The Era of DAAs: Assessing the Patients’ Characteristics, Clinical Impact, and Emergence of Comorbidities in HIV/HCV-Coinfected versus HIV-Infected Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measures
- Demographics and lifestyle habits—Data on participants’ age, gender, ethnicity, and sexual orientation were collected. Similarly, data on patients’ lifestyle habits, including smoking, alcohol consumption, and drug use, were obtained from their clinical history. Information regarding the purposes of parenteral drug use (nonconsumer, conventional use, or ChemSex) and the specifics of drug consumption were also gathered.
- HIV infection—HIV infection was characterized by the date of diagnosis, route of transmission, HIV clinical stage, CD4+ cell count at ART initiation (cells/µL), CD4+/CD8+ ratio at ART initiation, baseline HIV-1 VL (copies/mL), and history of AIDS. The presence of AIDS-related illnesses (tuberculosis, recurrent pneumonia, Pneumocystis pneumonia, esophageal candidiasis, Kaposi’s sarcoma, cryptococcosis, cerebral toxoplasmosis, non-Hodgkin lymphoma and progressive multifocal leuco-encephalopathy) was also recorded.
- HIV treatment—The date when the patient started ART, the patient’s time spent on ART, and the time elapsed from HIV diagnosis to the initiation of ART were recorded. Given that previous treatment with rilpivirine-based regimens is associated with clinical benefit for improving liver stiffness (LS) [37], data were also gathered on whether the patient had ever been exposed to rilpivirine or not.
- HVC infection—HCV infection was characterized by the route of transmission (sexual, parenteral), date of diagnosis, stage of hepatitis C at diagnosis (acute or chronic), HCV genotype, HCV VL at DAA initiation (IU/mL) and range (<800,000 IU/mL, ≥800,000 IU/mL), antiviral regimen used (DAAs), time from diagnosis to treatment, and achievement of SVR at 12 weeks (SVR12). Additionally, the number of subsequent HCV reinfections was recorded.
- Biochemistry and LS assessment—The patients’ biochemical data included liver chemistry (aspartate aminotransferase [AST], alanine aminotransferase [ALT], gamma glutamyl transpeptidase [GGT]), renal function (glomerular filtration rate [GFR]), lipid profile (triglycerides, low-density lipoprotein [LDL], high-density lipoprotein [HDL] and total cholesterol levels), and complete blood count data, as well as HIV status (quantitative HIV RNA data, and CD4+ and CD8+ T-cell counts). The APRI score and fibrosis stage (no fibrosis: APRI < 0.5; moderate fibrosis: APRI 0.5–1.5; cirrhosis: APRI > 1.5) and the FIB-4 score and fibrosis stage (no fibrosis: FIB-4 < 1.45; moderate fibrosis: FIB-4 1.45–3.25; cirrhosis: FIB-4 > 3.25) were calculated as surrogate markers of liver disease. Biochemistry test results and fibrosis measurements (APRI and FIB-4) were collected for the HIV/HCV cohort before starting DAA therapy and after SVR12. Additionally, in HIV/HCV-coinfected patients, LS was assessed using transient elastography (FibroScan), the gold standard technique, before DAA therapy. Fibrosis classification was determined using the METAVIR scale, where the LS cutoff values were LS < 7.1 KPa for stage F0–F1, LS between 7.1 and 9.4 KPa for stage F2, LS between 9.5 and 12.4 KPa for stage F3, and LS ≥ 12.5 KPa for stage F4 of cirrhosis. Since FibroScan is not used as a routine test in HIV-monoinfected patients, alternative biochemical markers (APRI and FIB-4) were used to compare the groups [38].
- Patients’ baseline comorbidities—The data on HIV infection risk factors included the number and type of previous comorbidities at the time of diagnosis, such as hypertension, diabetes mellitus (DM), dyslipidemia, cardiovascular disease (CVD), kidney disease, liver disease, and non-AIDS cancer. Additionally, the number of documented sexually transmitted infections (STIs), serological data on hepatitis A and B, and obesity (determined by a body mass index [BMI] over 30 Kg/m2) were recorded.
2.3. Outcomes
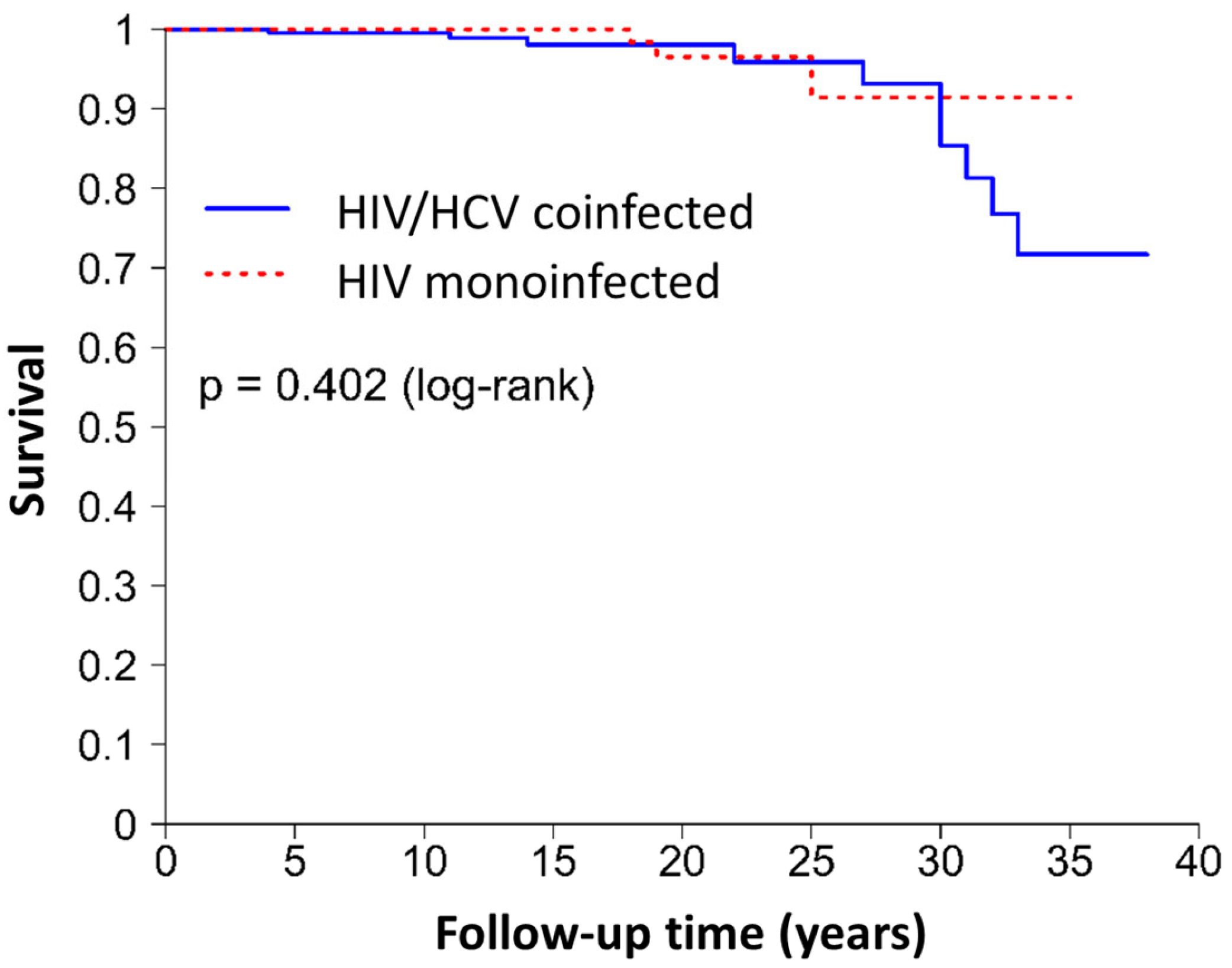
- Development of comorbidities—The clinical events registered in the medical history and gathered for this study were hypertension, DM, dyslipidemia, CVD, kidney disease, liver disease, non-AIDS cancer, and death. The number of clinical events that occurred during the follow-up and the overall follow-up time were also recorded. In HIV/HCV-coinfected patients, the comorbidities recorded were developed from HCV diagnosis to the present. In HIV-monoinfected patients, the comorbidities presented at baseline were not considered.
