Coronary Artery Anomalies: A Computed Tomography Angiography Pictorial Review
Abstract
1. Introduction
2. Embryology
3. Anatomy of the Coronary Arteries
4. Considerations of the Scanning Protocol
- Maximum intensity projections (MIP): these images highlight the most intense areas of contrast.
- Curved multiplanar reformats (cMPRs): allowing for visualization of the coronary arteries in any plane.
- Volume rendering technique (VRT): images provide a three-dimensional view of the coronary arteries (Videos S1–S3).
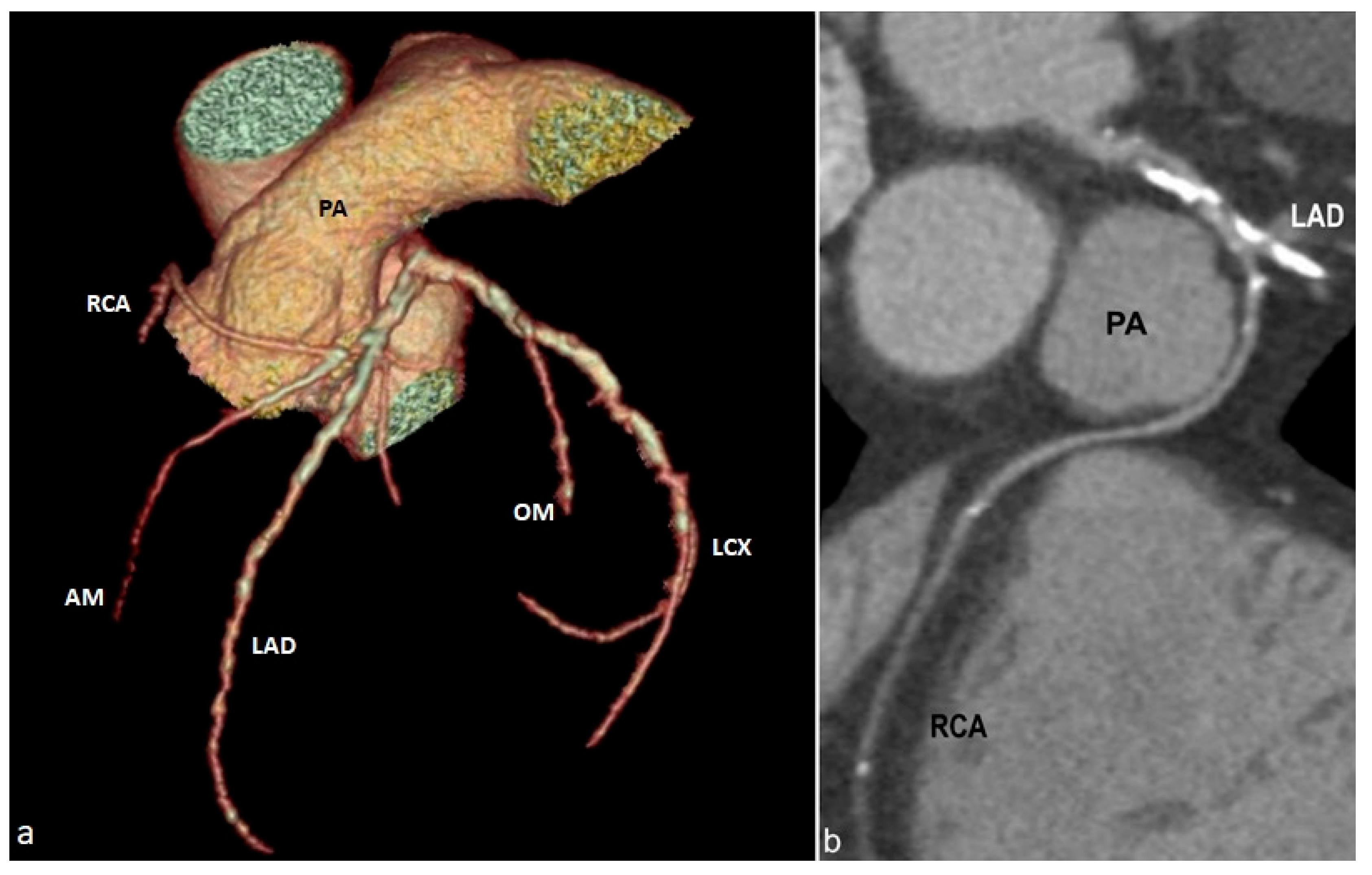
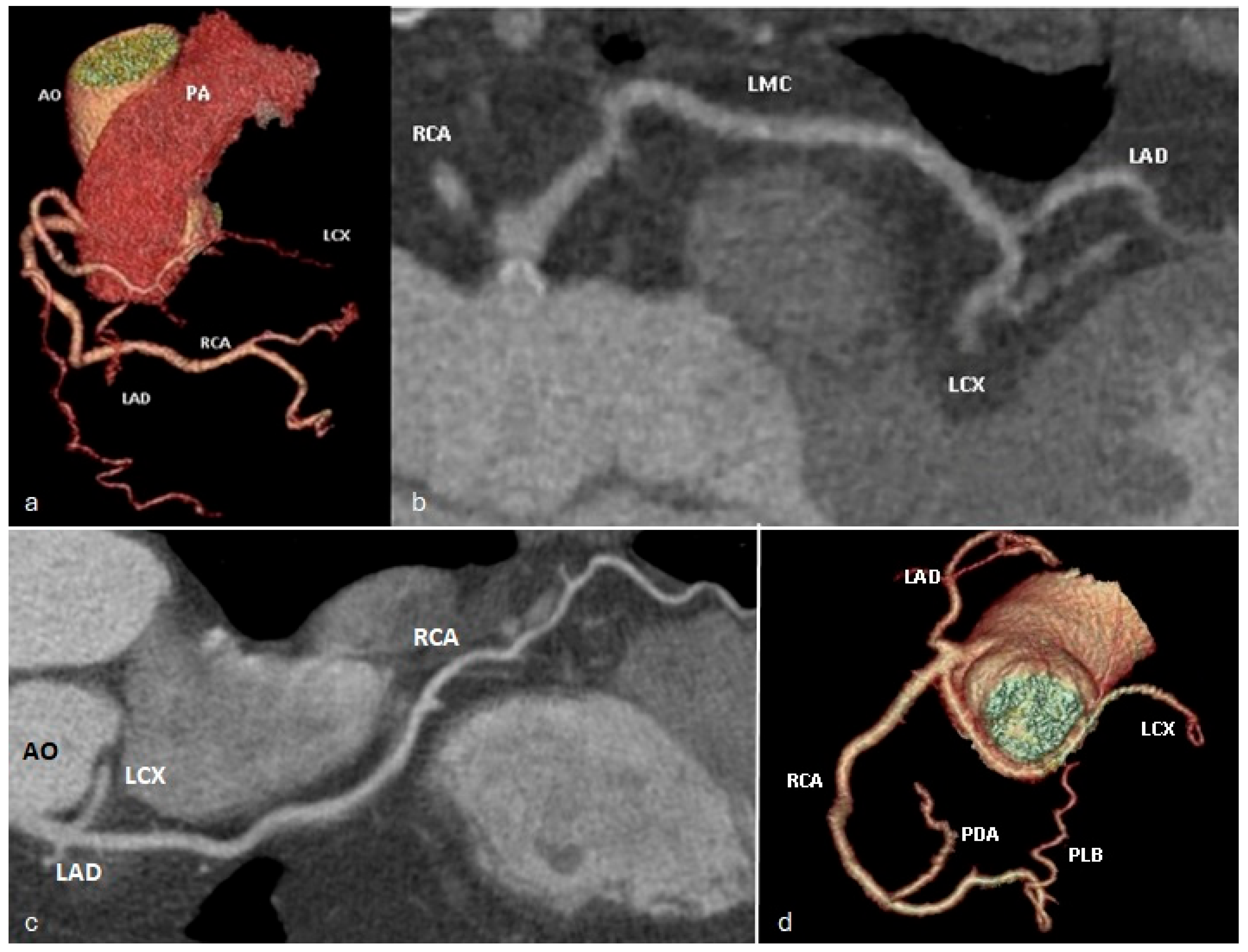
5. Pictorial Review of Coronary Artery Anomalies
- Ectopic origin of a coronary artery arising from the pulmonary trunk or directly from the right or left pulmonary arteries;
- Anomalous proximal course with the vessel running between the aorta and the pulmonary trunk (interarterial course), when arising from the opposite or non-coronary sinus of Valsalva;
- Coronary artery fistulae; however, only when resulting in a steal phenomenon (impaired myocardial perfusion due to the diversion of blood flow) or a substantial shunt.
- Anomalies of origin
- II.
- Anomalies of course
- III.
- Anomalies of termination
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALCAPA | Anomalous left coronary artery from the pulmonary artery |
| AM | Acute marginal branch |
| AO | Aorta |
| CAA | Coronary artery anomalies |
| cMPR | Curved multiplanar reformat |
| CONUS A | Conus artery |
| CTCA | CT coronary angiography |
| D1 | First diagonal branch |
| D2 | Second diagonal branch |
| IB | Intercostal branch |
| LAD | Left anterior descending |
| LCX | Left circumflex artery |
| L-LAD | Long branch of left anterior descending |
| LMC | Left main coronary artery |
| LPA | Left pulmonary artery |
| MIP | Maximum intensity projections |
| OM | Obtuse marginal branch |
| PA | Pulmonary artery |
| PDA | Posterior descending artery |
| PLB | Postero-lateral branch |
| RCA | Right coronary artery |
| RI | Ramus intermedius |
| RPA | Right pulmonary artery |
| S-LAD | Short branch of left anterior descending |
| VEGF | Vascular endothelial growth factor |
| VR | Volume rendering |
References
- Agarwal, P.P.; Dennie, C.; Pena, E.; Nguyen, E.; LaBounty, T.; Yang, B.; Patel, S. Anomalous Coronary Arteries That Need Intervention: Review of Pre- and Postoperative Imaging Appearances. Radiographics 2017, 37, 740–757. [Google Scholar] [CrossRef]
