Fundus-to-Antrum Ratio Measured with Fluoroscopy within One Week after Endoscopic Sleeve Gastroplasty Predicts Total Body Weight Loss over Time
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Follow-Up
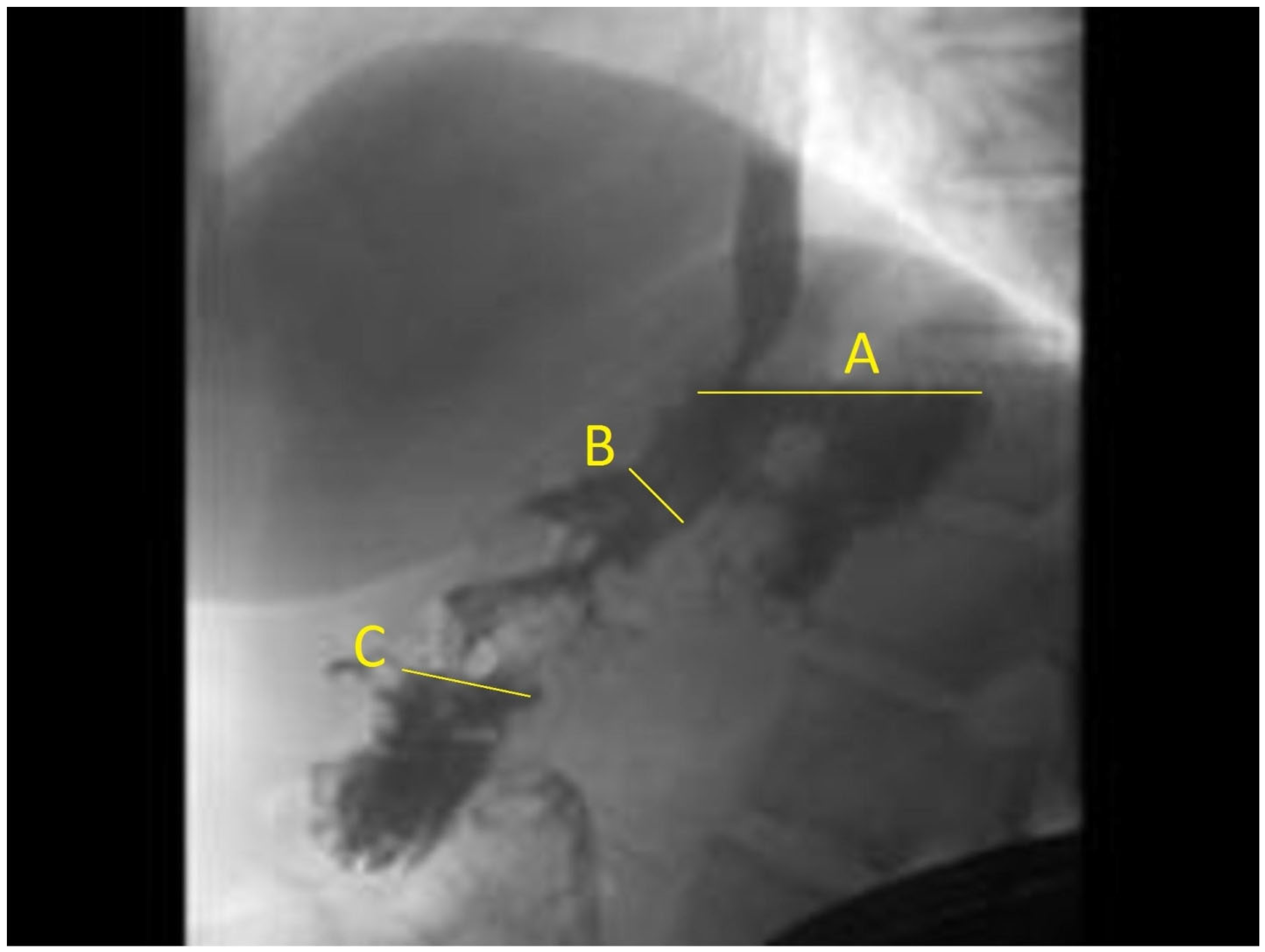
2.2. ESG Procedure
2.3. Definitions and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Prediction Model
3.2. Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, K.F.; Schatzkin, A.; Harris, T.B.; Kipnis, V.; Mouw, T.; Ballard-Barbash, R.; Hollenbeck, A.; Leitzmann, M.F. Overweight, Obesity, and Mortality in a Large Prospective Cohort of Persons 50 to 71 Years Old. N. Engl. J. Med. 2006, 355, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; for the POWER-UP Research Group; Volger, S.; Tsai, A.G.; Sarwer, D.B.; Berkowitz, R.I.; Diewald, L.K.; Carvajal, R.; Moran, C.H.; Vetter, M. Managing obesity in primary care practice: An overview with perspective from the POWER-UP study. Int. J. Obes. 2013, 37, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Garvey, W.T.; Hurley, D.L.; McMahon, M.M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S.; et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: Cosponsored by American association of clinical endocrinologists, The obesity society, and American society for metabolic & bariatric surgery. Obesity 2013, 21, S1–S27. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; de Moura, D.T.H.; Khan, A.; Bilal, M.; Ryan, M.B.; Thompson, C.C. Safety and efficacy of endoscopic sleeve gastroplasty worldwide for treatment of obesity: A systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2019, 16, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Dayyeh, B.K.A.; Bazerbachi, F.; Vargas, E.J.; Sharaiha, R.Z.; Thompson, C.C.; Thaemert, B.C.; Teixeira, A.F.; Chapman, C.G.; Kumbhari, V.; Ujiki, M.B.; et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): A prospective, multicentre, randomised trial. Lancet 2022, 400, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Sharaiha, R.Z.; Hajifathalian, K.; Kumar, R.; Saunders, K.; Mehta, A.; Ang, B.; Skaf, D.; Shah, S.; Herr, A.; Igel, L.; et al. Five-Year Outcomes of Endoscopic Sleeve Gastroplasty for the Treatment of Obesity. Clin. Gastroenterol. Hepatol. 2020, 19, 1051–1057.e2. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Gaeta, M.; Rausa, E.; Macchitella, Y.; Bonavina, L. Early Impact of Bariatric Surgery on Type II Diabetes, Hypertension, and Hyperlipidemia: A Systematic Review, Meta-Analysis and Meta-Regression on 6587 Patients. Obes. Surg. 2014, 24, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Flores, L.; Vidal, J.; Canivell, S.; Delgado, S.; Lacy, A.; Esmatjes, E. Hypertension remission 1 year after bariatric surgery: Predictive factors. Surg. Obes. Relat. Dis. 2014, 10, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Young, J.; Kale-Pradhan, P.B. Effect of Bariatric Surgery on Hypertension. Ann. Pharmacother. 2014, 48, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Mohan, B.P.; Asokkumar, R.; Khan, S.R.; Kotagiri, R.; Sridharan, G.K.; Chandan, S.; Ravikumar, N.P.; Ponnada, S.; Jayaraj, M.; Adler, D.G. Outcomes of endoscopic sleeve gastroplasty; how does it compare to laparoscopic sleeve gastrectomy? A systematic review and meta-analysis. Endosc. Int. Open 2020, 8, E558–E565. [Google Scholar] [CrossRef] [PubMed]
