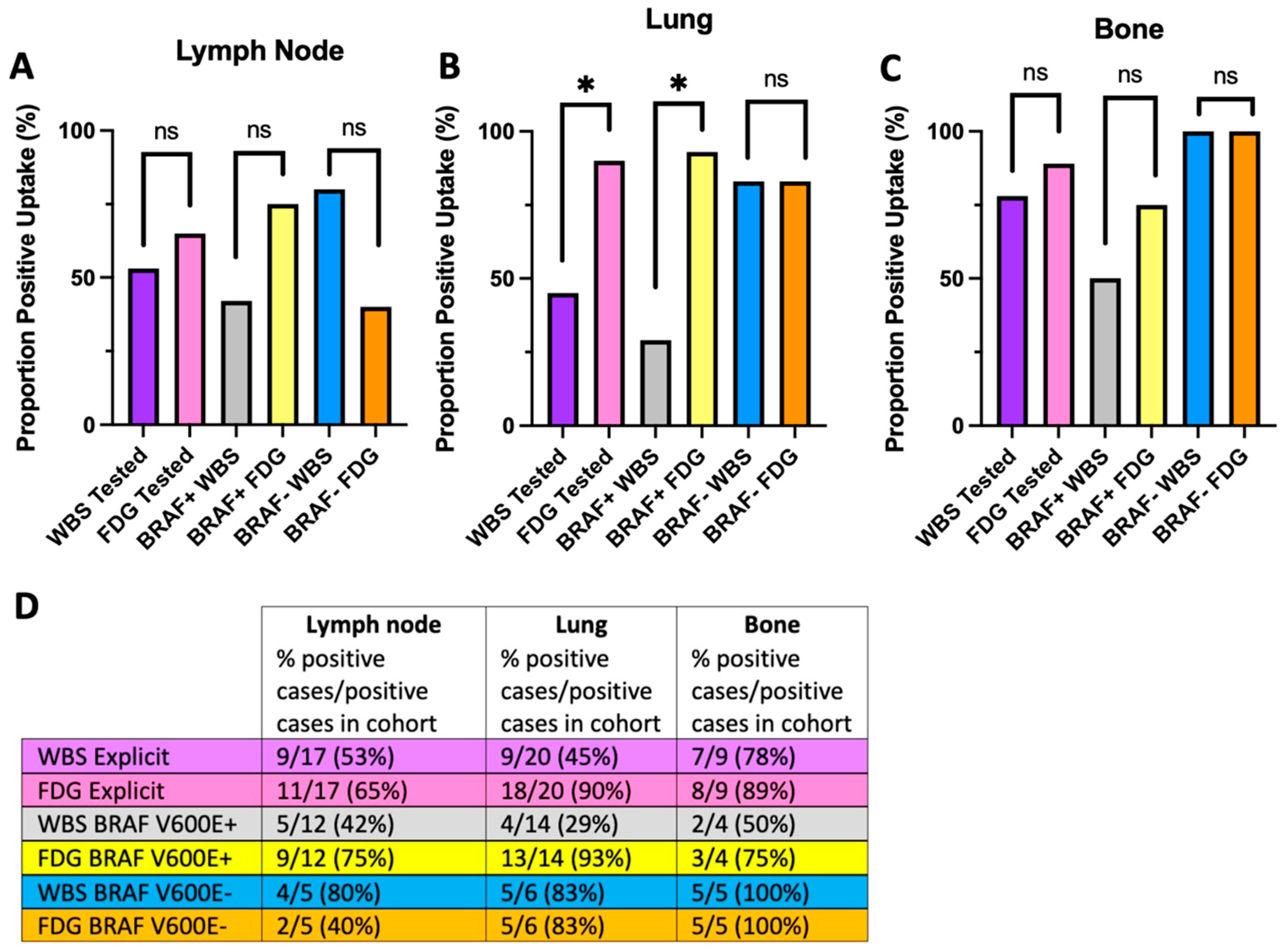
4.1. The Flip-Flop Phenomenon Varies by Metastatic Site and Molecular Status
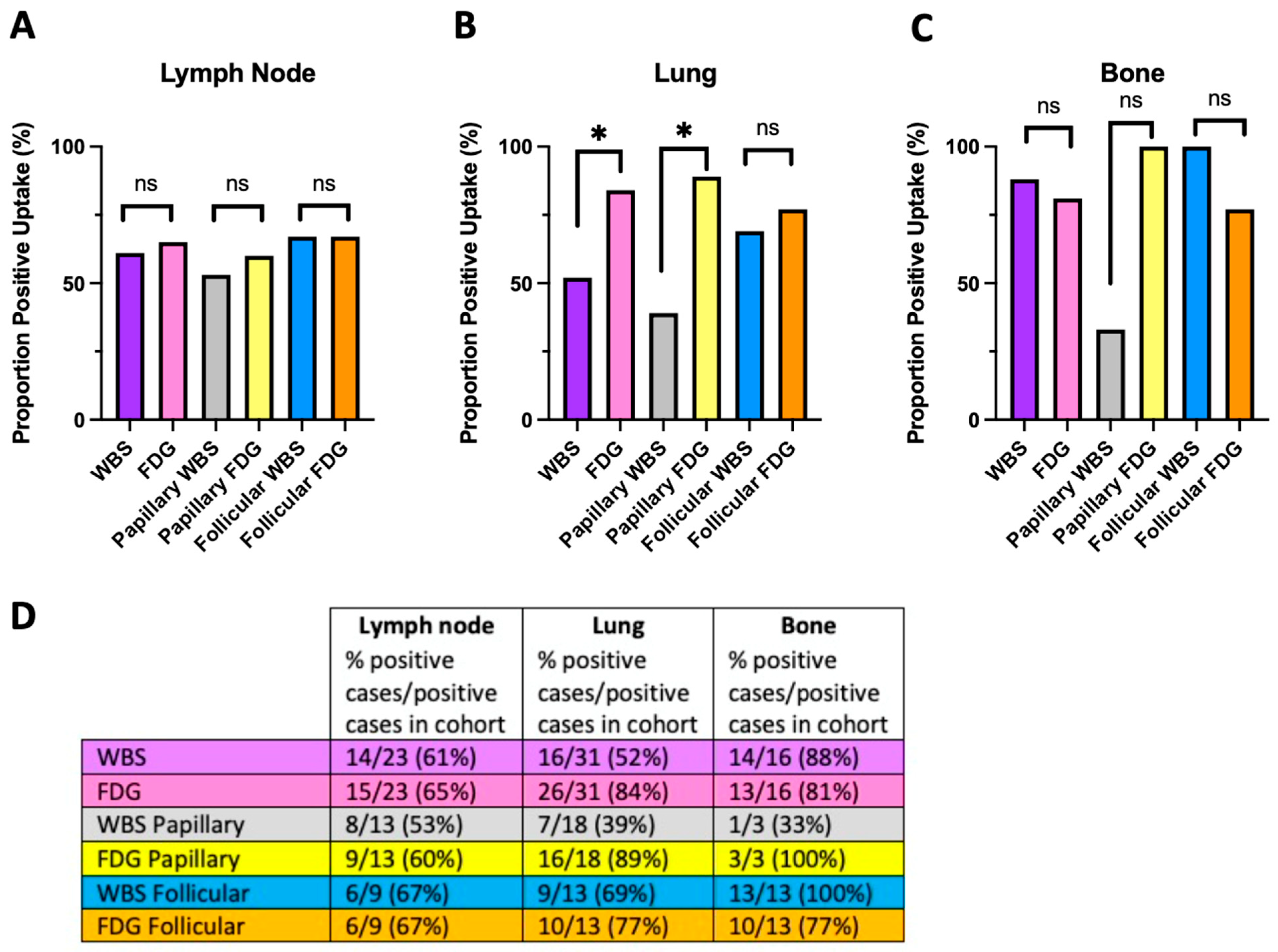
4.2. The Flip-Flop Phenomenon and Histologic Classification
4.3. Agreement between Imaging Modalities
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BVL | BRAF V600E-Like |
| DTC | Differentiated thyroid cancer |
| FDG | 18Fluorodeoxyglucose |
| FP | Follicular pattern |
| MAPK | Mitogen-activated protein kinases |
| PET | Positron emission technology |
| PP | Papillary pattern |
| RAI | Radioactive iodine |
| RL | RAS-like |
| TERT | Telomerase reverse transcriptase |
| WBS | Whole body scan |
References
- Fagin, J.A.; Wells, S.A., Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simoes, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr Pathol 2022, 33, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research, N. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef]
- Suh, S.; Goh, T.S.; Kim, Y.H.; Oh, S.O.; Pak, K.; Seok, J.W.; Kim, I.J. Development and Validation of a Risk Scoring System Derived from Meta-Analyses of Papillary Thyroid Cancer. Endocrinol. Metab. 2020, 35, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.W.; Choi, H.S.; Yeom, G.J.; Lim, J.A.; Moon, J.H.; Park, D.J.; Chung, J.K.; Cho, B.Y.; Yi, K.H.; Park, Y.J. Long-term prognosis of differentiated thyroid cancer with lung metastasis in Korea and its prognostic factors. Thyroid 2014, 24, 277–286. [Google Scholar] [CrossRef]
- Wang, H.; Dai, H.; Li, Q.; Shen, G.; Shi, L.; Tian, R. Investigating (18)F-FDG PET/CT Parameters as Prognostic Markers for Differentiated Thyroid Cancer: A Systematic Review. Front. Oncol. 2021, 11, 648658. [Google Scholar] [CrossRef]
- Goffredo, P.; Sosa, J.A.; Roman, S.A. Differentiated thyroid cancer presenting with distant metastases: A population analysis over two decades. World J. Surg. 2013, 37, 1599–1605. [Google Scholar] [CrossRef]
- Robbins, R.J.; Wan, Q.; Grewal, R.K.; Reibke, R.; Gonen, M.; Strauss, H.W.; Tuttle, R.M.; Drucker, W.; Larson, S.M. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J. Clin. Endocrinol. Metab. 2006, 91, 498–505. [Google Scholar] [CrossRef]