2.4. Statistical Analyses
3. Results
4. Discussion
4.1. Patients’ Clinical Characteristics and Comorbidities at Baseline
4.2. Clinical Impact and Emergence of Comorbidities Onset
4.3. Limitations
4.4. Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). HIV and AIDS; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- de Coninck, Z.; Hussain-Alkhateeb, L.; Bratt, G.; Ekström, A.M.; Gisslén, M.; Petzold, M.; Svedhem, V. Non-AIDS Mortality Is Higher among Successfully Treated People Living with HIV Compared with Matched HIV-Negative Control Persons: A 15-Year Follow-Up Cohort Study in Sweden. AIDS Patient Care STDs 2018, 32, 297–305. [Google Scholar] [CrossRef]
- Marcus, J.L.; Leyden, W.A.; Alexeeff, S.E.; Anderson, A.N.; Hechter, R.C.; Hu, H.; Lam, J.O.; Towner, W.J.; Yuan, Q.; Horberg, M.A.; et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults with and without HIV Infection, 2000–2016. JAMA Netw. Open 2020, 3, e207954. [Google Scholar] [CrossRef] [PubMed]
- Smit, C.; Boyd, A.; Rijnders, B.J.A.; van de Laar, T.J.W.; Leyten, E.M.; Bierman, W.F.; Brinkman, K.; Claassen, M.A.A.; den Hollander, J.; Boerekamps, A.; et al. HCV Micro-Elimination in Individuals with HIV in the Netherlands 4 Years after Universal Access to Direct-Acting Antivirals: A Retrospective Cohort Study. Lancet HIV 2021, 8, e96–e105. [Google Scholar] [CrossRef] [PubMed]
- Schouten, J.; Wit, F.W.; Stolte, I.G.; Kootstra, N.A.; van der Valk, M.; Geerlings, S.E.; Prins, M.; Reiss, P. AGEhIV Cohort Study Group Cross-Sectional Comparison of the Prevalence of Age-Associated Comorbidities and Their Risk Factors between HIV-Infected and Uninfected Individuals: The AGEhIV Cohort Study. Clin. Infect. Dis. 2014, 59, 1787–1797. [Google Scholar] [CrossRef]
- Sellier, P.; Hamet, G.; Brun, A.; Ponscarme, D.; De Castro, N.; Alexandre, G.; Rozenbaum, W.; Molina, J.-M.; Abgrall, S.; COREVIH Ile-de-France-Est research group. Mortality of People Living with HIV in Paris Area from 2011 to 2015. AIDS Res. Hum. Retroviruses 2020, 36, 373–380. [Google Scholar] [CrossRef]
- Borkowska, T.; Chkhartishvili, N.; Karkashadze, E.; Chokoshvili, O.; Gabunia, P.; Sharvadze, L.; Tsertsvadze, T. The Prevalence of Hyperglycemia and Its Impact on Mortality among People Living with HIV in Georgia. PLoS ONE 2022, 17, e0276749. [Google Scholar] [CrossRef]
- Proulx, J.; Ghaly, M.; Park, I.-W.; Borgmann, K. HIV-1-Mediated Acceleration of Oncovirus-Related Non-AIDS-Defining Cancers. Biomedicines 2022, 10, 768. [Google Scholar] [CrossRef]
- Lohse, N.; Hansen, A.-B.E.; Pedersen, G.; Kronborg, G.; Gerstoft, J.; Sørensen, H.T.; Vaeth, M.; Obel, N. Survival of Persons with and without HIV Infection in Denmark, 1995–2005. Ann. Intern. Med. 2007, 146, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Jacobson, L.P.; Cohen, M.; French, A.; Phair, J.; Muñoz, A. Cause-Specific Life Expectancies After 35 Years of Age for Human Immunodeficiency Syndrome-Infected and Human Immunodeficiency Syndrome-Negative Individuals Followed Simultaneously in Long-Term Cohort Studies, 1984–2008. Am. J. Epidemiol. 2013, 177, 116–125. [Google Scholar] [CrossRef]
- Antiretroviral Therapy Cohort Collaboration Life. Expectancy of Individuals on Combination Antiretroviral Therapy in High-Income Countries: A Collaborative Analysis of 14 Cohort Studies. Lancet 2008, 372, 293–299. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Wandeler, G.; Dufour, J.-F.; Bruggmann, P.; Rauch, A. Hepatitis C: A Changing Epidemic. Swiss Med. Wkly. 2015, 145, w14093. [Google Scholar] [CrossRef]
- Chan, D.P.C.; Sun, H.-Y.; Wong, H.T.H.; Lee, S.-S.; Hung, C.-C. Sexually Acquired Hepatitis C Virus Infection: A Review. Int. J. Infect. Dis. 2016, 49, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Ferrer, A.; Fierer, D.S.; Alvarez-Alvarez, B.; de Gorgolas, M.; Fernandez-Guerrero, M.L. Acute Hepatitis C Outbreak among HIV-Infected Men, Madrid, Spain. Emerg. Infect. Dis. 2011, 17, 1560–1562. [Google Scholar] [CrossRef] [PubMed]
- Drückler, S.; van Rooijen, M.S.; de Vries, H.J.C. Chemsex Among Men Who Have Sex with Men: A Sexualized Drug Use Survey Among Clients of the Sexually Transmitted Infection Outpatient Clinic and Users of a Gay Dating App in Amsterdam, the Netherlands. Sex. Transm. Dis. 2018, 45, 325–331. [Google Scholar] [CrossRef] [PubMed]