- Beecham, R.; Prater, S.; Batlle, J. Coronary Artery Anomalies. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Chen, Q.; Pan, T.; Wang, Y.N.; Schoepf, U.J.; Bidwell, S.L.; Qiao, H.; Feng, Y.; Xu, C.; Xu, H.; Xie, G.; et al. A Coronary CT Angiography Radiomics Model to Identify Vulnerable Plaque and Predict Cardiovascular Events. Radiology 2023, 307, e221693. [Google Scholar] [CrossRef]
- Baz, R.O.; Gherghescu, G.; Mustafa, A.; Enyedi, M.; Scheau, C.; Baz, R.A. The Role of CT Imaging in a Fractured Coronary Stent with Pseudoaneurysm Formation. Diagnostics 2024, 14, 840. [Google Scholar] [CrossRef]
- Cademartiri, F.; Casolo, G.; Clemente, A.; Seitun, S.; Mantini, C.; Bossone, E.; Saba, L.; Sverzellati, N.; Nistri, S.; Punzo, B.; et al. Coronary CT angiography: A guide to examination, interpretation, and clinical indications. Expert Rev. Cardiovasc. Ther. 2021, 19, 413–425. [Google Scholar] [CrossRef]
- Sharma, B.; Chang, A.; Red-Horse, K. Coronary Artery Development: Progenitor Cells and Differentiation Pathways. Annu. Rev. Physiol. 2017, 79, 1–19. [Google Scholar] [CrossRef]
- Ramai, D.; Lai, J.; Monzidelis, C.; Reddy, S. Coronary Artery Development: Origin, Malformations, and Translational Vascular Reparative Therapy. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 292–300. [Google Scholar] [CrossRef]
- Tomanek, R.; Angelini, P. Embryology of coronary arteries and anatomy/pathophysiology of coronary anomalies. A comprehensive update. Int. J. Cardiol. 2019, 281, 28–34. [Google Scholar] [CrossRef]
- Walker, D.L.; Vacha, S.J.; Kirby, M.L.; Lo, C.W. Connexin43 deficiency causes dysregulation of coronary vasculogenesis. Dev. Biol. 2005, 284, 479–498. [Google Scholar] [CrossRef]
- Spicer, D.E.; Henderson, D.J.; Chaudhry, B.; Mohun, T.J.; Anderson, R.H. The anatomy and development of normal and abnormal coronary arteries. Cardiol. Young 2015, 25, 1493–1503. [Google Scholar] [CrossRef]
- Pérez-Pomares, J.M.; de la Pompa, J.L.; Franco, D.; Henderson, D.; Ho, S.Y.; Houyel, L.; Kelly, R.G.; Sedmera, D.; Sheppard, M.; Sperling, S.; et al. Congenital coronary artery anomalies: A bridge from embryology to anatomy and pathophysiology—A position statement of the development, anatomy, and pathology ESC Working Group. Cardiovasc. Res. 2016, 109, 204–216. [Google Scholar] [CrossRef]
- Phillips, H.M.; Rhee, H.J.; Murdoch, J.N.; Hildreth, V.; Peat, J.D.; Anderson, R.H.; Copp, A.J.; Chaudhry, B.; Henderson, D.J. Disruption of Planar Cell Polarity Signaling Results in Congenital Heart Defects and Cardiomyopathy Attributable to Early Cardiomyocyte Disorganization. Circ. Res. 2007, 101, 137–145. [Google Scholar] [CrossRef]
- Kiefer, T.L.; Crowley, A.L.; Jaggers, J.; Harrison, J.K. Coronary arteriovenous fistulae: The complexity of coronary artery-to-coronary sinus connections. Tex. Heart Inst. J. 2012, 39, 218–222. [Google Scholar]