- Deandreis, D.; Al Ghuzlan, A.; Leboulleux, S.; Lacroix, L.; Garsi, J.P.; Talbot, M.; Lumbroso, J.; Baudin, E.; Caillou, B.; Bidart, J.M.; et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr. Relat. Cancer 2011, 18, 159–169. [Google Scholar] [CrossRef]
- Gaertner, F.C.; Okamoto, S.; Shiga, T.; Ito, Y.; Uchiyama, Y.; Manabe, O.; Hattori, N.; Tamaki, N. FDG PET Performed at Thyroid Remnant Ablation Has a Higher Predictive Value for a Long-Term Survival of High-Risk Patients with Well-Differentiated Thyroid Cancer Than Radioiodine Uptake. Clin. Nucl. Med. 2015, 40, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, B.; Klain, M.; Nicolai, E.; D’Amico, D.; De Matteis, G.; Raddi, M.; Fonti, R.; Pellegrino, T.; Storto, G.; Cuocolo, A.; et al. Prognostic role of FDG PET/CT in patients with differentiated thyroid cancer treated with 131-iodine empiric therapy. Medicine 2017, 96, e8344. [Google Scholar] [CrossRef] [PubMed]
- Feine, U.; Lietzenmayer, R.; Hanke, J.-P.; Held, J.; Wohrle, H.; Muller-Schauenburg, W. Fluorine-18-FDG and Iodine-131-Iodide Uptake in Thyroid Cancer. J. Nucl. Med. 1996, 37, 1468–1472. [Google Scholar]
- Kang, S.Y.; Bang, J.I.; Kang, K.W.; Lee, H.Y.; Chung, J.K. FDG PET/CT for the early prediction of RAI therapy response in patients with metastatic differentiated thyroid carcinoma. PLoS ONE 2019, 14, e0218416. [Google Scholar] [CrossRef] [PubMed]
- Flavell, R.; Naeger, D.; Aparici, C.; Hawkins, R.; Pampaloni, M.; Behr, S. Malignancies with Low Fluoro-deoxyglucose Uptake at PET/CT: Pitfalls and Prognostic Importance. Radiographics 2016, 36, 293–294. [Google Scholar] [CrossRef]
- Bahri, H.; Laurence, L.; Edeline, J.; Leghzali, H.; Devillers, A.; Raoul, J.L.; Cuggia, M.; Mesbah, H.; Clement, B.; Boucher, E.; et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: A long-term evaluation. J. Nucl. Med. 2014, 55, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Larson, S.M.; Tuttle, R.M.; Kalaigian, H.; Kolbert, K.; Sonenberg, M.; Robbins, R.J. Resistance of [18F]-Fluorodeoxyglucose-Avid Metastatic Thyroid Cancer Lesions to Treatment with High-Dose Radioactive Iodine. Thyroid 2001, 11, 1169–1175. [Google Scholar] [CrossRef]