- Sharaiha, R.Z.; Kumta, N.A.; Saumoy, M.; Desai, A.P.; Sarkisian, A.M.; Benevenuto, A.; Tyberg, A.; Kumar, R.; Igel, L.; Verna, E.C.; et al. Endoscopic Sleeve Gastroplasty Significantly Reduces Body Mass Index and Metabolic Complications in Obese Patients. Clin. Gastroenterol. Hepatol. 2017, 15, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Sharaiha, R.Z.; Kedia, P.; Kumta, N.; DeFilippis, E.M.; Gaidhane, M.; Shukla, A.; Aronne, L.J.; Kahaleh, M. Initial experience with endoscopic sleeve gastroplasty: Technical success and reproducibility in the bariatric population. Endoscopy 2012, 47, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Brethauer, S.A.; Kim, J.; el Chaar, M.; Papasavas, P.; Eisenberg, D.; Rogers, A.; Ballem, N.; Kligman, M.; Kothari, S. Standardized outcomes reporting in metabolic and bariatric surgery. Surg. Obes. Relat. Dis. 2015, 11, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Arau, R.T.; The study group for endoscopic bariatric and metabolic therapies in the Korean Society of Gastrointestinal Endoscopy. The Efficacy and Safety of Endoscopic Sleeve Gastroplasty as an Alternative to Laparoscopic Sleeve Gastrectomy. Clin. Endosc. 2021, 54, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nava, G.; Asokkumar, R.; Lacruz, T.; Rull, A.; Beltran, L.; Bautista-Castaño, I. The effect of weight loss and exercise on Health-Related Quality of Life (HRQOL) following Endoscopic Bariatric Therapies (EBT) for obesity. Health Qual. Life Outcomes 2020, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abu Dayyeh, B.K.; Rajan, E.; Gostout, C.J. Endoscopic sleeve gastroplasty: A potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest. Endosc. 2013, 78, 530–535. [Google Scholar] [CrossRef]
- Majumder, S.; Birk, J. A review of the current status of endoluminal therapy as a primary approach to obesity management. Surg. Endosc. 2013, 27, 2305–2311. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nava, G.; Negi, A.; Bautista-Castaño, I.; Rubio, M.A.; Asokkumar, R. Gut and Metabolic Hormones Changes after Endoscopic Sleeve Gastroplasty (ESG) vs. Laparoscopic Sleeve Gastrectomy (LSG). Obes. Surg. 2020, 30, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Vargas, E.J.; Rizk, M.; Gomez-Villa, J.; Edwards, P.K.; Jaruvongvanich, V.; Storm, A.C.; Acosta, A.; Lake, D.; Fidler, J.; Bharucha, A.E.; et al. Effect of endoscopic sleeve gastroplasty on gastric emptying, motility and hormones: A comparative prospective study. Gut 2023, 72, 1073–1080. [Google Scholar] [CrossRef]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef]