- Díez-Villanueva, P.; Gutiérrez-Ibañes, E.; Cuerpo-Caballero, G.P.; Sanz-Ruiz, R.; Abeytua, M.; Soriano, J.; Sarnago, F.; Elízaga, J.; González-Pinto, A.; Fernández-Avilés, F. Direct injury to right coronary artery in patients undergoing tricuspid annuloplasty. Ann. Thorac. Surg. 2014, 97, 1300–1305. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Adverse Cardiac Remodelling after Acute Myocardial Infarction: Old and New Biomarkers. Dis. Markers 2020, 2020, 1215802. [Google Scholar] [CrossRef]
- Aggarwal, R.; Potel, K.N.; Shao, A.; So, S.W.; Swingen, C.; Reyes, C.P.; Rose, R.; Wright, C.; Hocum Stone, L.L.; McFalls, E.O.; et al. An Adjuvant Stem Cell Patch with Coronary Artery Bypass Graft Surgery Improves Diastolic Recovery in Porcine Hibernating Myocardium. Int. J. Mol. Sci. 2023, 24, 5475. [Google Scholar] [CrossRef]
- Chen, J.; Ceholski, D.K.; Liang, L.; Fish, K.; Hajjar, R.J. Variability in coronary artery anatomy affects consistency of cardiac damage after myocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H275–H282. [Google Scholar] [CrossRef]
- Baz, R.A.; Jurja, S.; Ciuluvica, R.; Scheau, C.; Baz, R. Morphometric study regarding ophthalmic and internal carotid arteries utilizing computed tomography angiography. Exp. Ther. Med. 2022, 23, 112. [Google Scholar] [CrossRef]
- Baquero, G.A.; Azarrafiy, R.; de Marchena, E.J.; Carrillo, R.G. Hybrid off-pump coronary artery bypass grafting surgery and transaortic transcatheter aortic valve replacement: Literature review of a feasible bailout for patients with complex coronary anatomy and poor femoral access. J. Card. Surg. 2019, 34, 591–597. [Google Scholar] [CrossRef]
- Enyedi, M.; Scheau, C.; Baz, R.O.; Didilescu, A.C. Circle of Willis: Anatomical variations of configuration. A magnetic resonance angiography study. Folia Morphol. 2023, 82, 24–29. [Google Scholar] [CrossRef]
- Baz, R.A.; Scheau, C.; Niscoveanu, C.; Bordei, P. Morphometry of the Entire Internal Carotid Artery on CT Angiography. Medicina 2021, 57, 832. [Google Scholar] [CrossRef]
- Shahoud, J.S.; Ambalavanan, M.; Tivakaran, V.S. Cardiac Dominance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Angelini, P. Normal and anomalous coronary arteries: Definitions and classification. Am. Heart J. 1989, 117, 418–434. [Google Scholar] [CrossRef]
- Chiribiri, A.; Ishida, M.; Nagel, E.; Botnar, R.M. Coronary Imaging With Cardiovascular Magnetic Resonance: Current State of the Art. Prog. Cardiovasc. Dis. 2011, 54, 240–252. [Google Scholar] [CrossRef]
- Khalid, N.; Chhabra, L.; Ahmad, S.A.; Sareen, P. A Large-Sized Left Main Coronary Artery with Quadfurcation: A Rare Anatomy. Conn. Med. 2015, 79, 211–212. [Google Scholar]