- Rosario, P.W.; Mourao, G.F.; dos Santos, J.B.; Calsolari, M.R. Is empirical radioactive iodine therapy still a valid approach to patients with thyroid cancer and elevated thyroglobulin? Thyroid 2014, 24, 533–536. [Google Scholar] [CrossRef]
- Blaser, D.; Maschauer, S.; Kuwert, T.; Prante, O. In Vitro Studies on the Signal Transduction of Thyroidal Uptake of 18F-FDG and 131I-Iodide. J. Nucl. Med. 2006, 47, 1382–1388. [Google Scholar]
- Giordano, T.J. Follicular cell thyroid neoplasia: Insights from genomics and The Cancer Genome Atlas research network. Curr. Opin. Oncol. 2016, 28, 1–4. [Google Scholar] [CrossRef]
- Al-Qurayshi, Z.; Sullivan, C.B.; Pagedar, N.; Lee, G.S.; Tufano, R.; Kandil, E. Prevalence and Risk of Metastatic Thyroid Cancers and Management Outcomes: A National Perspective. Laryngoscope 2021, 131, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.; Paone, G.; Ceriani, L.; Pusztaszeri, M. Cellular and molecular basis for thyroid cancer imaging in nuclear medicine. Clin. Transl. Imaging 2013, 1, 149–161. [Google Scholar] [CrossRef]
- Liu, J.; Liu, R.; Shen, X.; Zhu, G.; Li, B.; Mingzhao, Z. The Genetic Duet of BRAF V600E and TERT Promoter Mutations Robustly Predicts Loss of Radioiodine Avidity in Recurrent Papillary Thyroid Cancer. J. Nucl. Med. 2020, 61, 177–182. [Google Scholar] [CrossRef]
- Soe, M.H.; Chiang, J.M.; Flavell, R.R.; Khanafshar, E.; Mendoza, L.; Kang, H.; Liu, C. Non-Iodine-Avid Disease Is Highly Prevalent in Distant Metastatic Differentiated Thyroid Cancer With Papillary Histology. J. Clin. Endocrinol. Metab. 2022, 107, e3206–e3216. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Li, X.; Liang, Z.; Gao, W.; Liang, J.; Cheng, S.; Lin, Y. TERT Promoter Mutation Predicts Radioiodine-Refractory Character in Distant Metastatic Differentiated Thyroid Cancer. J. Nucl. Med. 2017, 58, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Puxeddu, E.; Ferretti, E.; Morisi, R.; Moretti, S.; Bruno, R.; Barbi, F.; Avenia, N.; Scipioni, A.; Verrienti, A.; et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 2840–2843. [Google Scholar] [CrossRef]