- González-Baeza, A.; Dolengevich-Segal, H.; Pérez-Valero, I.; Cabello, A.; Téllez, M.J.; Sanz, J.; Pérez-Latorre, L.; Bernardino, J.I.; Troya, J.; De La Fuente, S.; et al. Sexualized Drug Use (Chemsex) Is Associated with High-Risk Sexual Behaviors and Sexually Transmitted Infections in HIV-Positive Men Who Have Sex with Men: Data from the U-SEX GESIDA 9416 Study. AIDS Patient Care STDs 2018, 32, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.; Stoové, M.; Wilson, D.P.; Keiser, O.; El-Hayek, C.; Doyle, J.; Hellard, M. Eliminating Hepatitis C Virus as a Public Health Threat among HIV-Positive Men Who Have Sex with Men: A Multi-Modelling Approach to Understand Differences in Sexual Risk Behaviour. J. Int. AIDS Soc. 2018, 21, e25059. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The End of AIDS: HIV Infection as a Chronic Disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Blanco, J.-R.; Negredo, E.; Bernal, E.; Blanco, J. Impact of HIV Infection on Aging and Immune Status. Expert Rev. Anti-Infect. Ther. 2021, 19, 719–731. [Google Scholar] [CrossRef]
- Rider, P.J.; Liu, F. Crosstalk between HIV and Hepatitis C Virus during Co-Infection. BMC Med. 2012, 10, 32. [Google Scholar] [CrossRef]
- Graham, C.S.; Baden, L.R.; Yu, E.; Mrus, J.M.; Carnie, J.; Heeren, T.; Koziel, M.J. Influence of Human Immunodeficiency Virus Infection on the Course of Hepatitis C Virus Infection: A Meta-Analysis. Clin. Infect. Dis. 2001, 33, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Martin-Carbonero, L.; de Ledinghen, V.; Moreno, A.; Maida, I.; Foucher, J.; Barreiro, P.; Romero, M.; Satta, G.; Garcia-Samaniego, J.; Gonzalez-Lahoz, J.; et al. Liver Fibrosis in Patients with Chronic Hepatitis C and Persistently Normal Liver Enzymes: Influence of HIV Infection. J. Viral Hepat. 2009, 16, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Sherman, K.E.; O’Brien, J.; Gutierrez, A.G.; Harrison, S.; Urdea, M.; Neuwald, P.; Wilber, J. Quantitative Evaluation of Hepatitis C Virus RNA in Patients with Concurrent Human Immunodeficiency Virus Infections. J. Clin. Microbiol. 1993, 31, 2679–2682. [Google Scholar] [CrossRef]
- van der Helm, J.; Geskus, R.; Sabin, C.; Meyer, L.; Del Amo, J.; Chêne, G.; Dorrucci, M.; Muga, R.; Porter, K.; Prins, M.; et al. Effect of HCV Infection on Cause-Specific Mortality after HIV Seroconversion, before and after 1997. Gastroenterology 2013, 144, 751–760.e2. [Google Scholar] [CrossRef]
- Alvaro-Meca, A.; Berenguer, J.; Díaz, A.; Micheloud, D.; Aldámiz-Echevarría, T.; Fanciulli, C.; Resino, S. Stroke in HIV-Infected Individuals with and without HCV Coinfection in Spain in the Combination Antiretroviral Therapy Era. PLoS ONE 2017, 12, e0179493. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, A.; Al-Harthi, L.; Christensen, S.; Mack, W.; Cohen, M.; Landay, A. CD8+ T Cell Activation in Women Coinfected with Human Immunodeficiency Virus Type 1 and Hepatitis C Virus. J. Infect. Dis. 2008, 197, 1402–1407. [Google Scholar] [CrossRef]
- Kovacs, A.; Karim, R.; Mack, W.J.; Xu, J.; Chen, Z.; Operskalski, E.; Frederick, T.; Landay, A.; Voris, J.; Spencer, L.S.; et al. Activation of CD8 T Cells Predicts Progression of HIV Infection in Women Coinfected with Hepatitis C Virus. J. Infect. Dis. 2010, 201, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, C.; Álvarez, B.; Valencia, J.L.; García, M.; Navarrete-Muñoz, M.A.; Ligos, J.M.; Cabello, A.; Prieto, L.; Nistal, S.; Montoya, M.; et al. Both HCV Infection and Elevated Liver Stiffness Significantly Impacts on Several Parameters of T-Cells Homeostasis in HIV-Infected Patients. J. Clin. Med. 2020, 9, 2978. [Google Scholar] [CrossRef] [PubMed]
- Medrano, L.M.; Garcia-Broncano, P.; Berenguer, J.; González-García, J.; Jiménez-Sousa, M.Á.; Guardiola, J.M.; Crespo, M.; Quereda, C.; Sanz, J.; Canorea, I.; et al. Elevated Liver Stiffness Is Linked to Increased Biomarkers of Inflammation and Immune Activation in HIV/Hepatitis C Virus-Coinfected Patients. AIDS 2018, 32, 1095–1105. [Google Scholar] [CrossRef]
- Sengupta, S.; Powell, E.; Kong, L.; Blackard, J.T. Effects of HCV on Basal and Tat-Induced HIV LTR Activation. PLoS ONE 2013, 8, e64956. [Google Scholar] [CrossRef]
- López-Cortés, L.F.; Trujillo-Rodríguez, M.; Báez-Palomo, A.; Benmarzouk-Hidalgo, O.J.; Dominguez-Molina, B.; Milanés-Guisado, Y.; Espinosa, N.; Viciana, P.; Gutiérrez-Valencia, A. Eradication of Hepatitis C Virus (HCV) Reduces Immune Activation, Microbial Translocation, and the HIV DNA Level in HIV/HCV-Coinfected Patients. J. Infect. Dis. 2018, 218, 624–632. [Google Scholar] [CrossRef]