- Tyczyński, P.; Pręgowski, J.; Skowroński, J.; Wojtkowska, I.; Brutkiewicz, A.; Witkowski, A. Left main coronary artery quadrifurcation and acute coronary syndrome. Pol. Heart J. 2017, 75, 398. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef]
- Ispas, V.; Iliescu, D.; Baz, R.; Bordei, P. Specific morphological characteristics of the coronary arteries. ARS Medica Tomitana 2013, 2, 112–116. [Google Scholar] [CrossRef]
- Sun, Z.; Choo, G.H.; Ng, K.H. Coronary CT angiography: Current status and continuing challenges. Br. J. Radiol. 2012, 85, 495–510. [Google Scholar] [CrossRef]
- Timofticiuc, I.-A.; Călinescu, O.; Iftime, A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Biomaterials Adapted to Vat Photopolymerization in 3D Printing: Characteristics and Medical Applications. J. Funct. Biomater. 2024, 15, 7. [Google Scholar] [CrossRef]
- Sun, Z.; Ng, K.H. Multislice CT angiography in cardiac imaging. Part II: Clinical applications in coronary artery disease. Singap. Med. J. 2010, 51, 282–289. [Google Scholar]
- Graidis, C.; Dimitriadis, D.; Karasavvidis, V.; Dimitriadis, G.; Argyropoulou, E.; Economou, F.; George, D.; Antoniou, A.; Karakostas, G. Prevalence and characteristics of coronary artery anomalies in an adult population undergoing multidetector-row computed tomography for the evaluation of coronary artery disease. BMC Cardiovasc. Disord. 2015, 15, 112. [Google Scholar] [CrossRef]
- Gräni, C.; Benz, D.C.; Schmied, C.; Vontobel, J.; Possner, M.; Clerc, O.F.; Mikulicic, F.; Stehli, J.; Fuchs, T.A.; Pazhenkottil, A.P.; et al. Prevalence and characteristics of coronary artery anomalies detected by coronary computed tomography angiography in 5634 consecutive patients in a single centre in Switzerland. Swiss Med. Wkly. 2016, 146, w14294. [Google Scholar] [CrossRef]
- Ilić, D.S.; Stojanov, D.; Koraćević, G.; Petrović, S.; Radovanović, Z.; Arsić, S. The prevalence of coronary artery anomalies in adults: Studied with computed tomography coronary angiography. Vojnosanit. Pregl. 2018, 75, 16–22. [Google Scholar] [CrossRef]
- Schmitt, R.; Froehner, S.; Brunn, J.; Wagner, M.; Brunner, H.; Cherevatyy, O.; Gietzen, F.; Christopoulos, G.; Kerber, S.; Fellner, F. Congenital anomalies of the coronary arteries: Imaging with contrast-enhanced, multidetector computed tomography. Eur. Radiol. 2005, 15, 1110–1121. [Google Scholar] [CrossRef]
- Onciu, M.; Baz, R.; Onciu, C. Particularités de distribution des branches des artères coronnaires. Morphologie 2005, 89, 189. [Google Scholar] [CrossRef]