- Kebebew, E.; Weng, J.; Bauer, J.; Ranvier, G.; Clark, O.H.; Duh, Q.Y.; Shibru, D.; Bastian, B.; Griffin, A. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann. Surg. 2007, 246, 466–470. [Google Scholar] [CrossRef]
- Boucai, L.; Saqcena, M.; Kuo, F.; Grewal, R.K.; Socci, N.; Knauf, J.A.; Krishnamoorthy, G.P.; Ryder, M.; Ho, A.L.; Ghossein, R.A.; et al. Genomic and Transcriptomic Characteristics of Metastatic Thyroid Cancers with Exceptional Responses to Radioactive Iodine Therapy. Clin. Cancer Res. 2023, 29, 1620–1630. [Google Scholar] [CrossRef]
- Chakravarty, D.; Santos, E.; Ryder, M.; Knauf, J.A.; Liao, X.H.; West, B.L.; Bollag, G.; Kolesnick, R.; Thin, T.H.; Rosen, N.; et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J. Clin. Investig. 2011, 121, 4700–4711. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Grewal, R.K.; Leboeuf, R.; Sherman, E.J.; Pfister, D.G.; Deandreis, D.; Pentlow, K.S.; Zanzonico, P.B.; Haque, S.; Gavane, S.; et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N. Engl. J. Med. 2013, 368, 623–632. [Google Scholar] [CrossRef]
- Rothenberg, S.M.; McFadden, D.G.; Palmer, E.L.; Daniels, G.H.; Wirth, L.J. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin. Cancer Res. 2015, 21, 1028–1035. [Google Scholar] [CrossRef]
- Jaber, T.; Waguespack, S.G.; Cabanillas, M.E.; Elbanan, M.; Vu, T.; Dadu, R.; Sherman, S.I.; Amit, M.; Santos, E.B.; Zafereo, M.; et al. Targeted Therapy in Advanced Thyroid Cancer to Resensitize Tumors to Radioactive Iodine. J. Clin. Endocrinol. Metab. 2018, 103, 3698–3705. [Google Scholar] [CrossRef]
- Iravani, A.; Solomon, B.; Pattison, D.A.; Jackson, P.; Ravi Kumar, A.; Kong, G.; Hofman, M.S.; Akhurst, T.; Hicks, R.J. Mitogen-Activated Protein Kinase Pathway Inhibition for Redifferentiation of Radioiodine Refractory Differentiated Thyroid Cancer: An Evolving Protocol. Thyroid 2019, 29, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.A.; Sherman, E.J.; Baxi, S.S.; Tchekmedyian, V.; Grewal, R.K.; Larson, S.M.; Pentlow, K.S.; Haque, S.; Tuttle, R.M.; Sabra, M.M.; et al. Vemurafenib Redifferentiation of BRAF Mutant, RAI-Refractory Thyroid Cancers. J. Clin. Endocrinol. Metab. 2019, 104, 1417–1428. [Google Scholar] [CrossRef]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef]