- López-Huertas, M.R.; Palladino, C.; Garrido-Arquero, M.; Esteban-Cartelle, B.; Sánchez-Carrillo, M.; Martínez-Román, P.; Martín-Carbonero, L.; Ryan, P.; Domínguez-Domínguez, L.; Santos, I.D.L.; et al. HCV-Coinfection Is Related to an Increased HIV-1 Reservoir Size in cART-Treated HIV Patients: A Cross-Sectional Study. Sci. Rep. 2019, 9, 5606. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Bernal, R.S.; Jimenez-Leon, M.R.; Tarancon-Diez, L.; Gutierrez-Valencia, A.; Serna-Gallego, A.; Trujillo-Rodriguez, M.; Alvarez-Rios, A.I.; Milanes-Guisado, Y.; Espinosa, N.; Roca-Oporto, C.; et al. Modulation of Monocyte Activation and Function during Direct Antiviral Agent Treatment in Patients Coinfected with HIV and Hepatitis C Virus. Antimicrob. Agents Chemother. 2020, 64, e00773-20. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Buzon, M.J.; Shaw, A.; Berg, R.K.; Yu, X.G.; Ferrando-Martinez, S.; Leal, M.; Ruiz-Mateos, E.; Lichterfeld, M. Hepatitis C Therapy with Interferon-α and Ribavirin Reduces CD4 T-Cell-Associated HIV-1 DNA in HIV-1/Hepatitis C Virus-Coinfected Patients. J. Infect. Dis. 2014, 209, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Busca Arenzana, C.; González-García, J.; Blas-García, A.; Esplugues, J.V.; Olveira Martín, A.; Montes Ramírez, M.L. Benefits of Rilpivirine for Liver Stiffness in HIV/HCV-Coinfected Patients. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2024, 42, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Castéra, L.; Vergniol, J.; Foucher, J.; Le Bail, B.; Chanteloup, E.; Haaser, M.; Darriet, M.; Couzigou, P.; De Lédinghen, V. Prospective Comparison of Transient Elastography, Fibrotest, APRI, and Liver Biopsy for the Assessment of Fibrosis in Chronic Hepatitis C. Gastroenterology 2005, 128, 343–350. [Google Scholar] [CrossRef]
- Benedetto, U.; Head, S.J.; Angelini, G.D.; Blackstone, E.H. Statistical Primer: Propensity Score Matching and Its Alternatives. Eur. J. Cardio-Thorac. Surg. 2018, 53, 1112–1117. [Google Scholar] [CrossRef]
- Soltani, S.; Saraf-Bank, S.; Basirat, R.; Salehi-Abargouei, A.; Mohammadifard, N.; Sadeghi, M.; Khosravi, A.; Fadhil, I.; Puska, P.; Sarrafzadegan, N. Community-based cardiovascular disease prevention programmes and cardiovascular risk factors: A systematic review and meta-analysis. Public Health 2021, 200, 59–70. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Rosticci, M.; Baronio, C.; Morbini, M.; Parini, A.; Grandi, E.; D’Addato, S.; Borghi, C.; Brisighella Heart Study Group. Serum LDL cholesterol levels and new onset of arterial hypertension: An 8-year follow-up. Eur. J. Clin. Investig. 2014, 44, 926–932. [Google Scholar] [CrossRef]
- Packard, C.; Chapman, M.J.; Sibartie, M.; Laufs, U.; Masana, L. Intensive low-density lipoprotein cholesterol lowering in cardiovascular disease prevention: Opportunities and challenges. Heart 2021, 107, 1369–1375. [Google Scholar] [CrossRef]
- Berberich, A.J.; Hegele, R.A. A Modern Approach to Dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef] [PubMed]
- Murton, M.; Goff-Leggett, D.; Bobrowska, A.; Garcia Sanchez, J.J.; James, G.; Wittbrodt, E.; Nolan, S.; Sörstadius, E.; Pecoits-Filho, R.; Tuttle, K. Burden of Chronic Kidney Disease by KDIGO Categories of Glomerular Filtration Rate and Albuminuria: A Systematic Review. Adv. Ther. 2021, 38, 180–200. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; De Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.V.; Webber, L.; Sheron, N.; EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Oztumer, C.A.; Chaudhry, R.M.; Alrubaiy, L. Association between behavioural risk factors for chronic liver disease and transient elastography measurements across the UK: A cross-sectional study. BMJ Open Gastroenterol. 2020, 7, e000524. [Google Scholar] [CrossRef] [PubMed]
- La Fazia, V.M.; Pierucci, N.; Mohanty, S.; Gianni, C.; Della Rocca, D.G.; Compagnucci, P.; MacDonald, B.; Mayedo, A.; Torlapati, P.G.; Bassiouny, M.; et al. Catheter ablation approach and outcome in HIV+ patients with recurrent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2023, 34, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Liakoni, E. Tobacco use disorder and cardiovascular health. Addiction 2022, 117, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, G.; Mastrorosa, I.; Vergori, A.; Timelli, L.; Lorenzini, P.; Zaccarelli, M.; Cicalini, S.; Bellagamba, R.; Plazzi, M.M.; Mazzotta, V.; et al. Liver Stiffness Reduction and Serum Fibrosis Score Improvement in HIV/Hepatitis C Virus-Coinfected Patients Treated with Direct-Acting Antivirals. HIV Med. 2018, 19, 578–584. [Google Scholar] [CrossRef]
- Álvarez, B.; Restrepo, C.; García, M.; Navarrete-Muñoz, M.A.; Jiménez-Sousa, M.A.; Prieto, L.; Cabello, A.; Nistal, S.; Resino, S.; Górgolas, M.; et al. Liver Stiffness Hinders Normalization of Systemic Inflammation and Endothelial Activation after Hepatitis C Virus (HCV) Eradication in HIV/HCV Coinfected Patients. Vaccines 2020, 8, 323. [Google Scholar] [CrossRef]
- Vos, A.G.; Idris, N.S.; Barth, R.E.; Klipstein-Grobusch, K.; Grobbee, D.E. Pro-Inflammatory Markers in Relation to Cardiovascular Disease in HIV Infection. A Systematic Review. PLoS ONE 2016, 11, e0147484. [Google Scholar] [CrossRef]