- Greenberg, M.A.; Fish, B.G.; Spindola-Franco, H. Congenital anomalies of the coronary arteries. Classification and significance. Radiol. Clin. N. Am. 1989, 27, 1127–1146. [Google Scholar] [CrossRef]
- Angelini, P. Coronary Artery Anomalies: An entity in search of an identity. Circulation 2007, 115, 1296–1305. [Google Scholar] [CrossRef]
- Villa, A.D.; Sammut, E.; Nair, A.; Rajani, R.; Bonamini, R.; Chiribiri, A. Coronary artery anomalies overview: The normal and the abnormal. World J. Radiol. 2016, 8, 537–555. [Google Scholar] [CrossRef]
- Gentile, F.; Castiglione, V.; Caterina, R.D. Coronary Artery Anomalies. Circulation 2021, 144, 983–996. [Google Scholar] [CrossRef]
- Yamanaka, O.; Hobbs, R.E. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Catheter. Cardiovasc. Diagn. 1990, 21, 28–40. [Google Scholar] [CrossRef]
- Ogden, J.A. Congenital anomalies of the coronary arteries. Am. J. Cardiol. 1970, 25, 474–479. [Google Scholar] [CrossRef]
- Levin, D.C.; Fellows, K.E.; Abrams, H.L. Hemodynamically significant primary anomalies of the coronary arteries. Angiographic aspects. Circulation 1978, 58, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Rigatelli, G.; Docali, G.; Rossi, P.; Bandello, A.; Rigatelli, G. Validation of a Clinical-Significance-Based Classification of Coronary Artery Anomalies. Angiology 2005, 56, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Reagan, K.; Boxt, L.M.; Katz, J. Introduction to Coronary Arteriography. Radiol. Clin. N. Am. 1994, 32, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Peña, E.; Nguyen, E.T.; Merchant, N.; Dennie, C. ALCAPA Syndrome: Not Just a Pediatric Disease. Radiographics 2009, 29, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C.; Siegel, R.J.; Zipes, D.P. Origin of the right coronary artery from the left sinus of Valsalva and its functional consequences: Analysis of 10 necropsy patients. Am. J. Cardiol. 1982, 49, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Çitaku, H.; Kamberi, L.; Gorani, D.; Koçinaj, D.; Krasniqi, X. Anomalous Origin of Left Circumflex Artery. Med. Arch. 2015, 69, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Bhattad, P.B.; Ramsaran, E. Anomalous Origin of the Left Circumflex Coronary Artery: Approach in Acute Coronary Syndrome. Cureus 2022, 14, e29330. [Google Scholar] [CrossRef] [PubMed]
- Cheezum, M.K.; Liberthson, R.R.; Shah, N.R.; Villines, T.C.; O’Gara, P.T.; Landzberg, M.J.; Blankstein, R. Anomalous Aortic Origin of a Coronary Artery From the Inappropriate Sinus of Valsalva. J. Am. Coll. Cardiol. 2017, 69, 1592–1608. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.P.; Kazerooni, E.A. Dual Left Anterior Descending Coronary Artery: CT Findings. Am. J. Roentgenol. 2008, 191, 1698–1701. [Google Scholar] [CrossRef]