- Ganly, I.; Ricarte Filho, J.; Eng, S.; Ghossein, R.; Morris, L.G.; Liang, Y.; Socci, N.; Kannan, K.; Mo, Q.; Fagin, J.A.; et al. Genomic dissection of Hurthle cell carcinoma reveals a unique class of thyroid malignancy. J. Clin. Endocrinol. Metab. 2013, 98, E962–E972. [Google Scholar] [CrossRef]
- Ha, L.N.; Iravani, A.; Nhung, N.T.; Hanh, N.T.M.; Hutomo, F.; Son, M.H. Relationship between clinicopathologic factors and FDG avidity in radioiodine-negative recurrent or metastatic differentiated thyroid carcinoma. Cancer Imaging 2021, 21, 8. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, S.; Yuan, X.; Wang, H.; Ma, C. Progression free survival related to 18F-FDG PET/CT uptake and 131I uptake in lung metastases of differentiated thyroid cancer. Hell. J. Nucl. Med. 2019, 22, 123–130. [Google Scholar]
- Wang, D.; Bai, Y.; Huo, Y.; Ma, C. FDG PET Predicts the Effects of (131)I and Prognosis for Patients with Bone Metastases from Differentiated Thyroid Carcinoma. Cancer Manag. Res. 2020, 12, 13223–13232. [Google Scholar] [CrossRef]
- Leboulleux, S.; Dupuy, C.; Lacroix, L.; Attard, M.; Grimaldi, S.; Corre, R.; Ricard, M.; Nasr, S.; Berdelou, A.; Hadoux, J.; et al. Redifferentiation of a BRAF(K601E)-Mutated Poorly Differentiated Thyroid Cancer Patient with Dabrafenib and Trametinib Treatment. Thyroid 2019, 29, 735–742. [Google Scholar] [CrossRef]



| Characteristics | Value |
|---|---|
| Number of patients (N) | 46 |
| Female | 24 (52%) |
| Male | 22 (48%) |
| Age at imaging [median (interquartile range)] | 63 (55–73%) |
| Prior RAI (% of cohort) | 12 (26%) |
| Primary Histology (% of cohort) | |
| Papillary (Tall, Classical, Sclerosing, Unknown Subtypes) | 24 (52%) |
| Follicular (Follicular, Papillary-Follicular, Mixed Subtypes) | 22 (48%) |
| Histology by Subtype (% of cohort) | |
| Papillary-Follicular (PTC-FV) | 9 (20%) |
| Papillary-Tall | 4 (9%) |
| Papillary Classical | 13 (28%) |