- Potter, M.; Odueyungbo, A.; Yang, H.; Saeed, S.; Klein, M.B. Canadian Co-infection Cohort Study Investigators Impact of Hepatitis C Viral Replication on CD4+ T-Lymphocyte Progression in HIV-HCV Coinfection before and after Antiretroviral Therapy. AIDS 2010, 24, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Rallón, N.; García, M.; García-Samaniego, J.; Rodríguez, N.; Cabello, A.; Restrepo, C.; Álvarez, B.; García, R.; Górgolas, M.; Benito, J.M. HCV Coinfection Contributes to HIV Pathogenesis by Increasing Immune Exhaustion in CD8 T-Cells. PLoS ONE 2017, 12, e0173943. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khaitan, A.; Unutmaz, D. Revisiting Immune Exhaustion during HIV Infection. Curr. HIV/AIDS Rep. 2011, 8, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Villar, S.; Sainz, T.; Lee, S.A.; Hunt, P.W.; Sinclair, E.; Shacklett, B.L.; Ferre, A.L.; Hayes, T.L.; Somsouk, M.; Hsue, P.Y.; et al. HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality. PLoS Pathog. 2014, 10, e1004078. [Google Scholar] [CrossRef] [PubMed]
- Osude, N.; Durazo-Arvizu, R.; Markossian, T.; Liu, K.; Michos, E.D.; Rakotz, M.; Wozniak, G.; Egan, B.; Kramer, H. Age and sex disparities in hypertension control: The multi-ethnic study of atherosclerosis (MESA). Am. J. Prev. Cardiol. 2021, 8, 100230. [Google Scholar] [CrossRef] [PubMed]
- Cowie, C.C.; Casagrande, S.S.; Geiss, L.S. Prevalence and Incidence of Type 2 Diabetes and Prediabetes. In Diabetes in America, 3rd ed.; Cowie, C.C., Casagrande, S.S., Menke, A., Cissell, M.A., Eberhardt, M.S., Meigs, J.B., Gregg, E.W., Knowler, W.C., Barrett-Connor, E., Becker, D.J., et al., Eds.; National Institute of Diabetes and Digestive and Kidney Diseases (US): Bethesda, MD, USA, 2018; Chapter 3. [Google Scholar]
- Li, Q.; Jiang, Y.; Song, A.; Li, Y.; Xu, X.; Xu, R. The Association Between Chronological Age and Dyslipidemia: A Cross-Sectional Study in Chinese Aged Population. Clin. Interv. Aging 2023, 18, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.L.; Rothenbacher, D. Prevalence of chronic kidney disease in population-based studies: Systematic review. BMC Public Health 2008, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Guddati, A.K. Bimodal Age Distribution in Cancer Incidence. World J. Oncol. 2022, 13, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Drozdz, D.; Alvarez-Pitti, J.; Wójcik, M.; Borghi, C.; Gabbianelli, R.; Mazur, A.; Herceg-Čavrak, V.; Lopez-Valcarcel, B.G.; Brzeziński, M.; Lurbe, E.; et al. Obesity and Cardiometabolic Risk Factors: From Childhood to Adulthood. Nutrients 2021, 13, 4176. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Álvarez, B.; Navarrete-Muñoz, M.A.; Briz, V.; Olmedillas-López, S.; Nistal, S.; Cabello, A.; Prieto, L.; Górgolas, M.; García-Arranz, M.; Benito, J.M.; et al. HIV-Reservoir Size Is Not Affected Either by HCV Coinfection or by Direct Acting Antivirals (DAAs) Therapy. Sci. Rep. 2022, 12, 5095. [Google Scholar] [CrossRef] [PubMed]
- Sikavi, C.; Chen, P.H.; Lee, A.D.; Saab, E.G.; Choi, G.; Saab, S. Hepatitis C and Human Immunodeficiency Virus Coinfection in the Era of Direct-Acting Antiviral Agents: No Longer a Difficult-to-Treat Population. Hepatology 2018, 67, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Meissner, E.G. Update in HIV-Hepatitis C Virus Coinfection in the Direct Acting Antiviral Era. Curr. Opin. Gastroenterol. 2017, 33, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Sulkowski, M.; Naggie, S.; Puoti, M.; Orkin, C.; Hunt, S.L. Sofosbuvir and Ribavirin for Treatment of Chronic Hepatitis C in Patients Coinfected with Hepatitis C Virus and HIV: The Impact on Patient-Reported Outcomes. J. Infect. Dis. 2015, 212, 367–377. [Google Scholar] [CrossRef][Green Version]
- Younossi, Z.M.; Stepanova, M.; Sulkowski, M.; Naggie, S.; Henry, L.; Hunt, S. Sofosbuvir and Ledipasvir Improve Patient-Reported Outcomes in Patients Co-Infected with Hepatitis C and Human Immunodeficiency Virus. J. Viral Hepat. 2016, 23, 857–865. [Google Scholar] [CrossRef] [PubMed]
| HIV/HCV Group (N = 229) | HIV Group (N = 229) | p-Value | ||
|---|---|---|---|---|
| Demographics | ||||
| Age | Mean (SD) | 49.1 (10.8) | 49.6 (10.4) | 0.627 1 |
| Gender | 1.000 2 | |||
| Male | N (%) | 218 (95.2%) | 218 (95.2%) | |
| Female | N (%) | 11 (4.8%) | 10 (4.4%) | |
| Transgender | N (%) | 0 (0.0%) | 1 (0.4%) | |
| Ethnicity | 0.934 2 | |||
| African | N (%) | 0 (0.0%) | 1 (0.4%) | |
| Arab | N (%) | 1 (0.4%) | 0 (0.0%) | |
| Asian | N (%) | 1 (0.4%) | 1 (0.4%) | |
| Caucasian | N (%) | 174 (76.0%) | 176 (76.9%) | |