- Frescura, C.; Basso, C.; Thiene, G.; Corrado, D.; Pennelli, T.; Angelini, A.; Daliento, L. Anomalous origin of coronary arteries and risk of sudden death: A study based on an autopsy population of congenital heart disease. Hum. Pathol. 1998, 29, 689–695. [Google Scholar] [CrossRef]
- Menke, D.M.; Waller, B.F.; Bless, J.E. Hypoplastic Coronary Arteries and High Takeoff Position of the Right Coronary Ostium: A Fatal Combination of Congenital Coronary Artery Anomalies in an Amateur Athlete. Chest 1985, 88, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Shriki, J.E.; Shinbane, J.S.; Rashid, M.A.; Hindoyan, A.; Withey, J.G.; DeFrance, A.; Cunningham, M.; Oliveira, G.R.; Warren, B.H.; Wilcox, A. Identifying, Characterizing, and Classifying Congenital Anomalies of the Coronary Arteries. Radiographics 2012, 32, 453–468. [Google Scholar] [CrossRef]
- Van Geuns, R.J.M.; Cademartiri, F. Anatomy of the Coronary Arteries and Veins in CT Imaging. In CT of the Heart: Principles and Applications; Schoepf, U.J., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 219–227. [Google Scholar] [CrossRef]
- Ispas, V.; Bordei, P.; Iliescu, D.; Baz, R. Coronary arteries morphometry and their vascular territories. ARS Medica Tomitana 2013, 3, 130–135. [Google Scholar] [CrossRef]
- Al-Mohaissen, M.; Heilbron, B.; Leipsic, J.; Ignaszewski, A. Anomalous origin of the entire coronary system by three separate ostia within the right coronary sinus—A rarely observed coronary anomaly. Can. J. Cardiol. 2010, 26, e206–e208. [Google Scholar] [CrossRef] [PubMed]
- Al Umairi, R.; Al-khouri, M. Prevalence, Spectrum, and Outcomes of Single Coronary Artery Detected on Coronary Computed Tomography Angiography (CCTA). Radiol. Res. Pract. 2019, 2019, 2940148. [Google Scholar] [CrossRef] [PubMed]
- Zeina, A.R.; Blinder, J.; Sharif, D.; Rosenschein, U.; Barmeir, E. Congenital coronary artery anomalies in adults: Non-invasive assessment with multidetector CT. Br. J. Radiol. 2014, 82, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Salih, M.; Abdel-Hafez, O.; Ibrahim, R.; Halabi, A.R.; Aloka, F. A Single Coronary Artery Anomaly: Right Coronary Artery as a Branch From the Left Anterior Descending Artery. Cureus 2020, 12, e9801. [Google Scholar] [CrossRef] [PubMed]
- Yurtdaş, M.; Gülen, O. Anomalous origin of the right coronary artery from the left anterior descending artery: Review of the literature. Cardiol. J. 2012, 19, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Bordei, P.; Iliescua, D.M.; Dina, C.; Niţu, R.; Baz, R. Particularités morphologiques des ponts myocardiques diagnostiqués par coronarographie CT. Morphologie 2016, 100, 135–136. [Google Scholar] [CrossRef]