- Blom, W.A.M.; Lluch, A.; Vinoy, S.; Stafleu, A.; Berg, R.v.D.; Holst, J.J.; Kok, F.J.; Hendriks, H.F.J. Effects of gastric emptying on the postprandial ghrelin response. Am. J. Physiol. Metab. 2006, 290, E389–E395. [Google Scholar] [CrossRef]

| Patient Characteristic | Total, n = 162 | Random Model Building Sample, n = 81 | Random Validation Sample, n = 81 |
|---|---|---|---|
| Age, mean (SD), years | 46 (13) | 48 (12) | 44 (13) |
| Female, n (%) | 106 (65) | 55 (68) | 51 (63) |
| BMI, kg/m2, mean (SD) | 39 (6) | 39 (6) | 38 (6) |
| Fundus-to-antrum ratio, mean (SD) | 1.2 (0.6) | 1.3 (0.7) | 1.1 (0.6) |
| Fundus-to-body ratio, mean (SD) | 3.3 (1.1) | 3.5 (1.1) | 3.2 (1.0) |
| TBWL%, mean (SD) | 16.5 (8.3) | 15.9 (7.8) | 17.1 (8.8) |
| TBWL ≥ 5%, (%) | 92 | 90 | 94 |
| TBWL ≥ 10%, n (%) | 75 | 70 | 79 |
| TBWL ≥ 15%, (%) | 50 | 48 | 52 |
| Outcome | Fundus-to-Antrum Ratio | Fundus-to-Body Ratio | ||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| TBWL 5% or more | 1.93 (0.94–3.97) | 0.075 | 1.02 (0.67–1.55) | 0.929 |
| TBWL 10% or more | 2.49 (1.31–4.71) | 0.005 | 0.84 (0.58–1.22) | 0.361 |
| TBWL 15% or more | 2.78 (1.28–6.07) | 0.010 | 0.67 (0.42–1.09) | 0.106 |
| Variable | Coefficient | Standard Error (SE) | 95% CI |
|---|---|---|---|
| Fundus-to-antrum ratio | 0.911 | 0.326 | −0.082 to −0.055 |
| Compliance | 2.038 | 0.410 | 0.273 to 1.549 |
| Baseline BMI | 0.036 | 0.032 | 1.234 to 2.843 |
| Time since procedure | −0.069 | 0.007 | −0.027 to 0.099 |
| Intercept | −2.531 | 1.354 | −5.186 to 0.124 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajifathalian, K.; Amer, K.; Shamoon, D.; Westerveld, D.; Aronne, L.; Mehta, A.; Wong, A.; Lo, G.; Oh, S.; Kierans, A.S.; et al. Fundus-to-Antrum Ratio Measured with Fluoroscopy within One Week after Endoscopic Sleeve Gastroplasty Predicts Total Body Weight Loss over Time. J. Clin. Med. 2024, 13, 3933. https://doi.org/10.3390/jcm13133933
Hajifathalian K, Amer K, Shamoon D, Westerveld D, Aronne L, Mehta A, Wong A, Lo G, Oh S, Kierans AS, et al. Fundus-to-Antrum Ratio Measured with Fluoroscopy within One Week after Endoscopic Sleeve Gastroplasty Predicts Total Body Weight Loss over Time. Journal of Clinical Medicine. 2024; 13(13):3933. https://doi.org/10.3390/jcm13133933
Chicago/Turabian StyleHajifathalian, Kaveh, Kamal Amer, Dema Shamoon, Donevan Westerveld, Louis Aronne, Amit Mehta, Angela Wong, Grace Lo, Sarah Oh, Andrea Siobhan Kierans, and et al. 2024. "Fundus-to-Antrum Ratio Measured with Fluoroscopy within One Week after Endoscopic Sleeve Gastroplasty Predicts Total Body Weight Loss over Time" Journal of Clinical Medicine 13, no. 13: 3933. https://doi.org/10.3390/jcm13133933
APA StyleHajifathalian, K., Amer, K., Shamoon, D., Westerveld, D., Aronne, L., Mehta, A., Wong, A., Lo, G., Oh, S., Kierans, A. S., Hassan, K. M., Lahooti, A., & Sharaiha, R. Z. (2024). Fundus-to-Antrum Ratio Measured with Fluoroscopy within One Week after Endoscopic Sleeve Gastroplasty Predicts Total Body Weight Loss over Time. Journal of Clinical Medicine, 13(13), 3933. https://doi.org/10.3390/jcm13133933