| Latino | N (%) | 53 (23.1%) | 51 (22.3%) | |
| Sexual orientation | <0.001 2 | |||
| Heterosexual | N (%) | 51 (22.3%) | 17 (7.4%) | |
| Homosexual | N (%) | 173 (75.5%) | 212 (92.6%) | |
| Bisexual | N (%) | 5 (2.2%) | 0 (0.0%) | |
| Lifestyle habits | ||||
| Smoking habit | 0.001 2 | |||
| No | N (%) | 97 (42.9%) | 122 (53.3%) | |
| Yes | N (%) | 126 (55.8%) | 93 (40.6%) | |
| Former smoker | N (%) | 3 (1.3%) | 14 (6.1%) | |
| Alcohol consumption | <0.001 2 | |||
| No | N (%) | 120 (53.1%) | 167 (72.9%) | |
| Yes | N (%) | 106 (46.9%) | 62 (27.1%) | |
| Drug consumption (ever) | <0.001 2 | |||
| No | N (%) | 54 (25.5%) | 203 (89.0%) | |
| Yes | N (%) | 157 (74.1%) | 25 (11.0%) | |
| Former consumer | N (%) | 1 (0.5%) | 0 (0.0%) | |
| Purpose of parenteral drugs’ use | <0.001 2 | |||
| Nonconsumer | N (%) | 117 (55.2%) | 226 (98.7%) | |
| Conventional use | N (%) | 48 (22.6%) | 2 (0.9%) | |
| ChemSex | N (%) | 47 (22.2%) | 1 (0.4%) | |
| HIV infection | ||||
| Time since diagnosis (years) | Median (IQR) | 14.0 (9.0–20.0) | 13.0 (9.0–18.0) | 0.092 3 |
| HIV route of transmission | <0.001 2 | |||
| Parenteral | N (%) | 50 (21.8%) | 7 (3.1%) | |
| Sexual | N (%) | 179 (78.2%) | 222 (96.9%) | |
| HIV clinical stage | <0.001 2 | |||
| Stage A | N (%) | 140 (61.1%) | 163 (71.2%) | |
| Stage B | N (%) | 42 (18.3%) | 36 (15.7%) | |
| Stage C | N (%) | 47 (20.5%) | 30 (13.1%) | |
| CD4+ cell count at ART initiation (cells/µL) | Median (IQR) | 306.0 (180.0–472.0) | 314.0 (216.0–440.0) | 0.845 3 |
| CD4+ cell count at ART initiation (range) | 0.012 2 | |||
| 1–199 cells/µL | N (%) | 61 (30.0%) | 44 (20.2%) | |
| 200–499 cells/µL | N (%) | 98 (48.3%) | 136 (62.4%) | |
| ≥500 cells/µL | N (%) | 44 (21.7%) | 38 (17.4%) | |
| CD4+/CD8+ ratio at ART initiation | Median (IQR) | 0.28 (0.14–0.47) | 0.30 (0.18–0.45) | 0.313 3 |
| Baseline HIV-1 VL (copies/mL) | Median (IQR) | 107.730 (31.960–271.000) | 86.743 (23.147–258.250) | 0.282 3 |
| Baseline HIV-1VL (range) | 0.102 2 | |||
| <100.000 copies/mL | N (%) | 93 (48.2%) | 114 (54.0%) | |
| 100.000–500.000 copies/mL | N (%) | 67 (34.7%) | 76 (36.0%) | |
| <500.000 copies/mL | N (%) | 33 (17.1%) | 21 (10.0%) | |
| History of AIDS | N (%) | 48 (21.0%) | 29 (12.7%) | 0.025 2 |
| HIV treatment | ||||
| Time since HIV diagnosis to treatment (years) | Median (IQR) | 1.0 (0.0–4.0) | 1.0 (0.0–3.0) | 0.155 3 |
| Time on HIV treatment (years) | Median (IQR) | 11.0 (7.0–15.0) | 11.0 (7.0–15.0) | 0.573 3 |
| Ever exposed to rilpivirine | N (%) | 65 (28.4%) | 54 (23.6%) | 0.287 2 |
| Poor previous ART adherence | N (%) | 29 (12.7%) | 9 (3.9%) | 0.001 2 |
| HCV infection | ||||
| HCV route of transmission | - | |||
| Parenteral | N (%) | 51 (22.3%) | - | |
| Sexual | N (%) | 178 (77.7%) | - | |
| HCV clinical stage at diagnosis | - | |||
| Acute | N (%) | 150 (65.5%) | - | |
| Chronic | N (%) | 79 (34.5%) | - | |
| Time since HCV diagnosis to successful treatment (years) | Mean (SD) | 5.66 (9.13) | - | |
| Median (IQR) | 1.0 (0.0–6.0) | - | ||
| HCV genotypes | - | |||
| 1a/b | N (%) | 159 (69.5%) | - | |
| 2/3 | N (%) | 13 (5.6%) | - | |
| 4 | N (%) | 57 (24.9%) | - | |
| HCV viral load at DAA initiation (IU/mL) | - | |||
| <800.000 | N (%) | 88 (38.4%) | - | |
| ≥800.000 | N (%) | 141 (61.6%) | - | |
| Achieved SVR12 | N (%) | 225 (98.2%) | - | |
| Transient elastography (FibroScan) LS before DAAs (kPa) | Mean (SD) | 7.13 (5.32) | - | - |
| Transient elastography (FibroScan) LS before DAAs (Metavir fibrosis score stages) | - | |||
| F0–F1 (<7.1 kPa) | N (%) | 153 (66.8%) | - | |
| F2 (7.1–9.4 kPa) | N (%) | 33 (14.4%) | - | |
| F3 (9.5–12.4 kPa) | N (%) | 20 (8.7%) | - | |
| F4 (≥12.5 kPa) | N (%) | 13 (5.7%) | - | |
| No data | N (%) | 10 (4.4%) | - | |
| HCV reinfections (number) | - | |||
| 2 HCV infections | N (%) | 25 (10.9%) | - | |
| 3 HCV infections | N (%) | 5 (2.2%) | - | |
| Baseline biochemistry results | ||||
| AST (IU/L) | Median (IQR) | 48.0 (35.0–74.0) | 21.0 (17.0–27.0) | <0.001 3 |
| ALT (IU/L) | Median (IQR) | 69.0 (45.0–123.0) | 21.0 (16.0–30.0) | <0.001 3 |
| GGT (IU/L) | Median (IQR) | 60.5 (32.8–114.0) | 21.0 (15.0–32.0) | <0.001 3 |
| Total cholesterol (mg/dL) | Mean (SD) | 159.0 (33.2) | 181.0 (35.5) | <0.001 1 |
| LDL-cholesterol (mg/dL) | Mean (SD) | 90.4 (27.5) | 110.0 (34.1) | <0.001 1 |
| HDL-cholesterol (mg/dL) | Mean (SD) | 47.3 (13.7) | 49.8 (15.2) | 0.063 1 |
| Triglycerides (mg/dL) | Median (IQR) | 101.0 (74.0–138.0) | 106.0 (83.0–151.0) | 0.136 3 |
| GFR (mL/min/1.73 m2) | Mean (SD) | 94.0 (15.1) | 83.0 (17.8) | <0.001 3 |
| Platelet (x103 µL) | Mean (SD) | 231.0 (66.6) | 251.0 (62.7) | 0.001 1 |
| CD4+ (cells/µL) | Mean (SD) | 703.0 (350.) | 812.0 (328.0) | 0.001 1 |