- Nakanishi, R.; Rajani, R.; Ishikawa, Y.; Ishii, T.; Berman, D.S. Myocardial bridging on coronary CTA: An innocent bystander or a culprit in myocardial infarction? J. Cardiovasc. Comput. Tomogr. 2012, 6, 3–13. [Google Scholar] [CrossRef]
- Rajendran, R.; Hegde, M. The prevalence of myocardial bridging on multidetector computed tomography and its relation to coronary plaques. Pol. J. Radiol. 2019, 84, 478–483. [Google Scholar] [CrossRef]
- Sternheim, D.; Power, D.A.; Samtani, R.; Kini, A.; Fuster, V.; Sharma, S. Myocardial Bridging: Diagnosis, Functional Assessment, and Management: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 2196–2212. [Google Scholar] [CrossRef] [PubMed]
- Celik, T.; Bozlar, U.; Ozturk, C.; Balta, S.; Verim, S.; Demir, M.; Demirkol, S.; Iyisoy, A. A new anomaly of the left anterior descending artery: Type X dual LAD. Indian Heart J. 2015, 67, S14–S17. [Google Scholar] [CrossRef]
- Bozlar, U.; Uğurel, M.; Sarı, S.; Akgün, V.; Örs, F.; Taşar, M. Prevalence of dual left anterior descending artery variations in CT angiography. Diagn. Interv. Radiol. 2015, 21, 34–41. [Google Scholar] [CrossRef]
- Jariwala, P.; Jadhav, K.P.; Koduganti, S. Dual left anterior descending artery: Diagnostic criteria and novel classification. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Nepal, S.; Annamaraju, P. Coronary Arteriovenous Fistula. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Zenooz, N.A.; Habibi, R.; Mammen, L.; Finn, J.P.; Gilkeson, R.C. Coronary Artery Fistulas: CT Findings. Radiographics 2009, 29, 781–789. [Google Scholar] [CrossRef]
- Angelini, P. Coronary artery anomalies--current clinical issues: Definitions, classification, incidence, clinical relevance, and treatment guidelines. Tex. Heart Inst. J. 2002, 29, 271–278. [Google Scholar] [PubMed]
- Young, P.M.; Gerber, T.C.; Williamson, E.E.; Julsrud, P.R.; Herfkens, R.J. Cardiac Imaging: Part 2, Normal, Variant, and Anomalous Configurations of the Coronary Vasculature. Am. J. Roentgenol. 2011, 197, 816–826. [Google Scholar] [CrossRef]
- Pleva, L.; Jonszta, T.; Kukla, P. Congenital coronary anomalies. Cor Et Vasa 2014, 56, e27–e36. [Google Scholar] [CrossRef]
- Kim, S.Y.; Seo, J.B.; Do, K.-H.; Heo, J.-N.; Lee, J.S.; Song, J.-W.; Choe, Y.H.; Kim, T.H.; Yong, H.S.; Choi, S.I.; et al. Coronary Artery Anomalies: Classification and ECG-gated Multi–Detector Row CT Findings with Angiographic Correlation. Radiographics 2006, 26, 317–333. [Google Scholar] [CrossRef]