| Papillary-Mixed | 3 (7%) |
| Papillary-Sclerosing | 1 (2%) |
| Papillary-Unknown | 6 (13%) |
| Follicular (FTC) | 10 (22%) |
| Integrated Classification (% of cohort) | |
| Papillary Pattern | 27 (59%) |
| Follicular Pattern | 19 (41%) |
| Tumor Molecular Characteristics | |
| BRAF V600E+ | 20 (44%) |
| BRAF V600E− | 9 (20%) |
| BRAF V600E Untested | 17 (37%) |
| RAS Mutation+ | 5 (11%) |
| RAS Mutation– | 9 (20%) |
| RAS Untested | 32 (70%) |
| TERT Mutation+ | 10 (22%) |
| TERT Mutation– | 4 (9%) |
| TERT Untested | 32 (70%) |
| Metastatic Uptake (on either WBS or FDG) | |
| Lymph node (% of cohort) | 24 (52%) |
| Lung (% of cohort) | 31 (67%) |
| Bone (% of cohort) | 16 (35%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diwanji, D.; Carrodeguas, E.; Seo, Y.; Kang, H.; Soe, M.H.; Chiang, J.M.; Zhang, L.; Liu, C.; Behr, S.C.; Flavell, R.R. Comparative Uptake Patterns of Radioactive Iodine and [18F]-Fluorodeoxyglucose (FDG) in Metastatic Differentiated Thyroid Cancers. J. Clin. Med. 2024, 13, 3963. https://doi.org/10.3390/jcm13133963
Diwanji D, Carrodeguas E, Seo Y, Kang H, Soe MH, Chiang JM, Zhang L, Liu C, Behr SC, Flavell RR. Comparative Uptake Patterns of Radioactive Iodine and [18F]-Fluorodeoxyglucose (FDG) in Metastatic Differentiated Thyroid Cancers. Journal of Clinical Medicine. 2024; 13(13):3963. https://doi.org/10.3390/jcm13133963
Chicago/Turabian StyleDiwanji, Devan, Emmanuel Carrodeguas, Youngho Seo, Hyunseok Kang, Myat Han Soe, Janet M. Chiang, Li Zhang, Chienying Liu, Spencer C. Behr, and Robert R. Flavell. 2024. "Comparative Uptake Patterns of Radioactive Iodine and [18F]-Fluorodeoxyglucose (FDG) in Metastatic Differentiated Thyroid Cancers" Journal of Clinical Medicine 13, no. 13: 3963. https://doi.org/10.3390/jcm13133963
APA StyleDiwanji, D., Carrodeguas, E., Seo, Y., Kang, H., Soe, M. H., Chiang, J. M., Zhang, L., Liu, C., Behr, S. C., & Flavell, R. R. (2024). Comparative Uptake Patterns of Radioactive Iodine and [18F]-Fluorodeoxyglucose (FDG) in Metastatic Differentiated Thyroid Cancers. Journal of Clinical Medicine, 13(13), 3963. https://doi.org/10.3390/jcm13133963