| CD8+ (cells/µL) | Mean (SD) | 1074.0 (510.0) | 968.0 (442.0) | 0.018 1 |
| CD4+/CD8+ | Mean (SD) | 0.73 (0.37) | 0.96 (0.49) | <0.001 1 |
| APRI score | Median (IQR) | 0.55 (0.35–0.90) | 0.09 (0.05–0.13) | <0.001 3 |
| APRI fibrosis stage | <0.001 2 | |||
| No fibrosis | N (%) | 102 (44.5%) | 226 (98.7%) | |
| Moderate fibrosis | N (%) | 94 (41.0%) | 3 (1.3%) | |
| Cirrhosis | N (%) | 33 (14.4%) | 0 (0.0%) | |
| FIB-4 score | Median (IQR) | 1.12 (0.78–1.57) | 0.33 (0.22–0.57) | <0.001 3 |
| FIB-4 fibrosis stage | <0.001 2 | |||
| No fibrosis | N (%) | 159 (69.4%) | 224 (97.8%) | |
| Moderate fibrosis | N (%) | 53 (23.2%) | 5 (2.2%) | |
| Cirrhosis | N (%) | 17 (7.4%) | 0 (0.0%) | |
| Baseline comorbidities | ||||
| Number of previous comorbidities | Mean (SD) | 0.30 (0.70) | 0.10 (0.30) | <0.001 1 |
| Number of documented STIs | Mean (SD) | 4.20 (3.60) | 2.60 (2.40) | <0.001 1 |
| Hepatitis A test (Positive) | N (%) | 163 (74.4%) | 155 (76.4%) | 0.730 2 |
| HBsAg (Positive) | N (%) | 9 (3.9%) | 6 (2.7%) | 0.662 2 |
| HBsAb (Positive) | N (%) | 136 (59.4%) | 147 (66.8%) | 0.125 2 |
| HBcAb (Positive) | N (%) | 97 (42.4%) | 81 (37.0%) | 0.287 2 |
| Obesity (BMI > 30 kg/m2) | N (%) | 19 (8.3%) | 21 (10.8%) | 0.483 2 |
| Hypertension at diagnosis | N (%) | 18 (7.9%) | 3 (1.3%) | 0.002 2 |
| DM at diagnosis | N (%) | 18 (7.9%) | 3 (1.3%) | 0.040 2 |
| Dyslipidemia at diagnosis | N (%) | 15 (6.6%) | 12 (5.2%) | 0.692 2 |
| CVD at diagnosis | N (%) | 7 (3.1%) | 1 (0.4%) | 0.075 2 |
| Kidney disease at diagnosis | N (%) | 6 (2.6%) | 0 (0.0%) | 0.040 2 |
| Hepatic disease at diagnosis | N (%) | 15 (6.6%) | 0 (0.0%) | <0.001 2 |
| Non-AIDS cancer at diagnosis | N (%) | 9 (3.9%) | 0 (0.0%) | 0.007 2 |
| HIV/HCV Group (N = 229) | HIV Group (N = 229) | p-Value | ||
|---|---|---|---|---|
| AST (IU/L) | Median (IQR) | 21.0 (17.0–26.0) | 21.0 (17.0–27.0) | 0.991 1 |
| ALT (IU/L) | Median (IQR) | 18.0 (14.0–23.0) | 21.0 (16.0–30.0) | <0.001 1 |
| GGT (IU/L) | Median (IQR) | 18.0 (13.0–31.0) | 21.0 (15.0–32.0) | 0.059 1 |
| Total cholesterol (mg/dL) | Mean (SD) | 172.0 (36.2) | 181.0 (35.5) | 0.010 2 |
| LDL-cholesterol (mg/dL) | Mean (SD) | 102.0 (33.3) | 110.0 (34.1) | 0.007 2 |
| HDL-cholesterol (mg/dL) | Mean (SD) | 46.5 (14.0) | 49.8 (15.2) | 0.015 2 |
| Triglycerides (mg/dL) | Median (IQR) | 107.0 (79.0–156.0) | 106.0 (83.0–151.0) | 0.842 1 |
| GFR (mL/min/1.73 m2) | Mean (SD) | 92.2 (15.2) | 83.0 (17.8) | <0.001 2 |
| Platelet (×103 µL) | Mean (SD) | 235.0 (72.6) | 251.0 (62.7) | 0.011 2 |
| CD4+ (cells/µL) | Mean (SD) | 752.0 (399.0) | 812.0 (328.0) | 0.082 2 |
| CD8+ (cells/µL) | Mean (SD) | 1119.0 (497.0) | 968.0 (442.0) | 0.001 2 |
| CD4+/CD8+ | Mean (SD) | 0.74 (0.36) | 0.96 (0.49) | <0.001 2 |
| APRI score | Median (IQR) | 0.23 (0.17–0.30) | 0.09 (0.05–0.13) | <0.001 1 |
| APRI fibrosis stage | 0.001 3 | |||
| No fibrosis | N (%) | 208 (90.8%) | 226 (98.7%) | |
| Moderate fibrosis | N (%) | 18 (7.9%) | 3 (1.3%) | |
| Cirrhosis | N (%) | 3 (1.3%) | 0 (0.0%) | |
| FIB-4 score | Median (IQR) | 0.94 (0.69–1.29) | 0.33 (0.22–0.57) | <0.001 1 |
| FIB-4 fibrosis stage | 0.001 3 | |||
| No fibrosis | N (%) | 182 (79.5%) | 224 (97.8%) | |
| Moderate fibrosis | N (%) | 40 (17.5%) | 5 (2.2%) | |
| Cirrhosis | N (%) | 7 (3.1%) | 0 (0.0%) |
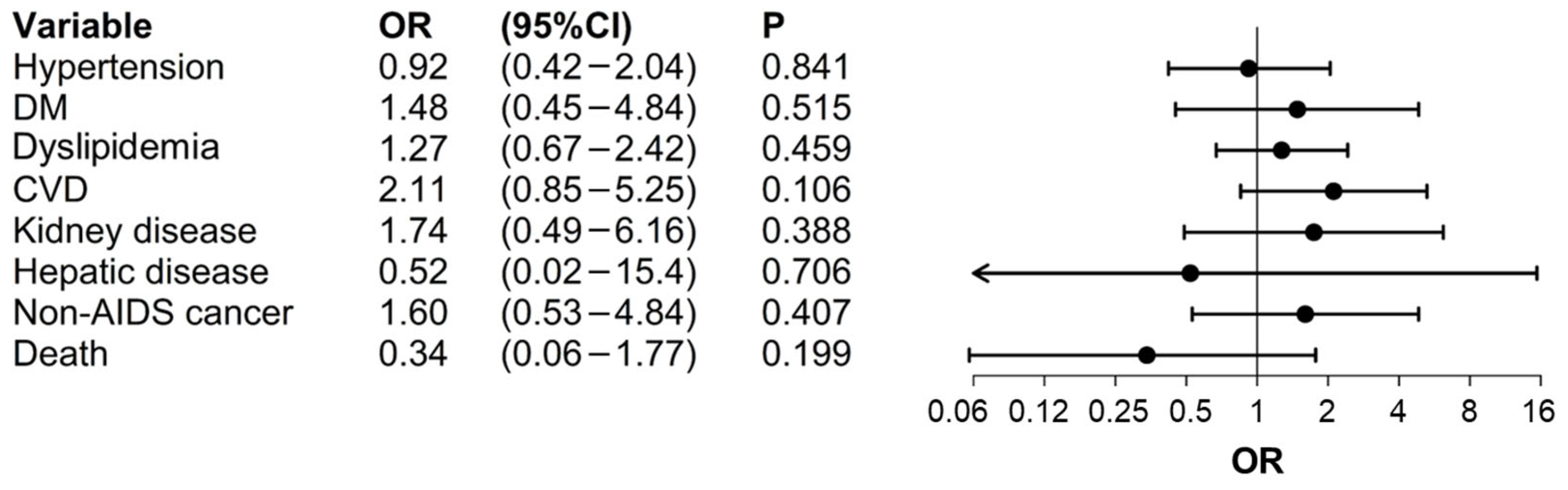
| Variables Included 1 | OR | CI 95% | p-Value | |
|---|---|---|---|---|
| Hypertension | Group (HIV vs. HIV/HCV) | 0.94 | 0.42–2.10 | 0.878 |
| Number of documented STIs | 0.84 | 0.73–0.96 | 0.011 | |
| Drugs consumption (ever) | 0.41 | 0.16–1.07 | 0.068 | |
| Alcohol consumption | 0.89 | 0.43–1.82 | 0.742 | |
| DM | Group (HIV vs. HIV/HCV) | 1.85 | 0.52–6.63 | 0.345 |
| Number of previous comorbidities | 3.76 | 2.03–6.95 | <0.001 | |
| Number of documented STIs | 0.76 | 0.58–1.00 | 0.051 | |
| CD8+ (cells/µL) 2 | 1.10 | 1.01–1.19 | 0.024 | |
| HIV route of transmission | 1.33 | 0.30–5.93 | 0.711 | |
| Dyslipidemia | Group (HIV vs. HIV/HCV) | 1.06 | 0.56–1.99 | 0.857 |
| Number of documented STIs | 0.91 | 0.83–0.99 | 0.044 | |
| Drugs consumption (ever) | 0.49 | 0.25–0.97 | 0.041 | |
| CVD | Group (HIV vs. HIV/HCV) | 1.60 | 0.69–3.71 | 0.269 |
| Number of previous comorbidities | 2.39 | 1.45–3.96 | 0.001 | |
| Kidney disease | Group (HIV vs. HIV/HCV) | 2.50 | 0.88–7.07 | 0.084 |
| Number of documented STIs | 0.76 | 0.60–0.96 | 0.022 | |
| Hepatic disease | Group (HIV vs. HIV/HCV) | 0.59 | 0.05–7.15 | 0.681 |
| Time since HIV diagnosis to treatment | 1.11 | 1.02–1.19 | 0.010 | |
| FIB-4 fibrosis stage (fibrosis vs. no fibrosis) | 8.51 | 1.23–59.00 | 0.030 | |
| Non-AIDS cancer | Group (HIV vs. HIV/HCV) | 2.38 | 0.91–6.21 | 0.076 |