- Abreu, G.; Nabais, S.; Enes, V.; Marques, J.; Costa, J.; Correia, A. Arcada coronária—Uma anomalia rara da circulação coronária. Rev. Port. Cardiol. 2014, 33, 241.e1. [Google Scholar] [CrossRef] [PubMed]
- Carangal, V.P.; Dehmer, G.J. Intercoronary communication between the circumflex and right coronary arteries. Clin. Cardiol. 2000, 23, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.D.; Tu, P.; Gong, Y.; Song, W.H.; Zhang, L.; Wang, H.R. Two cases of intercoronary communication between circumflex artery and right coronary artery. J. Geriatr. Cardiol. 2019, 16, 384–386. [Google Scholar] [CrossRef] [PubMed]
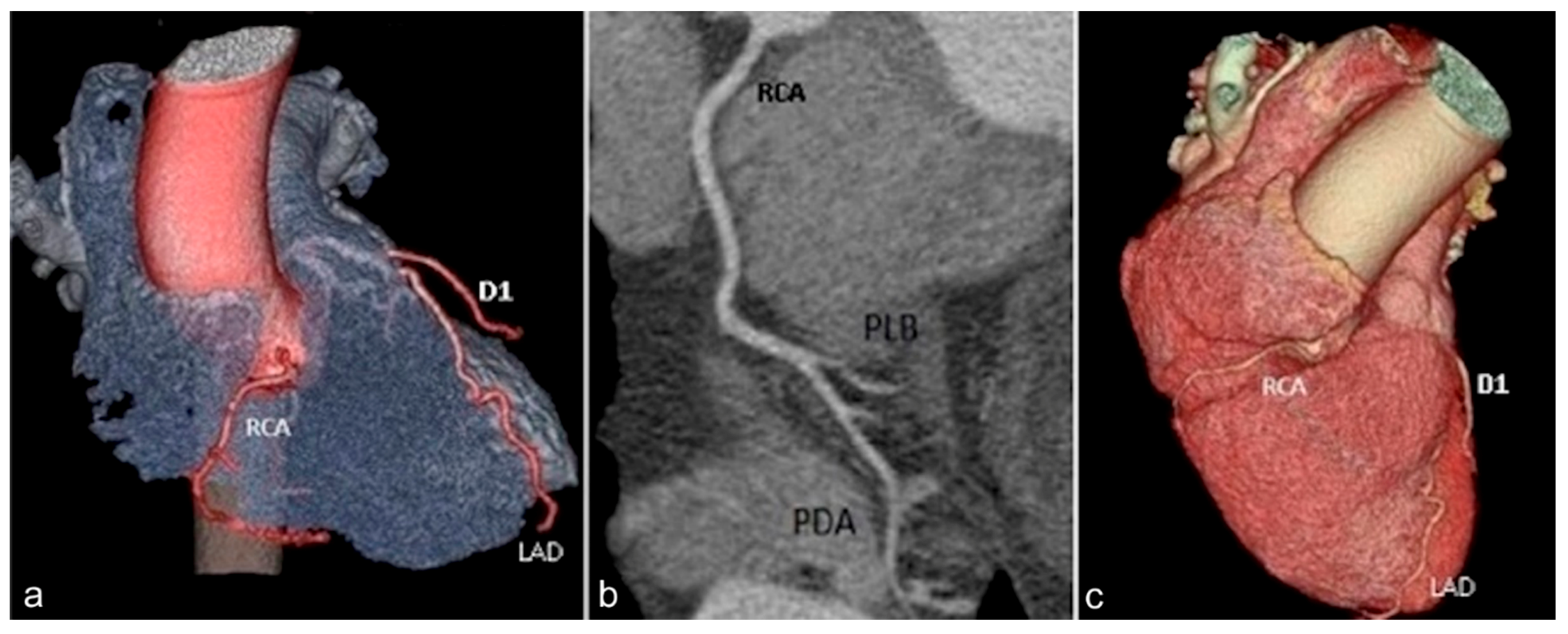





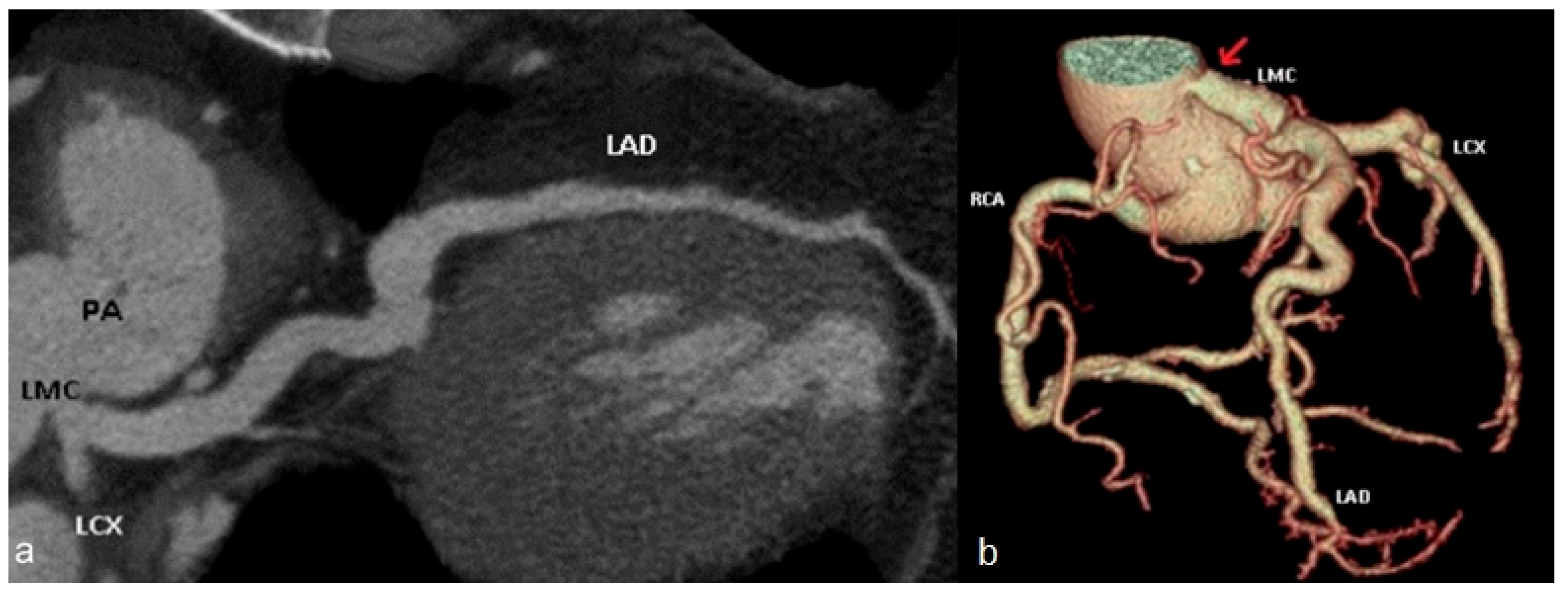

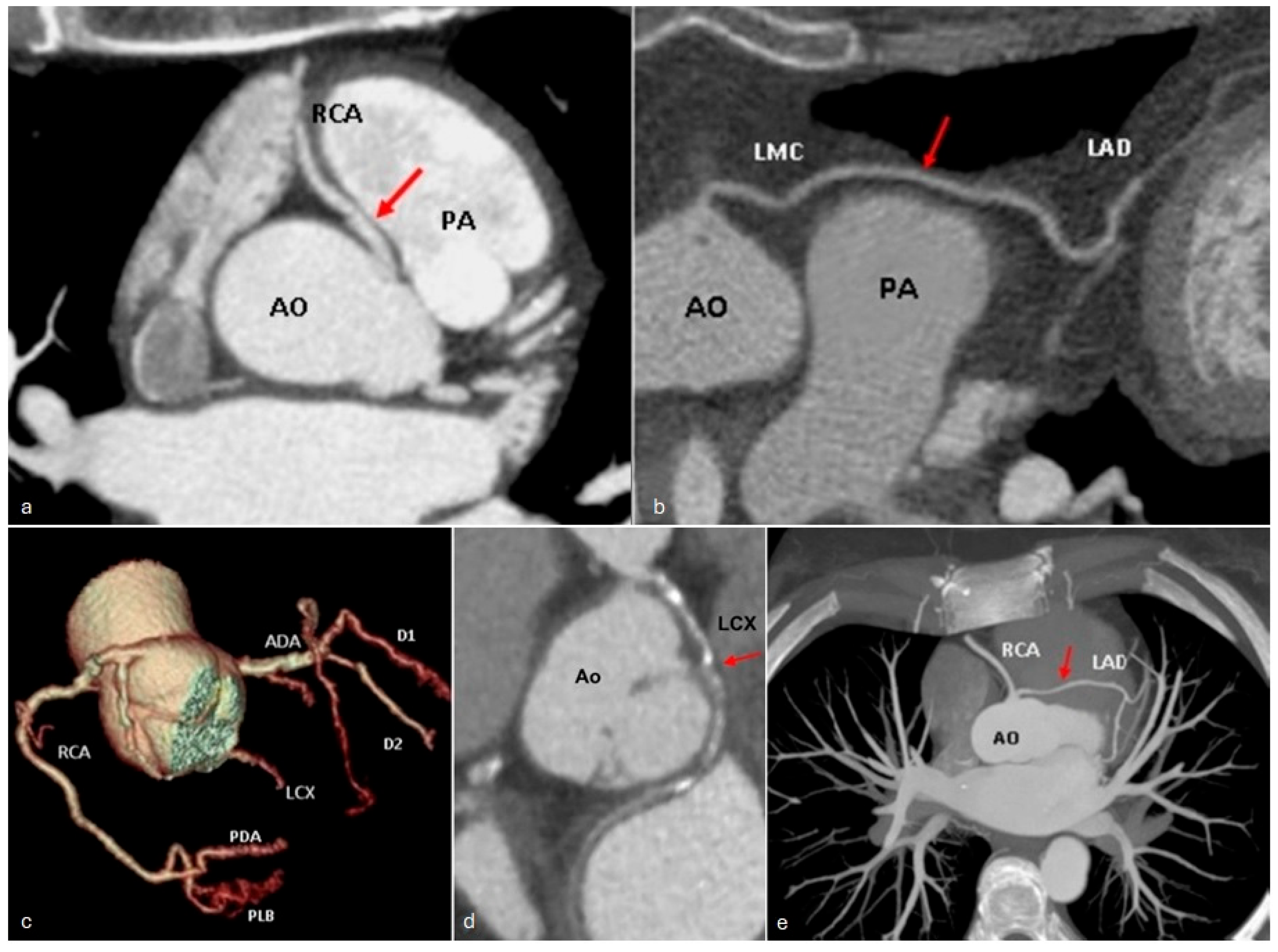
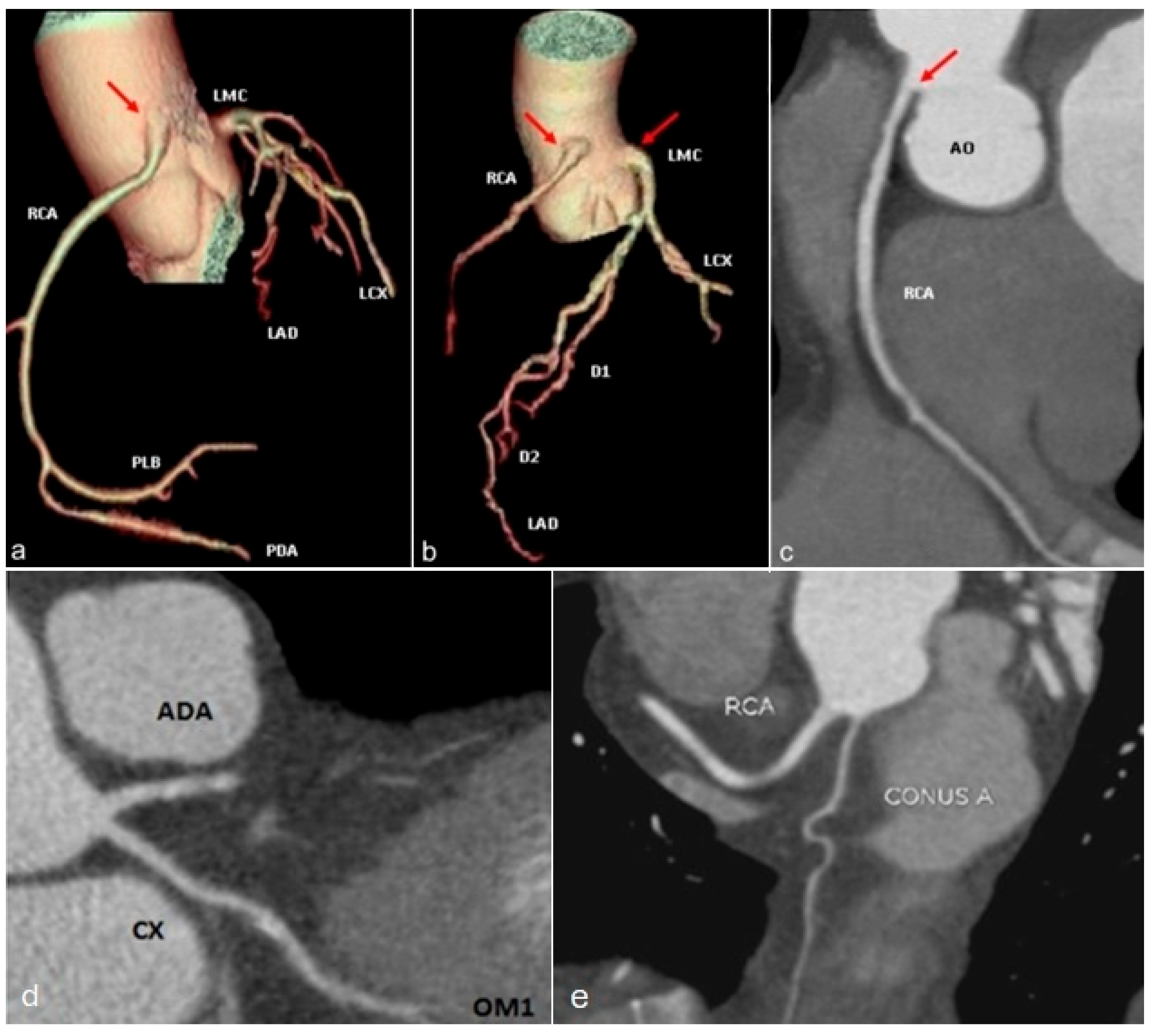




| Hemodynamically Significant | Hemodynamically Insignificant |
|---|---|
| Anomalies of origin | |
| Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) | Multiple ostia |
| Abnormal origin of a coronary artery from the opposite coronary sinus or non-coronary sinus with an interarterial course | High take-off |
Abnormal origin of a coronary artery from the opposite coronary sinus or non-coronary sinus with:
| |
| Separate emergence of the three coronary arteries through distinct ostia from the right coronary sinus | |
| Single coronary artery | |
| Anomalies of course | |
| Myocardial bridging * | |
| Duplication | |
| Anomalies of termination | |
| Coronary artery fistula * | |
| Coronary arcade | |
| Extracardiac systemic termination | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baz, R.O.; Refi, D.; Scheau, C.; Savulescu-Fiedler, I.; Baz, R.A.; Niscoveanu, C. Coronary Artery Anomalies: A Computed Tomography Angiography Pictorial Review. J. Clin. Med. 2024, 13, 3920. https://doi.org/10.3390/jcm13133920
Baz RO, Refi D, Scheau C, Savulescu-Fiedler I, Baz RA, Niscoveanu C. Coronary Artery Anomalies: A Computed Tomography Angiography Pictorial Review. Journal of Clinical Medicine. 2024; 13(13):3920. https://doi.org/10.3390/jcm13133920
Chicago/Turabian StyleBaz, Radu Octavian, Deria Refi, Cristian Scheau, Ilinca Savulescu-Fiedler, Radu Andrei Baz, and Cosmin Niscoveanu. 2024. "Coronary Artery Anomalies: A Computed Tomography Angiography Pictorial Review" Journal of Clinical Medicine 13, no. 13: 3920. https://doi.org/10.3390/jcm13133920
APA StyleBaz, R. O., Refi, D., Scheau, C., Savulescu-Fiedler, I., Baz, R. A., & Niscoveanu, C. (2024). Coronary Artery Anomalies: A Computed Tomography Angiography Pictorial Review. Journal of Clinical Medicine, 13(13), 3920. https://doi.org/10.3390/jcm13133920