| Number of previous comorbidities | 1.97 | 1.09–3.56 | 0.025 | |
| Number of documented STIs | 0.89 | 0.75–0.172 | 0.172 | |
| Death | Group (HIV vs. HIV/HCV) | 0.25 | 0.04–1.49 | 0.128 |
| Number of previous comorbidities | 1.49 | 0.76–2.93 | 0.240 | |
| Number of documented STIs | 0.41 | 0.21–0.82 | 0.011 | |
| HIV route of transmission | 3.43 | 0.34–34.35 | 0.293 | |
| History of AIDS | 2.05 | 0.58–7.22 | 0.262 | |
| Purpose of parental drugs’ use (No consumption) | 1.84 | 0.19–17.79 | 0.598 | |
| Comorbidities | Variables Included 1 | HIV-Monoinfected Patients | HIV/HCV-Coinfected Patients | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| Hypertension | Age | 1.09 | 1.04–1.14 | <0.001 | 1.09 | 1.03–1.16 | 0.002 |
| Time on HIV treatment | 1.08 | 1.01–1.16 | 0.038 | - 2 | - 2 | - 2 | |
| DM | Age | 1.10 | 1.03–1.17 | 0.005 | - 2 | - 2 | - 2 |
| Obesity (BMI ≥ 30 kg/m2) | 6.76 | 1.77–25.90 | 0.005 | - 2 | - 2 | - 2 | |
| Number of previous comorbidities | - 2 | - 2 | - 2 | 5.50 | 2.27–8.93 | <0.001 | |
| Time on HIV treatment | - 2 | - 2 | - 2 | 1.14 | 1.02–1.28 | 0.019 | |
| Dyslipidemia | Time on HIV treatment | 1.13 | 1.05–1.21 | 0.001 | - 2 | - 2 | - 2 |
| Obesity (BMI ≥ 30 kg/m2) | 7.17 | 2.42–21.25 | <0.001 | 3.82 | 1.18–12.37 | 0.025 | |
| Number of previous comorbidities | 0.03 | 0.01–0.44 | 0.010 | - 2 | - 2 | - 2 | |
| Age | 1.06 | 1.02–1.11 | 0.006 | - 2 | - 2 | - 2 | |
| Treatment regimen with rilpivirine | 3.20 | 1.37–7.48 | 0.007 | - 2 | - 2 | - 2 | |
| CD4+ (cells/µL) | 1.14 | 1.02–1.28 | 0.019 | - 2 | - 2 | - 2 | |
| Gender (Female vs. Male) 3 | - 2 | - 2 | - 2 | 11.5 | 2.60–50.60 | 0.001 | |
| HCV VL (≥800,000 IU/mL vs. <800,000 IU/mL) | - 2 | - 2 | - 2 | 3.48 | 1.29–9.36 | 0.014 | |
| HIV clinical stage (vs. A) | |||||||
| Stage B | - 2 | - 2 | - 2 | 2.27 | 0.88–5.86 | 0.068 | |
| Stage C | - 2 | - 2 | - 2 | 0.38 | 0.10–1.42 | 0.174 | |
| CVD | HIV follow-up time | 1.14 | 1.06–1.23 | 0.001 | - 2 | - 2 | - 2 |
| Number of previous comorbidities | 4.87 | 1.40–16.89 | 0.013 | 2.04 | 1.16–3.58 | 0.013 | |
| Number of HCV infections | - 2 | - 2 | - 2 | 2.57 | 1.03–6.38 | 0.042 | |
| Kidney disease | Age | 1.11 | 1.05–1.17 | <0.001 | - 2 | - 2 | - 2 |
| Time on HIV treatment | - 2 | - 2 | - 2 | 1.15 | 1.02–1.30 | 0.027 | |
| Hepatic disease | CD4+ (cells/µL) | 1.46 | 1.03–2.05 | 0.032 | - 2 | - 2 | - 2 |
| Time since HIV diagnosis | - 2 | - 2 | - 2 | 1.17 | 1.07–1.28 | 0.001 | |
| Non-AIDS cancer | Age | 1.16 | 1.09–1.23 | <0.001 | - 2 | - 2 | - 2 |
| HCV diagnosis phase (chronic vs. acute) | - 2 | - 2 | - 2 | 16.20 | 2.00–138.3 | 0.009 | |
| Death | Age | 1.18 | 1.04–1.33 | 0.011 | - 2 | - 2 | - 2 |
| Time since HCV diagnosis to DAA’s treatment | - 2 | - 2 | - 2 | 1.13 | 1.05–1.20 | 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Álvarez, B.; Prieto-Pérez, L.; de la Cuadra-Grande, A.; Casado, M.Á.; Cabello Úbeda, A.; Al-Hayani, A.W.; Carrillo Acosta, I.; Mahillo-Fernández, I.; Górgolas Hernández-Mora, M.; Benito, J.M.; et al. The Era of DAAs: Assessing the Patients’ Characteristics, Clinical Impact, and Emergence of Comorbidities in HIV/HCV-Coinfected versus HIV-Infected Individuals. J. Clin. Med. 2024, 13, 3936. https://doi.org/10.3390/jcm13133936
Álvarez-Álvarez B, Prieto-Pérez L, de la Cuadra-Grande A, Casado MÁ, Cabello Úbeda A, Al-Hayani AW, Carrillo Acosta I, Mahillo-Fernández I, Górgolas Hernández-Mora M, Benito JM, et al. The Era of DAAs: Assessing the Patients’ Characteristics, Clinical Impact, and Emergence of Comorbidities in HIV/HCV-Coinfected versus HIV-Infected Individuals. Journal of Clinical Medicine. 2024; 13(13):3936. https://doi.org/10.3390/jcm13133936
Chicago/Turabian StyleÁlvarez-Álvarez, Beatriz, Laura Prieto-Pérez, Alberto de la Cuadra-Grande, Miguel Ángel Casado, Alfonso Cabello Úbeda, Aws W. Al-Hayani, Irene Carrillo Acosta, Ignacio Mahillo-Fernández, Miguel Górgolas Hernández-Mora, Jose M. Benito, and et al. 2024. "The Era of DAAs: Assessing the Patients’ Characteristics, Clinical Impact, and Emergence of Comorbidities in HIV/HCV-Coinfected versus HIV-Infected Individuals" Journal of Clinical Medicine 13, no. 13: 3936. https://doi.org/10.3390/jcm13133936
APA StyleÁlvarez-Álvarez, B., Prieto-Pérez, L., de la Cuadra-Grande, A., Casado, M. Á., Cabello Úbeda, A., Al-Hayani, A. W., Carrillo Acosta, I., Mahillo-Fernández, I., Górgolas Hernández-Mora, M., Benito, J. M., & Rallón, N. (2024). The Era of DAAs: Assessing the Patients’ Characteristics, Clinical Impact, and Emergence of Comorbidities in HIV/HCV-Coinfected versus HIV-Infected Individuals. Journal of Clinical Medicine, 13(13), 3936. https://doi.org/10.3390/jcm13133936






